Integumentary System Development
| Embryology - 26 Feb 2026 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Introduction
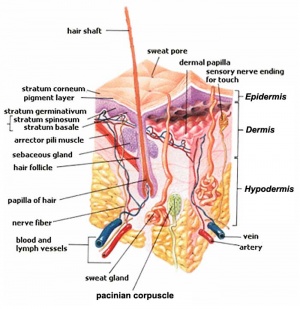
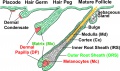
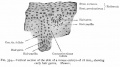
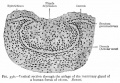
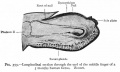
The integumentary system covers the surface of the embryo (skin) and its specialized skin structures including hair, nails, sweat glands, mammary glands and teeth. As a system it has contributions from all embryonic layers.
The skin provides a barrier between ourselves and our environment, it also contains specializations in different regions including hair, nails, glands and sensory receptors. In other species, additional specializations such as feathers, horns and shell can be seen.
The two major tissue organizations of epithelial (ectoderm, epidermis) and mesenchyme (mesoderm connective tissue, dermis and hypodermis) are shown within skin. In addition, we have aslo extensive populating by melanocytes (neural crest) and sensory nerve endings. It remains today as possibly the first epithelial specialization from which other epithelial specializations arose that are now located inside the body.
Ectoderm forms the surface epidermis and the associated glands. Mesoderm, from the somites, forms the underlying connective tissue of dermis and hypodermis. Neural crest cells also migrate into the forming epidermis and the skin is also populated by specialized sensory endings. Fetal skin also has the ability to heal wounds without a scar in contrast to adult skin, this may relate to differences in the fetal extracellular matrix structure. The adult epidermis contains keratinocytes, melanocytes and Langerhans cells.
Some Recent Findings
|
| More recent papers |
|---|
|
This table allows an automated computer search of the external PubMed database using the listed "Search term" text link.
More? References | Discussion Page | Journal Searches | 2019 References | 2020 References Search term: Integumentary Embryology | Integumentary Development |
| Older papers |
|---|
| These papers originally appeared in the Some Recent Findings table, but as that list grew in length have now been shuffled down to this collapsible table.
See also the Discussion Page for other references listed by year and References on this current page.
|
Textbooks
- Human Embryology (2nd ed.) Larson Chapter 14 p443-455
- The Developing Human: Clinically Oriented Embryology (6th ed.) Moore and Persaud Chapter 20: P513-529
- Before We Are Born (5th ed.) Moore and Persaud Chapter 21: P481-496
- Essentials of Human Embryology Larson Chapter 14: P303-315
- Human Embryology, Fitzgerald and Fitzgerald
- Color Atlas of Clinical Embryology Moore Persaud and Shiota Chapter 15: p231-236
Objectives
- Understand the differentiation of the epidermis and dermis.
- Understand the formation of hair and nails.
- Understand the formation of sweat glands, mammary glands.
- Understand the formation of teeth.
Development Overview
Ectoderm and Mesoderm Origin
4 weeks
- simple ectoderm epithelium over mesenchyme.
1-3 months
|
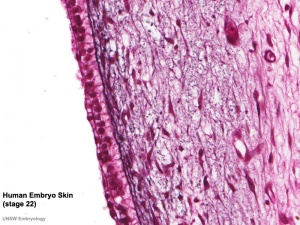
Integument Human Embryo (Week 8, Stage 22) |
4 months
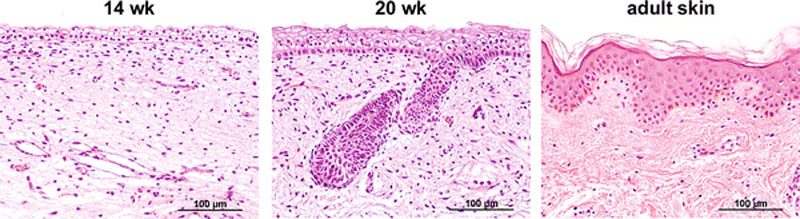
Fetal human integumentary histology[2](Weeks in figure are from LMP)
- Basal cell - proliferation generates folds in basement membrane.
