Paper - Congenital absence of the kidney
| Embryology - 27 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Boyden EA. Congenital absence of the kidney - an interpretation based on a 10-mm human embryo exhibiting unilateral renal agenesis. (1932) Anat. Rec. 52(4):325-349.
| Online Editor |
|---|
| This historic 1932 paper by Boyden describes the renal abnormality of congenital absence of the kidney.
|
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
Congenital Absence of the Kidney - An interpretation based on a 10 mm Human Embryo exhibiting Unilateral Renal Agenesis
Edward A. Boyden
Institute of Anatomy, University of Minnesota
Five Text Figures And Five Plates (Eighteen Figures)
Introduction
As various authors have pointed out, unilateral absence of the kidney has been known since the time of Aristotle, who observed that although an animal is never found without a heart yet, indeed, it may be found “without spleen, also with two spleens and with one kidney.” In man, according to the most recent survey of the literature (Collins, ’31), the incidence of cases is less than 1: 1000, in which respect it resembles the horseshoe kidney; also it seems to occur more often on the left than on the right side and somewhat more frequently in men than in women. But, as Braasch has pointed out in his discussion of Collins’ paper, it is impossible to rule out early destruction of one kidney with disappearance of most of the renal and ureteral tissue, so that all cases in which the kidney is lacking cannot be attributed to embryonic renal agenesis. Nor is it always possible, because of resorption of embryonic vestiges, to reconstruct the exact sequence of events. Hence, the discovery of a young human embryo in which the kidney of one side is in process of disappearance provides, for the first time, direct information about the cause of this malformation in man.
The embryo in question, collected by C. M. Jackson in 1905 (no. 134 of the Minnesota Collection), measured 10 mm., crown-rump, in alcohol. Although it was slightly macerated, having been deposited for a year in weak formalin by the attending physician, all its tissues were easily recognizable and suitable for reconstruction. Aside from the nephric system the only gross defect appeared in the spinal cord, the lumen of which, in the region below the heart, was cross shaped when viewed in transverse section. In addition, the ventral pancreas had not yet appeared and the dorsal pancreas was less developed than it should have been at this stage.
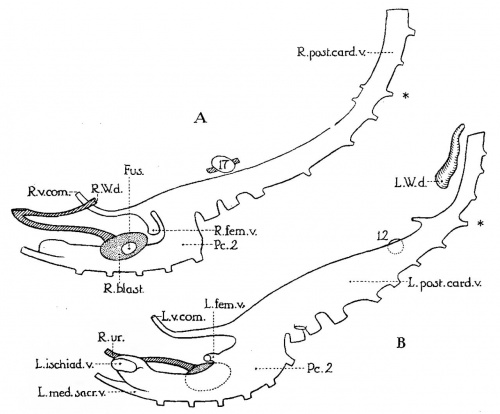
As revealed by graphic reconstruction (pl. 1) the right Wolflian duct has reached the cloaca and has given rise to a ureter which, in turn, has grown into the blastema of that side and is apparently developing into a normal kidney. On the left side (L.W.d.) the Wolffian duct, in its downgrowth from the pronephros, has reached no farther than the level of the vitelline artery. Furthermore, it has a larger diameter than the one on the right side (fig. 10), suggesting that it has been dilated by the excretions of mesonephric tubules to which it is attached. If so, this is the first physiological evidence of function in the human mesonephros.[1]
Following the failure of the left Wolflian duct to reach the cloaca, no ureter has developed on that side; nevertheless, the left blastema has appeared (figs. 6, 7, and 12), although it is not differentiating into tubules. The significance of this independent origin of the blastema is discussed in connection with experimental findings (p. 332), but it should be noted at this time that the inductive influence of the right ureter is not sufficient to activate the nephrogenic tissue of the left side even though the two blastemas are fused. This is in accord with Brown ’s observations on a strain of mouse embryos with inherited kidney defects. She states that it is necessary for the ureter to enter the blastema before the latter will differentiate into tubules.
Further examination of plate 1 reveals an incomplete development of the Wolflian bodies. In embryos of this length (between 8 and 12 mm.) there should be from thirtythree to thirty-eight mesonephric tubules (Felix; Shikinami). Yet in this embryo there are only twelve mesonephric corpuscles in the left Wolffian body and seventeen in the right. We are thus dealing with an arrest in development of the mesonephroi—presumably the factor underlying renal agenesis.
Apparently, also, this retardation in growth is manifested by the lower position of the kidney anlages which are just entering the umbilical crotch (compare fig. 4, McClure and Butler, illustrating a 11-mm. embryo in which the kidneys are more than halfway through the umbilical crotch).[2]
Although, normally, the umbilical arteries merely tend to bring the kidneys together as they migrate upward into the lumbar region, the two renal blastemas of this specimen (figs. 7 and 12) are so closely hemmed in by the surrounding arteries and veins that they cannot escape fusion. In addition to the general narrowing it should be noted that the left umbilical artery branches from the aorta at a more acute angle than the other, and that the left posterior cardinal vein (from the undeveloped portion of the Wolflian body down) is not only larger than usual, but larger than the corresponding vein on the right side (fig. 1).
Here, then, for the first time, may be viewed the critical stage in the formation of a horseshoe kidney. Besides confirming the theory of Lewis and Papez regarding the role played by the umbilical vessels in obstructing the ascent of the kidneys (Boyden, ’31), this potential case of horseshoe kidney offers alternative suggestions regarding the factors which underlie this narrowing of the pelvic isthmus. Either there is a lack ofexpansion of the caudal portion of the body cavity, following lack of development of the caudal portion of the Wolffian bodies or general retardation of caudal growth; or else the retarding of the upward migration of the kidney has permitted the surrounding vessels to encroach on the space normally occupied by the kidneys before the latter reach their destination. In either case, arrested development of the Wolflian bodies would seem to be the primary cause of both anomalies in this embryo—namely, unilateral renal agenesis and fusion of the renal anlages.[3]
Fig.1 Graphic reconstruction of right (A) and left (B) posterior cardinal veins of a 10—mm. human embryo (no. 134 of Minnesota Collection). X 33. Drawn from left side, to illustrate greater development of left veins (compare pls. 1 and 2 for orientation). Fus-., area of fusion between left and right blastemas; L. and R.'v.com., vena comitans of left and right umbilical arteries (each vein courses ventrolaterally to its respective umbilical artery until it reaches the caudal umbilical wall. There it anastomoses with the vein of the other side and with the umbilical veins.. These may be the veins which McClure and Butler designate in later stages as the “caudal mesonephric branches of the posterior cardinal veins”); P6..9, second portion of posterior cardinal vein; asterisk, designating level of vitelline artery; 12 and 17, lowest glomeruli of left and right Wolfian bodies, respectively (compare pl. 1).
Description of an Adult Case
Of special interest, in relation to the 10-mm. embryo just described, is a dissecting-room case of absence of the left kidney in a man seventy-two years of age. The patient had been a common laborer, always in good health until the attack of bronchopneumonia that resulted in his death. The genito-urinary history was entirely negative.
In dissecting this subject no vestige of the left kidney could be found and the left suprarenal gland was disc-shaped, indicating that it had never been molded by a kidney. On the right side, kidney and suprarenal gland were normal in every respect, as was also the bladder. But on the left side the ductus deferens, seminal vesicle, and ureter were absent (figs. 8 and 9). Gross examination of the left testis showed a normal organ with ductus deferens extending only as far as the subcutaneous ring. Since the seminal vesicle and adjacent portion of the ductus deferens are derived from the distal end of the Wolffian duct, there can be no doubt but that congenital absence of the left kidney in this case was due to defective development of the distal portion of the left Wolflian body.
To what extent such extensive arrest in development occurs cannot be estimated from a study of adult cases. For, as Collins has pointed out in a personal communication, the literature is very unreliable in its correlation of absence of the ductus deferens and seminal vesicle with absence of the kidney; and, in females, absence of the ureter does not give much information regarding the extent of mesonephric deficiency. Nevertheless it is significant that Collins’ data include forty-four cases of unilateral absence of ductus deferens and seminal vesicle on the same side as the missing kidney.
Regarding the ureter, Collins finds that in 581 cases of unilateral absence of the kidney, the urinary duct of the defective side was missing in 413 cases (71.07 per cent) and definitely reported as present in only forty-two cases (7.2 per cent); that is, it was absent in approximately 90 per cent of the cases in which the ureter was mentioned. Whether or not the remaining 126 cases (21.73 per cent) would cancel each other as a balanced error is debatable. Certainly, it is clear that all of the 581 cannot be cases of embryonic renal agenesis, but represent absorption of the kidney after it was formed. For in at least forty-nine instances (there is also mention of renal vessels in ninety—four other cases) renal veins were noted on the defective side; and we know from the Work of McClure and Butler that renal veins do not develop until the kidneys have formed and have reached the position which they will occupy in the adult (22-mm. stage). In the great majority of cases, however, it would seem certain that congenital absence of the kidney is associated with congenital absence of the ureter.
Agenesis of the Ureter Following Experimental Obstruction of the Wolffian Ducts
Turning now to the blastema, let us consider evidence of its persistence in the absence of the ureter. In the following experiments it will be shown that the nephrogenic component of the kidney (the blastema) does not differentiate into tubules in the absence of the ureteric component. Nevertheless, the former always appears as an anlage, in spite of lesions that prevent the formation of the ureter. Here, then, is striking evidence that failure of the kidney to develop is not due to failure of appearance of its nephrogenic component.