- Neural crest cells - melanoblasts migrate into epithelium. These are the future melanocyte pigment cell of the skin.
- Embryonic connective tissue- differentiates into dermis, a loose ct layer over a dense ct layer. Beneath the dense ct layer is another loose ct layer that will form the subcutaneous layer.
- Ectoderm contributes to nails, hair follictles and glands.
- Nails form as thickening of ectoderm epidermis at the tips of fingers and toes. These form germinative cells of nail field.
- Cords of these cells extend into mesoderm forming epithelial columns. These form hair follicles, sebaceous and sweat glands.
5 months
- Hair growth initiated at base of cord, lateral outgrowths form associated sebaceous glands.
- Other cords elongate and coil to form sweat glands.
- Cords in mammary region branch as they elongate to form mammary glands. These glands will complete development in females at puberty. Functional maturity only occurs in late pregnancy.
Newborn Infant
The following newborn information is from a recent review of Newborn infant skin: physiology, development, and care.[6]
Full-term newborn infant (healthy)
- skin well-developed and functional at birth, with a thick epidermis and well-formed stratum corneum layers.
- Transepidermal water loss is very low at birth
- equal to, or lower than adults, indicating a highly effective skin barrier.
- Vernix facilitates stratum corneum development in full-term infants
- through mechanisms including physical protection from amniotic fluid and enzymes, antimicrobial effects, skin surface pH lowering, provision of lipids, and hydration.
- stratum corneum barrier develops rapidly after birth
- complete maturation requires weeks to months.
Premature infants
- particularly those of very low birth weight
- have a poor skin barrier with few cornified layers and deficient dermal proteins.
- increased risk for skin damage, increased permeability to exogenous agents and infection.
Embryonic and Fetal Epidermis
Electron Micrographs of the Developing Human Epidermis[7]
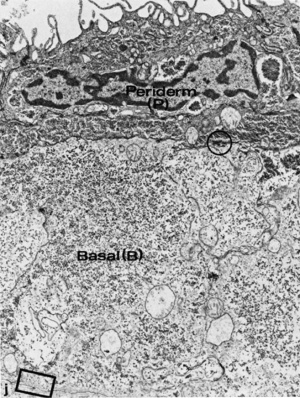
6 to 8 weeks (8-9 week EGA) |
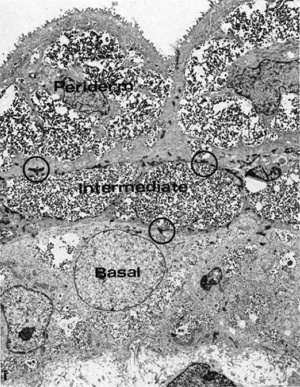
7 to 9 weeks (9-11 week EGA) |
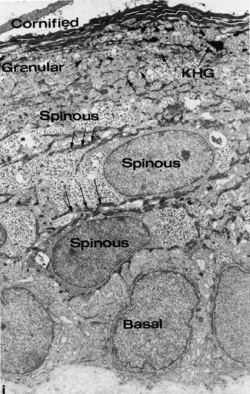
22 weeks (about 24 week EGA) |
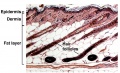
Dermis
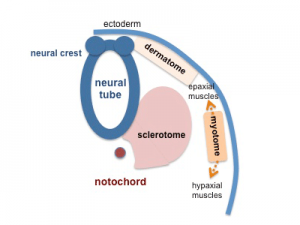
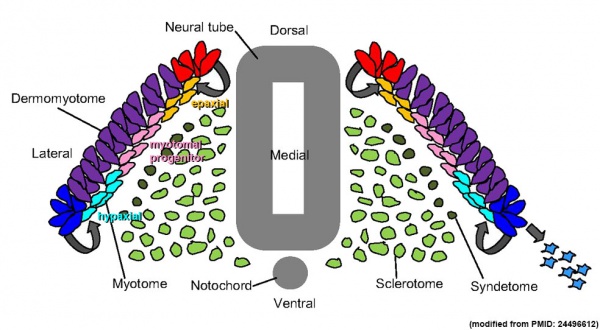
The underlying connective tissue layers of the skin (dermis and hypodermis) arise from the dermatome component of the developing somite.