The experiments here outlined representea continuation of those reported in 1924 and briefly mentioned in 1927—experi— ments in which the Wolffian ducts of young chick embryos of approximately thirty—six to fifty—six hours of incubation were
TABLE 1 Experimental agenesis of the ureter
”’§3?’‘ ] ‘E3333’ opiiitflin ptffmigx “mm B’-WEN
1. Both Wolfiian ducts interrupted terminally _ Wis‘-«mite Somitee _Houra 1 Du:/3+Hours‘-lm_1.."]§fm L. R. 215 V 19th ! 21 39 5 13 — — + + 216 24th 24 39 I 5 16 — —— + + 231 25th 22 40 5 4 14 — —— r + + 190 | 29th 25 50 ' 6 0 — —— + + b: Both ducts operated upon, terminally; one interrupted ' 23é 7' 10th I 10 40"“ 4 21 T" -11 1- + + 252 l 12th 12 35 4 23 + —— + + 212 : 19th 19 38 3 12 — + + + 29 5 29th 28 51 3 21 + — + + 2s E: 30th 27 51 __ 5 18 A — + g + + 0,. One duct operated upon, terminally
‘ 163-"? é.6£iT 28 E9 5 io + + 166 ' 30th 30 50 6 9 + — + + 174 2 30th 30 52 . 6 1 + —— + +
d:—l3oth ducts operatednupon -s-ubt-erminally; distal end of one re};{c1{e§' cloaca
'- 59 "|' " 26¢}? 27 52”” ' éb"'""" ZTTET
e. Both ducts operated upon subterminally; both distal ends reach cloaca -1-01 j 26th I 32 56" I 5 M8“ -2 + 1 + + 21 27th . 29 49 5 9 ! —’ —_|: E + —| ‘-S-tump of Wolflian duct reaches cloaca, but is too short to form ureter. ‘Long segment of Wolfiian duct reaches cloaca, but no ureter is formed.
injured, either at their growing points or behind the zone of differentiation; following which the eggs were reincubated for one to five days. From several hundred experiments numerous embryos were sectioned for study. Out‘ of twenty—nine that were old enough to contain the blastema, half of them showed that the Wolflian ducts of one or both sides had been successfully interrupted, with consequent failure in development of the ureteric bud (table 1). Yet in spite of absence of one or both ureters, and of the fact that in some cases the lesion was severe enough to sever the spinal cord (e.g., figs. 4 and 22) or to split the wing buds (e.g., figs. 21 and 3) or the leg buds (figs. 23, 19, and 5), nevertheless both blastemas appeared.
From a study of these embryos it therefore becomes apparent that differentiation of metanephric tubules is not a direct continuation of that repetitive process which gives rise to mesonephric tubules, even though the nephrogenic tissue of the two organs is at first continued (Sedgwick, ’80). For, whenever the nephrotome is interrupted experimentally at any level in the region of the future Wolffian body, the Wolffian duct and tubules fail to appear caudad to the lesion. Yet the metanephric blastema invariably appears, even when the lesion is as low as the thirtieth somite (table 1).[4]
Another characteristic of the metanephric blastema is its apparent power to assume the configuration of the embryonic kidney, notwithstanding the absence of the ureter. When one studies the reconstruction of such an embryo a.s is shown in figure 2, it becomes clear that the reason why the kidney
Fig.2 Reconstruction of chick embryo no. 21 (five days nine hours). X 37.5. Both Wolflian ducts interrupted subterminally at level of twenty—seventh somite, during twenty-nine-somite stage (forty-nine hours of incubation). Lower ends of each duct (L and R.W.d.) reached cloaca (CL). Upper ends are hydronephrotic (L. and R.hydr.W.d.). Right Wolffian duct (R.W.d.) gives rise to ureter (R.ur.). Left Wolfiian duct is probably too short to form ureter (compare position of ureteric bud in early stages: fig. 14, Boyden, ’22). Left blastema (L.bl.) assumes same shape as right, notwithstanding absence of left ureter. Left blastema is compressed by umbilical arteries (curved arrow). All., allantois; Bursa, bursa of Fabricius; Reot., rectum. (For sections through blastemas at level of umbilical arteries, see fig. 14.)
Fig.3 Graphic reconstruction of chick embryo no. 215 (five days thirteen hours. X 37.5. Wolflian ducts interrupted subterminally at level of nineteenth somite, during twenty-first somite stage (thirty-nine hours of incubation). Wing buds cleft by lesion (compare fig. 21). Ends of Wolffian ducts reach cloaca (9), but are too short to form ureteric buds. Nevertheless each blastema appears (L and R.blast.) in its usual place. At level of umbilical crotch (curved arrow) the left blastema. is obliterated, being compressed between the umbilical arteries. (For section through blastemas above level of arrow, see fig. 16.)
Fig.4 Graphic reconstruction of chick embryo no. 231 (four days fourteen hours). X 30. Both Wolffian ducts interrupted terminally at level of future twenty~fifth somite, during twenty-two~somite stage (forty hours of incubation). No trace of Wolffian ducts on cloaca. Lesion was severe enough to sever spinal cord and disloeate the two halves (see fig. 22). Nevertheless, both blastemas appeared in absence of ureteric buds. Note anomalous caudal diverticulum of cloaca (Din). All., allantois; Les., level of lesion; Rect., rectum; curved arrows, region where umbilical arteries compress right and left blastema (see fig. 17).
Fig.5 Graphic reconstruction of chick embryo no. 190 (six days). X 30. Both Wolffian ducts interrupted terminally, at level of future twenty-ninth somite, during twenty-five-somite stage (fifty hours of incubation). Right leg bud cleft by lesion (see fig. 19). Lesion sufficiently severe to intercept aorta and change direction of colon (see anomalous divertieulum, Di.v.). Note enormous distention of Wolfiian ducts (L. and R.hyd.W.d.) which greatly restricts available space for development of blastema, but does not prevent its appearance.
is devoid of tubules at the level of the umbilical crotch is not because the pelvis of the kidney is narrower there, but because there is hardly any blastema at that level. To be sure, the underlying cause of this configuration may well be the pressure exerted upon the blastema by the umbilical arteries, which seems to increase with age (compare figs. 4 and 3; 17 and 14). In figure 3, for instance, the isthmus of the left blastema is entirely obliterated by the arteries. In still other places it is prevented from developing in its usual position on the median side of the posterior cardinal vein by the pressure of one or both of the enormously distended hydronephrotic Wolffian ducts (figs. 4 and 18).[5]
The ultimate fate of the blastema that forms in the absence of a ureteric bud is not known with certainty. In the oldest embryo of the series (no. 166: six days nine hours; fig. 2, Boyden, ’27), it is still visible. But in view of its lessening density one may assume that it eventually disappears. This is rendered the more probable by the fact that in the 10-mm. human embryo described above, the left blastema was not differentiating even though it was in contact with the normal blastema of the other side. Secondly, Nicholson has made the observation that “in not a single case is there a record of a solid or, as we should expect if the blastema were self differentiated, cystic mass or isolated cyst at or near the level of the bifurcation of the aorta.”
It would seem therefore that while the blastema does not persist in the absence of the ureter, suflicient evidence has never been advanced to warrant the theory that congenital absence of the kidney is due primarily to lack of appearance of the blastema.
Discussion
There remains a third possible explanation of embryonic renal agenesis—namely, lack of union of blastema and ureteric bud. Such an occurrence has been clearly demon ‘In hydronephrosis of the Wolfiian body, it is of interest that the part of the mesonephric tubule which becomes most dilated is the glomerulus (arrow, fig. 18) ; whereas in hydronephrosis of the adult human kidney, only the collecting ducts dilate—~—the glomeruli and tubules becoming atrophic. Probably this is due to the fact that the mesonephros is not surrounded by an unyielding capsule so that the blood supply of the tubule is not interrupted and there is still room for dilatation of the glomeruli.
strated by Brown (’31), who has made a careful embryological study of a strain of brown mice (descended from a group of animals treated with comparatively light doses of unfiltered x—rays) in which the incidence of inherited eye, leg, and kidney defects had been increased by selection. Embryonic material was obtained by caesarian operations, so timed as to provide a graded series of embryos through the critical period of kidney formation.
Analysis of this extensive series shows retardation in development of the ureteric bud, in consequence of which it was unable to overcome the mechanical obstacles that prevented its union with the blastema. Brown classifies the defective ureters into six groups ranging from total absence of the bud to ducts that were in contact with the blastema. In thirteen- to fourteen-day embryos of the last group the ureter was so late in reaching its destination that tunics had begun to form around it and this may offer partial explanation for the fact that in some cases contact of the two anlages had induced only rudimentary, if any, differentiation of tubules. In other cases in which the ureter was far removed from the blastema the latter was in process of disappearance. But no case is recorded in which the blastema had failed to appear. In addition there were rudimentary kidneys in which retardation of development continued after union of renal anlages.
This interesting series described by Brown differs from the human and chick embryos described above,“ in that retardation of development operates directly upon the ureter instead of through its parent tissue, the Wolflian duct. Undoubtedly this ofiers an explanation for most human cases in which vestiges of the ureter remain on the side of the missing kidney.
‘With the exception of embryo 101, the only case in which the ureteric bud failed to develop after a sizable portion of the Wolffian duct had reached the cloaca (table 1).
Brown also describes constriction of the distal end of the Wolffian duct from the cloaca, after the ureteric bud had developed, also constriction of the ureter itself at the point
where it joins the cloaca. This defect recalls the recent important contribution of Chwalla, who in an elaborate restudy of the development of the human cloaca has demonstrated the existence of a septum that closes the end of the ureter at the very moment that it acquires a separate opening from the Wolflian duct. Normally, this septum persists from about the 12- to the 24-mm. stage, but has been found in a 7 9-mm. fetus. This septum undoubtedly explains an obscure point in human pathology, namely, the occurrence of congenital dilatation of the ureter.