Cartoon showing the dermatome component of the somite.[8]
Fetal Dermis
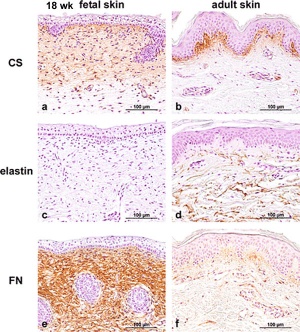
The following data is from an immunohistological study of fetal skin dermis layer.[2]
- Collagen type I is the principal component of extracellular matrix (ECM) (also in adult skin).
- Collagen type III high ratio to collagen type I (than adult skin).
- Glycosaminoglycans (GAGs) level higher (than adult skin).
- Hyaluronic acid and chondroitin sulfate both higher.
- Elastin was not present (found in adult skin).
Facial Skin
A recent study[1] has looked at the human fetus between GA 14-16 weeks specialised changes in face skin, the plugs in the nose and ear and the tongue surface.
Face Skin (zygomatic skin):
- thin stratum corneum and a stratum basale (CK5/6+, CK14+, and CK19+)
- intermediate layer, 2-3 layered large keratinocytes with nucleus.
- basal layer was lined by mono-layered mesenchymal cells (CD34+ and nestin+). Some of basal cells were PCNA-positive.
Plugs - external ear and nose:
- keratinocyte cell nuclei expressed PCNA, CK5/6, CK14, and CK19.
- Vimentin-positive mesenchymal cells migrated into the plug.
Tongue
- lingual epithelium CK7-positive stratum corneum as well as the thick mesenchymal papilla.
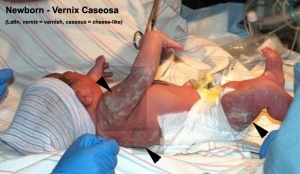
Vernix Caseosa
The vernix caseosa has several different potential functions and a variable composition.[9]
- a highly variable coating of the fetal skin
- high water content (80%) largely compartmentalized within fetal corneocytes (cells forming the stratum corneum)
- develops cranio-caudally production coincides in utero with terminal differentiation of the epidermis and formation of the stratum corneum
- primarily composed of sebum, cells that have sloughed off the fetus's skin and shed lanugo hair
- can be absent in preterm infants
- dehydration and rehydration processes occur two to four times faster at 37 degrees celcius than at room temperature[10]
- towards term fragments of vernix can mix into the amniotic fluid resulting in (normal) turbidity
- fetal swallowing of amniotic fluid mixed with fragments of vernix can also occur
- cathelicidin LL-37, alpha-defensins, and LL-37 in neutrophils.[11]
- Links: vernix caseosa | integumentary gland
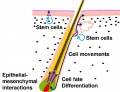
Adult Epidermal Stem Cells
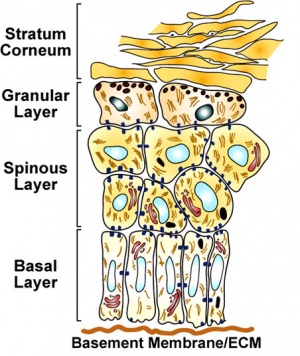
In adult human skin epithelium, keratinocytes take about a month to differentiate from the basal stem cell layer, through the different stages of differentiation and layers, to be finally sloughed off on the surface. As well as keratinocyte differentiation, this represents a specialised form of programmed cell death called "cornification".