Again, one may ask why retardation in development of these mouse embryos has not resulted in fusion of the kidneys, as noted in a previous article (Boyden, ’31)? Apparently this is due to the marked twisting of the body to the right which would tend to keep the kidneys apart. Brown considers that the mechanical obstruction resulting from this twisting of the body is a contributing factor in preventing the defective ureteric bud from reaching the blastema.
Finally, existence of this defective strain of mice raises the question as to whether or not kidney defects in man may not have a genetic basis, especially since we now have proof, at least in one human case, that unilateral renal agenesis can be attributed to arrest in development of the Wolfiian body.
Conclusions
A 10-mm. human embryo is described which exhibits the critical stagein the production of two congenital anomalies of the kidney—unilateral renal agenesis and horseshoe kidney. The underlying defect seems to be an arrest in development of the caudal portion of the mesonephros, as a result of which the left Wolflian duct fails to reach the cloaca and therefore to give rise to a ureteric bud. In its absence the left blastema fuses with that of the right to form a potential horseshoe kidney.
A review of adult cases of congenital absence of the kidney -—including a new case with associated absence of the ductus deferens and seminal vesicle—demonstrates that more than three—fourths of the cases of renal agenesis are characterized by the absence of the ureter.
Ureteral agenesis may be produced experimentally in chick embryos by interrupting the Wolflian duct in its zone of differentiation. Despite the absence of the ureter, the blastema appears and assumes the form of the embryonic kidney, but fails to differentiate into tubules.
An analysis of all data leads to the conclusion that no evidence has yet been presented which justifies the theory that absence of the kidney is due to lack of appearance of the metanephric blastema; on the contrary, it appears to be due to retardation in development of the ureter, to embryonic agenesis of the ureter, or to arrest in development of the parent tissue of the ureter.
Footnotes
- ↑ In chick embryos, it has been shown that mechanical distention of the allantois by mesonephric excretion is an essential factor in the development of that organ, for, with total absence of distending fluid, it remains an abortive organ (Boyden, ’24). In the human embryo with its much less developed mesonephros, there is a correspondingly less well-developed allantoic duct. Quite likely, however, in the light of the evidence presented here, there is sufiicient excretion to act as a mechanical factor‘ in the early development of the urinary bladder.
- ↑ McClure and Butler explain the presence of the abrupt bend in the posterior cardinal veins as having been brought about by the migration into this region of the permanent kidneys. But in embryo 134 (pl. 1) it may be noted that the bend occurs in the absence of a normal kidney on the left side and before either kidney has reached the bend. It seems more probable, therefore, that this acute bend should be attributed to the increasing pressure of the umbilical arteries and the placental circulation (compare figs. 1 and 7).
- ↑ This explanation should not be confused with the erroneous theory of arrested development of the kidney proposed by Meckel in 1812. According to Schlesinger, Meckel believed that fusion of right and left blastemas represented a. preliminary stage in development that was normally followed by separation of the two kidneys; and that arrest in development caused merely a persistence of this early stage of fusion.
- ↑ In the experiments of Willier and Rawles and of Hunt, which consisted of transplanting portions of the blastoderm of chick embryos (under the age of twelve somitcs) to the chorio-allantoic membrane, it is shown that the older the blastoderm (i.e., the more caudad Hensen’s node is at the time of transplantation) the more likely it is that the mesonephros will develop. Willier and Rawles interpret these interesting findings to mean not only that the segregation of the mesonephros is a repetitive process—each unit of tubules arising in serial order as a segregate-—but also that some relation exists between the number of units segregated and their capacity to survive in a graft. Yet in the writer’s experiments mesonephric tubules fail to appear in the absence of the Wolffian duct and no trace of nephrogenic tissue of the Wolffian body is visible (figs. 13 and 16) except in the earliest stages (fig. 1, Boyden, ’27). Hunt also notes that in grafts of Hensen’s node mesonephric tubules are found attached to the Wolffian duct. If this dependence of tubule formation upon the duct is substantiated, it would seem necessary to add another step to the interesting interpretation of Willier and Rawles regarding the organization of the mesonephros — namely, to postulate that the role of Hensen’s node is to organize the pronephros, the parent tissue of the Wolflian duct. The latter, as it moves caudad, will then induce serial formation of mesonephric tubules. As evidence of the dependence of the mesonephros upon the formation of the pronephros, it should be recalled that in normal development the Wolffian duct appears in advance of the mcsonephric tubule. Secondly, attention is called to embryos 239 and 252 (table 1) in which unilateral interruption of the nephrotome in the region just above the mesonephros has resulted in lack of formation of any mesonephric ducts or tubules on that side, caudad to the lesion.
- ↑ In hgdronephrosis of the Wolffian body, it is of interest that the part of the mesonephric tubule which becomes most dilated is the glomerulus (arrow, fig. 18) ; whereas in hydronephrosis of the adult human kidney, only the collecting duets dilate-the glomeruli and tubules becoming atrophic. Probably this is due to the fact that the mesonephros is not surrounded by an unyielding capsule so that the blood supply of the tubule is not interrupted and there is still room for dilatation of the glomeruli.