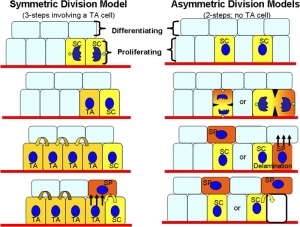
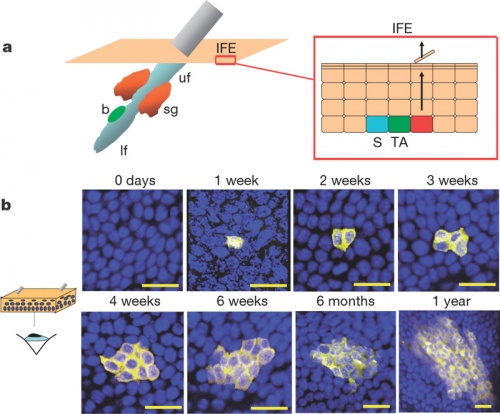
The following information is from a recent study on mouse skin using a single cell labelling system with longitudinal tracing and confocal imaging.[12]
Organization of the epidermis. Hair follicles contain stem cells located in the bulge (b, green), with the potential to generate lower hair follicle (lf), sebaceous gland (sg, orange) upper follicle (uf) and interfollicular epidermis (IFE, beige). The schematic shows the organization of keratinocytes in the IFE, as proposed by the stem/TA cell hypothesis. The basal layer comprises stem cells (S, blue), transit amplifying cells (TA, dark green), and post-mitotic basal cells (red), which migrate out of the basal layer as they differentiate (arrows).
Projected Z-stack confocal images of IFE wholemounts from AhcreERT R26EYFP/wt mice viewed from the basal surface at the times shown following induction. Yellow, EYFP; blue, DAPI nuclear stain. Scale bar, 20 microns.
Reprinted by permission from Macmillan Publishers Ltd: Nature. 2007 Mar 8;446(7132):185-9, copyright (2007)
Adult epidermal stem cells[12] "According to the current model of adult epidermal homeostasis, skin tissue is maintained by two discrete populations of progenitor cells. ...Here we show that clone-size distributions are consistent with a new model of homeostasis involving only one type of progenitor cell. These cells are found to undergo both symmetric and asymmetric division at rates that ensure epidermal homeostasis."
- Links: MRC - Phil Jones Laboratory
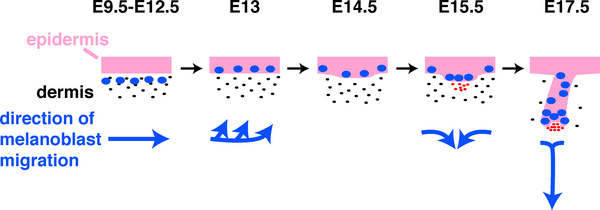
Melanocytes
|
|
Neural crest cells lying lateral to a region between the dermamyotome and the surface ectoderm (migration staging area) further migrate to populate the embryonic skin as proliferating melanoblasts. These initial melanoblasts are unpigmented containing only immature melanosomes without functional tyrosinase (TYR), the enzyme required for melanin synthesis.
See also modeling melanoblast development.[13]
Gene Regulatory Network (GRN)
- Sox10 in antagonising Mitfa-dependent melanocyte differentiation (zebrafish)[14]
- Melanocyte differentiation controlled by microphthalmia transcription factor (MITF).