Literature Cited
ARIsT0'rLE’s fiinf Biicher von der Zeugung und Entwickelung der Thiere. Aubert u. Winner, Leipzig, 1860; book IV, pp. 317 and 377.
BOYDEN, EDWARD A. 1922 The development of the cloaca in birds, with special reference to the origin of the bursa of Fabricius, the formation of a. urodaeal sinus, and the regular occurrence of a cloacal fenestra. Am. J. Anat., vol. 30, pp. 163-202.
1924 An experimental study of the development of the avian cloaca, with special reference to a mechanical factor in the growth of the allantois. J. Exp. Zool., vol. 40, pp. 437-472.
1927 Experimental obstruction of the mesonephric ducts. Proc. Soc. Exp. Bio]. and Med., vol. 24, pp. 572-576.
1931 Description of a horseshoe kidney associated with left inferior vena cava and disc-shaped suprarenal glands, together with a note on the occurrence of horseshoe kidneys in human embryos. Anat. Rec., vol. 51, pp. 187-212.
BROWN, ALICE L. 1931 An analysis of the developing metanephros in mouse embryos with abnormal kidneys. Am. J. Anat., vol. 47, pp. 117-172.
Cl-IWALLA, Runou‘ 1927 Ueber die Entwicklung der Harnblase und der primaren Harnriihre des Menschen mit besonderer Beriicksichtigung der Art und Weise, in der sich die Uretcren von den Urnierengangen trennen, nebst Bemerkungen iiber die Entwicklung der Muellerschen Gémge und des Mastdarms. Zeit. f. Anat. u. Entwick., Bd. 83, S. 615-733.
COLLINS, D. C. 1931 Congenital unilateral renal agenesia. Proc. Staff Meet. Mayo Cl., vol. 6, no. 39, pp. 581-583. (In press, Annals of Surgery.)
FELIX, W. 1912 The development of the urogenital organs. In Keibel and Mall, Manual of Human Embryology, vol. 2, pp. 752-979. Lippincott, Philadelphia.
HUNT, '1‘HoMAs E. 1931 An experimental study of the independent dilferentiation of the isolated Hensen’s node and its relation to the formation of axial and non—axial parts in the chick embryo. J. Exp. Zool., vol. 59, pp. 395-428.
MCCLURE, C. F. W., AND BU'l‘LER., E. G. 1925 The development of the vena
cava inferior in man. Am. J. Anat., vol. 35, pp. 331-384.
MECKEL, J. F. 1812 Lehrbuch der pathologischen Anatomic, S. 616. (Cited by Schlesinger, 1924, Virch. Arch., Bd. 248, S. 297-322.)
NICHOLSON, G. W. 1927 The kidneys and their development. Guys Hosp. Rep., vol. 77, pp. 362-385.
SEDGWICK, ADAM 1880 Development of the kidney in its relation to the wolfiian body in the chick. Studies Morph. Lab. Univ. Cambridge, p. 62. Williams & Norgate, London.
SHIKINAMI, J UJIRO 1926 Detailed form of the Wolffian body in human embryos of the first eight weeks. Contr. to Embr. no. 93, Publ. 363 of Carneg. Inst., pp. 49-61.
WILLIER, B. H., AND RAWLES, M. E. 1931 The relation of Hensen’s node to the differentiating capacity of whole chick blastoderms as studied in chorio-allantoic grafts. J. Exp. Zoi$l., vol. 59, pp. 429-466. 340
Plates
Plate 1
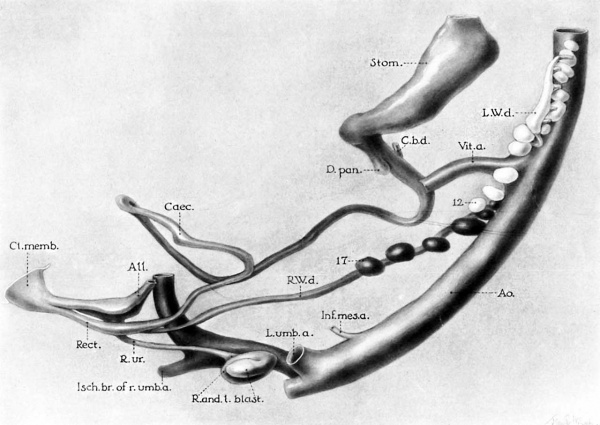
6 Graphic reconstruction of 10 mm human embryo (no. 134 of Minnesota Collection) illustrating arrested development of Wolffian bodies, failure of left Wolffian duct to reach cloaca, and fusion of left blastema with right kidney as latter is entering umbilical crotch. (For relations of veins, see figs. 1 and 7; for crosssections of embryo, see figs. 10 to 12). X 55.