- activates genes involved in pigment production - TYR, TRP-1 and TRP-2
Melanin pigments
The Fitzpatrick skin phototype (I-VI, light to dark) is used to classify skin colour based on the amount of melanin pigment in the skin. There are two mani forms of pigment:
- eumelanin - brown-black or dark insoluble polymer, (dark skin and hair)
- pheomelanin - red-yellow soluble polymer, formed by the conjugation of cysteine or glutathione. (red hair and skin phototypes I and II)
| Fitzpatrick Skin Phototype | ||
|---|---|---|
| Type | Features | Tanning ability |
| I | Pale white skin, blue/green eyes, blond/red hair | Always burns, does not tan |
| II | Fair skin, blue eyes | Burns easily, tans poorly |
| III | Darker white skin | Tans after initial burn |
| IV | Light brown skin | Burns minimally, tans easily |
| V | Brown skin | Rarely burns, tans darkly easily |
| VI | Dark brown or black skin | Never burns, always tans darkly |
| Reference: Fitzpatrick TB. Soleil et peau. (1975) J Med Esthet. 2: 33-34. | ||
- Links: Neural Crest Development | MITF
Keratin
Cytoskeleton intermediate filament protein of epithelial cells required for cell mechanical stability and integrity, humans have 54 functional keratin genes. The α-keratins are expressed in all vertebrates, while the β-keratins are specific to birds and reptiles.[15]
| Cell layer | Expression |
|---|---|
| Basal layer | K14, K5 |
| Spinous layer | K1, K10, caspase-14 |
| Granular layer | Filaggrin, involucrin, loricrin |
| Cornified layer | aggregated keratin filaments |
Intermediate Filaments Type I
Acidic keratins (pI < 5.7) 40–64 kDa (n = 28)
- K9-28 (epithelia)
- K31-40 (hair/nail)
Intermediate Filaments Type II
Basic keratins (pI ≥ 6.0) 53–67 kDa (n = 26)
- K1-8, K71-80 (epithelia)
- K81-86 (hair/nail)
- Keratins form heterodimers that assemble into heteropolymeric keratin filaments
Elaine Fuchs
A key researcher in the understanding of skin and keratin development Elaine Fuchs: A love for science that's more than skin deep. Interviewed by Ben Short[16] "Elaine Fuchs has collected many awards in her 30 years researching mammalian skin development, but it's hard to beat the two prizes she received in late 2009. Shortly before winning the prestigious L'Oreál-UNESCO award for women in science, Fuchs was awarded the National Medal of Science—the US's highest honor for outstanding scientific contributions."
Integrin Expression
The data below form a research article identifies expression of integrin subunits during development of human palm and sole skin.[17]
- All of the integrins expressed during development were also present in mature epidermis and were largely confined to the basal layer of keratinocytes in a pericellular distribution.
- alpha 3 and beta 1 subunits - were expressed prior to the initiation of stratification and did not change in abundance or distribution during subsequent development.
- alpha 4 and beta 3 - were not detected at any time in the epidermis.
Every other subunit examined showed spatial or temporal changes in expression.
- alpha 1 - was strong before stratification and until mid-development, but was greatly decreased in neonatal epidermis.
- alpha 2 - was first detected in small patches of basal cells prior to stratification, and thereafter was found in the entire basal layer, with greater staining in developing sweat glands.
- alpha 5 - was not expressed until mid-development, and then primarily in developing sweat glands, with faint expression in neonatal epidermis.
- alpha v - was detected following stratification, in developing sweat glands, and occasionally in neonatal epidermis.
- alpha 6 and beta 4 - were peribasally expressed before stratification. After stratification became concentrated at the basal cell surface in contact with the basement membrane, co-localizing with hemidesmosomes.
Three known ligands for keratinocyte integrins
- laminin and collagen type IV - present in the basement membrane zone at all stages of development
- fibronectin - only evident until about 13 weeks estimated gestational age.
Langerhans Cells
Langerhans cells (LCs) are immune system dendritic cells (myeloid antigen-presenting immune cells) found in the basal/suprabasal layers of stratified epidermis (and also in epithelia of the corneal and mucosal tissues). These migratory dendritic cells belong to the same cell family as microglia (see review[18])
In the embryo, LC precursors first form from the primitive yolk sac macrophages, and later in the fetal liver from a monocyte-like precursor. Langerhans cells then populate the single-layered epidermis and undergo local proliferation before birth.[19]
Postnatally, local self-renewal maintains or restores LC numbers following low grade or chronic inflammation.
Langerhans cells and Merkel cells within human hair follicle potentially interact with each other.[20]
History - Paul Langerhans (1847 – 1888)[21] in 1868 first (mis)identified these cells as neurons.
- Links: Immune System Development | Search Pubmed
Other Species
Chicken
The avian integumentary system includes integumentary specialisations such as feathers, scales, claws, and beaks.
- β-keratin - expressed in embryonic feathers[22]
- Links: Chicken Development
References
- ↑ 1.0 1.1 Kim JH, Jin ZW, Murakami G & Cho BH. (2016). Characterization of mesenchymal cells beneath cornification of the fetal epithelium and epidermis at the face: an immunohistochemical study using human fetal specimens. Anat Cell Biol , 49, 50-60. PMID: 27051567 DOI.
- ↑ 2.0 2.1 2.2 2.3 2.4 Coolen NA, Schouten KC, Middelkoop E & Ulrich MM. (2010). Comparison between human fetal and adult skin. Arch. Dermatol. Res. , 302, 47-55. PMID: 19701759 DOI.
- ↑ Vickaryous MK & Sire JY. (2009). The integumentary skeleton of tetrapods: origin, evolution, and development. J. Anat. , 214, 441-64. PMID: 19422424 DOI.
- ↑ Gong H, Wang H, Wang Y, Bai X, Liu B, He J, Wu J, Qi W & Zhang W. (2018). Skin transcriptome reveals the dynamic changes in the Wnt pathway during integument morphogenesis of chick embryos. PLoS ONE , 13, e0190933. PMID: 29351308 DOI.
- ↑ 5.0 5.1 Fuchs E. (2008). Skin stem cells: rising to the surface. J. Cell Biol. , 180, 273-84. PMID: 18209104 DOI.
- ↑ Visscher MO, Adam R, Brink S & Odio M. (2015). Newborn infant skin: physiology, development, and care. Clin. Dermatol. , 33, 271-80. PMID: 25889127 DOI.
- ↑ Dale BA, Holbrook KA, Kimball JR, Hoff M & Sun TT. (1985). Expression of epidermal keratins and filaggrin during human fetal skin development. J. Cell Biol. , 101, 1257-69. PMID: 2413039
- ↑ Blake JA & Ziman MR. (2014). Pax genes: regulators of lineage specification and progenitor cell maintenance. Development , 141, 737-51. PMID: 24496612 DOI.
- ↑ Pickens WL, Warner RR, Boissy YL, Boissy RE & Hoath SB. (2000). Characterization of vernix caseosa: water content, morphology, and elemental analysis. J. Invest. Dermatol. , 115, 875-81. PMID: 11069626 DOI.
- ↑ Rissmann R, Groenink HW, Gooris GS, Oudshoorn MH, Hennink WE, Ponec M & Bouwstra JA. (2008). Temperature-induced changes in structural and physicochemical properties of vernix caseosa. J. Invest. Dermatol. , 128, 292-9. PMID: 17671513 DOI.
- ↑ Yoshio H, Lagercrantz H, Gudmundsson GH & Agerberth B. (2004). First line of defense in early human life. Semin. Perinatol. , 28, 304-11. PMID: 15565791
- ↑ 12.0 12.1 Clayton E, Doupé DP, Klein AM, Winton DJ, Simons BD & Jones PH. (2007). A single type of progenitor cell maintains normal epidermis. Nature , 446, 185-9. PMID: 17330052 DOI.
- ↑ Larue L, de Vuyst F & Delmas V. (2013). Modeling melanoblast development. Cell. Mol. Life Sci. , 70, 1067-79. PMID: 22915137 DOI.
- ↑ Greenhill ER, Rocco A, Vibert L, Nikaido M & Kelsh RN. (2011). An iterative genetic and dynamical modelling approach identifies novel features of the gene regulatory network underlying melanocyte development. PLoS Genet. , 7, e1002265. PMID: 21909283 DOI.