Abbreviations
AIL, allantois Rect., rectum
Ao., aorta R. and L.blast., right and left renal Caec., caecum blastema
Cl.memb., cloacal membrane R.ur., right ureter
C.b.d., common bile duct R.W.d., right Wolffian duct
D.pcm., dorsal pancreas Stom., stomach
Inf.mes.a., inferior mesenteric artery V£t.a., vitelline artery
Isch.br.of 7*.u7nb.a.,rightischiatic artery 1%‘, lowest glomerulus of left Wolfiian L.umb.a., left umbilical artery body
L.W.d., hydronephrotic left Wolffian 1?’, lowest glomerulus of right side duct
Plate 2
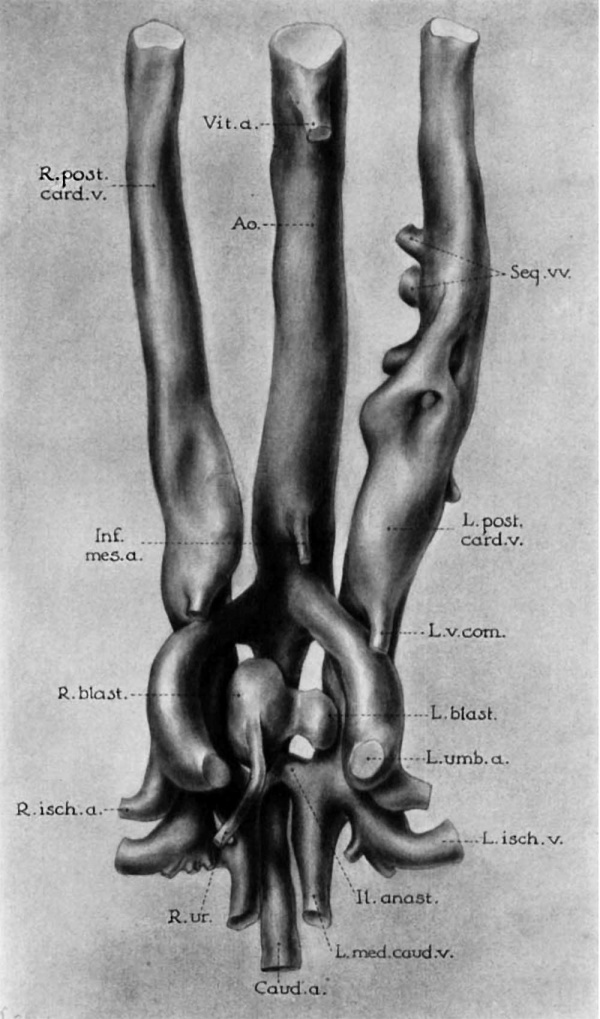
7 Wax reconstruction of blood vessels and kidneys of human embryo shown in plate 1, seen in ventral view. X 55. Note absence of left ureter, fusion of left and right blastemas, the more acute angle at which the left umbilical artery leaves the aorta, the greater size of the left posterior cardinal vein, and the actual contact of blastcmas with the second portion of the posterior cardinal veins (compare Pc./2, fig. 1; 17., fig. 12).
ABBREVIATIONS C’aud.a., caudal artery L.v.com., left vena comitans (compare Il.anast., anastomosis between posterior fig. 1) cardinal veins in iliac region R.isclmr., right ischiatic vein L.med.caud.v., left median caudal vein R.post.card.v., right posterior cardinal vein
(Other legends as in plate 1)
Plate 5
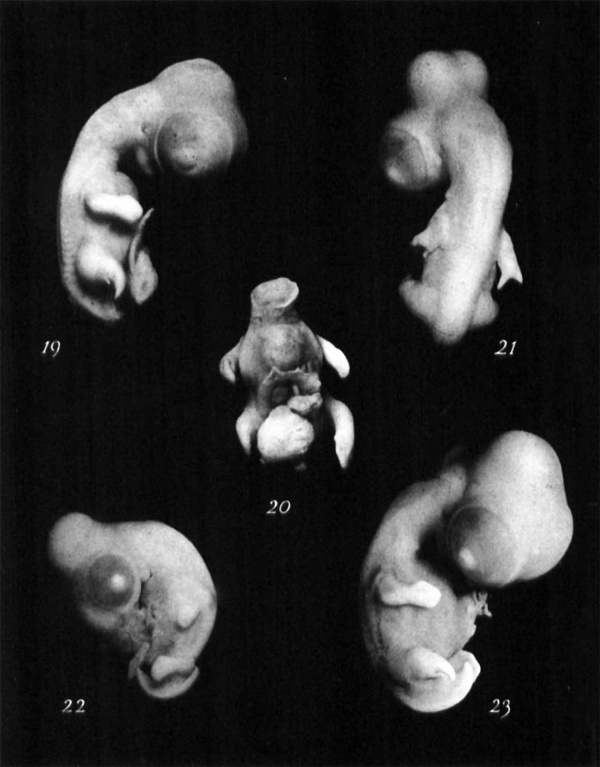
Chick embryos incubated from two to four days after the Wolffian ducts had been interruptcd experimentally. X ,5.
19 and 20 Enibryo no. 190 (six days). Both Wolffinn duets stopped ter~iiincllly :it level of tncnty-ninth somite. Note abortive allantois and leg bud clcft by operation (co~nparefig. 5).
21 Enibryo 110. 215 (five days thirteen 11ours). Both Wolfiaii ducts stoppcci terinin:rlly, at level of iiiiieteeiith soniitc. Note TI ing buds cleft by opcratioii (compare figs. 3 and 16).
23 Enibryo no. 231 (four days fourteen lioiirs). Both Wolffian ducts stopped trriiiin:illy at level of tneiity-fifth somite. Lesion sevcred spinal cord. Secl notch in back at level of leg bud. Note rudimentary allantois (eompare figs. 4 and 17).
23 Enibryo no. 171 (sir days one hour). Right Wolffian duct stopped terniinally a t Ieicl of thirticth somite. S o t e leg bud cleft by operation (compare fig. 18).
Figures 8 and 9. Bladder of man seveiity-two years of age with absence of the left kidney. X 3-.
8 Posterior view, illustrating absence of left ureter, (luctus deferens, and seminal vesicle. Note dilatation of right ureter (Rxun); and median spur at prostate gland.
9 Interior of same bladder, showing single ureteral orifice (arrow indicates opening of right ureter), the trabeeulated inner surface of bladder, and the embryonic character of the urachus.
Figures 10 to 12. Cross-sections (20 /.c) of ]0—u1u1. l1um:m enihryo shown in plates 1 and 2. X 39.
10 Section 339, at level of sixth mesonephric glomerulus of left side (compare pl. 1); illustrating dilatation of left VV0lfi‘ian (luct (L.).
11 Section 378, showing left Wolffian hody (W.d.) devoid of tubules, but containing enlarged posterior cardinal vein. 14, fourteenth iuesonephric glouierulus of right side (compare pl. 1).
12 Section 425, at level of tlmbilical crotch, /1., right umbilical artery; V., left posterior cardinal vein. The section passes through the ventral surfaces of the blastemas just before they fuse with each other (compare fig. 1 and pls. 1 and 2).
Transverse sections through chick embryos in which interruption of‘ the W olffian ducts has resulted in agenesis of the ureter, but persistence of the bl:-tsteina.
Figures 13 to 15. Three sections through embryo 21 (compare fig. 2). X 30.
14 Section at level of umbilical crotch. Arrow at left indicates right ureter; arrow at right, narrow portion of left blastoma.
13 Section above level of umhilieal arteries, showing la.rge blastema on either side of aorta (BL) and lower end of hydronephrotic left Wolffian duct (H1).
15 Section below level of umbilical arteries, showing difference in density of left blastema (BI) containing no ureter, and of right blastema, Wl)l("ll is being organized by the ureter.
16 Section through embryo no. 215 (compzire figs. 3 and 21). X 30. Shows expansion of renal blastemas (Bl.) above level of umbilical crotch, in absence of both ureters. l\'ote empty Wolflfian bodies.
17 Section through embryo no. 231 (compare figs. 4 and 22). X 36. Arrows indicate right and left blastema being compressed by umbilical arteries, but not as much as in older stages.
18 Section through embryo no. 174 (compare fig. 23). X 18. Right Wolfiian body is normal. Left Wolflian duct interrupted at level of thirtieth somite (table 1). Note enormously distended left Wolffian duct (Hyd.W.d.), which has prevented formation of left blustema at this level, between duct and left cardinal vein. Ureter present on right side (R.ur.). Arrow indicates hydronephrotic glomerulus. (See 1». 335.)
Chick embryos incubated from two to four days after the VVolffian duets had been interrupted experimentally. X 5.
19 and 20 Embryo no. 190 (six days). Both VVolfiia.n ducts stopped terminally at level of t\venty—ninth somite. Note abortive allantois and leg bud cleft. by operation (compare fig. 5).
31 Embryo no. 215 (five days thirteen hours). Both VVolflia.n ducts stopped t.ern1inn]l_v, at level of nineteenth somite. Note wing buds cleft by operation (compare figs. 3 and 16).
22 Embryo no. 231 (four days fourteen hours). Both Wolfiian ducts stopped t.ern1ina1l_v at level of t.went.y—fift.h somito. Lesion severed spinal cord. See notch in back at level of leg bud. Note rudimentary allantois (compare figs. 4 and 17).
23 Embryo no. 17-} (six days one hour). Right Wolffian duct stopped terminally at level of thirtieth somite. Note leg bud cleft by operation (compare fig. 18).
Cite this page: Hill, M.A. (2024, April 27) Embryology Paper - Congenital absence of the kidney. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Paper_-_Congenital_absence_of_the_kidney
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G