- ↑ Greenwold MJ, Bao W, Jarvis ED, Hu H, Li C, Gilbert MT, Zhang G & Sawyer RH. (2014). Dynamic evolution of the alpha (α) and beta (β) keratins has accompanied integument diversification and the adaptation of birds into novel lifestyles. BMC Evol. Biol. , 14, 249. PMID: 25496280 DOI.
- ↑ Fuchs E. (2009). Elaine Fuchs: A love for science that's more than skin deep. Interviewed by Ben Short. J. Cell Biol. , 187, 938-9. PMID: 20038675 DOI.
- ↑ <pubmed>1769328</pubmed>
- ↑ Collin M & Milne P. (2016). Langerhans cell origin and regulation. Curr. Opin. Hematol. , 23, 28-35. PMID: 26554892 DOI.
- ↑ Yasmin N, Bauer T, Modak M, Wagner K, Schuster C, Köffel R, Seyerl M, Stöckl J, Elbe-Bürger A, Graf D & Strobl H. (2013). Identification of bone morphogenetic protein 7 (BMP7) as an instructive factor for human epidermal Langerhans cell differentiation. J. Exp. Med. , 210, 2597-610. PMID: 24190429 DOI.
- ↑ Taira K, Narisawa Y, Nakafusa J, Misago N & Tanaka T. (2002). Spatial relationship between Merkel cells and Langerhans cells in human hair follicles. J. Dermatol. Sci. , 30, 195-204. PMID: 12443842
- ↑ Jolles S. (2002). Paul Langerhans. J. Clin. Pathol. , 55, 243. PMID: 11919207
- ↑ Wu P, Ng CS, Yan J, Lai YC, Chen CK, Lai YT, Wu SM, Chen JJ, Luo W, Widelitz RB, Li WH & Chuong CM. (2015). Topographical mapping of α- and β-keratins on developing chicken skin integuments: Functional interaction and evolutionary perspectives. Proc. Natl. Acad. Sci. U.S.A. , 112, E6770-9. PMID: 26598683 DOI.
Reviews
Visscher MO, Adam R, Brink S & Odio M. (2015). Newborn infant skin: physiology, development, and care. Clin. Dermatol. , 33, 271-80. PMID: 25889127 DOI.
Singh G & Archana G. (2008). Unraveling the mystery of vernix caseosa. Indian J Dermatol , 53, 54-60. PMID: 19881987 DOI.
Articles
Peltonen S, Raiko L & Peltonen J. (2010). Desmosomes in developing human epidermis. Dermatol Res Pract , 2010, 698761. PMID: 20592759 DOI.
Malminen M, Peltonen S, Koivunen J & Peltonen J. (2002). Functional expression of NF1 tumor suppressor protein: association with keratin intermediate filaments during the early development of human epidermis. BMC Dermatol. , 2, 10. PMID: 12199909
Dale BA, Holbrook KA, Kimball JR, Hoff M & Sun TT. (1985). Expression of epidermal keratins and filaggrin during human fetal skin development. J. Cell Biol. , 101, 1257-69. PMID: 2413039
Search PubMed
Search Pubmed: Integumentary Development | Skin Development | Hair Development | Tooth Development | Vernix Caseosa
NCBI - Policies and Guidelines | PubMed | Help:Reference Tutorial
Additional Images
Historic
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
Terms
| Integumentary Terms | ||
|---|---|---|
Integumentary Development
| ||
|
External Links
External Links Notice - The dynamic nature of the internet may mean that some of these listed links may no longer function. If the link no longer works search the web with the link text or name. Links to any external commercial sites are provided for information purposes only and should never be considered an endorsement. UNSW Embryology is provided as an educational resource with no clinical information or commercial affiliation.
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2026, February 26) Embryology Integumentary System Development. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Integumentary_System_Development
- © Dr Mark Hill 2026, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G