Book - Manual of Human Embryology 5
| Gastrulation links | ||||
|---|---|---|---|---|
|
Keibel F. V. The formation op the germ layers and the gastrulation problem in Keibel F. and Mall FP. Manual of Human Embryology I. (1910) J. B. Lippincott Company, Philadelphia.
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
V. The Formation of the Germ Layers and the Gastrulation Problem
By Franz Keibel, Freiburg i. Bb.
The formation of the germ layers, like the processes of segmentation, has not yet been observed in the human species; in the youngest known human ova the germ layers are already present. The middje layer, it is true, is still in process of formation f rdii the primitive streak in the younger ova; but it is a striking fact that in the very youngest ova, although a primitive streak cannot be recognized with certainty, nevertheless the middle germ layer is abundantly present. I have not employed the terms ectoderm, mesoderm, and entoderm — or ectoblast, mesoblast, and entoblast — because an accurate definition of them cannot be given so long as the middle layer is still in the process of formation; furthermore the nomenclature, influenced by theoretical considerations, is not altogether unambiguous and we must first understand its significance.
If we would understand the conditions in man, or even approach to an understanding of them, we must first briefly consider the corresponding conditions in other mammals, then those of other vertebrates, and, indeed, those of the invertebrates also. All the similarities that may be found in the processes back of the formation of the germ layers in the vertebrates and invertebrates we must regard as convergence phenomena, but with the formation of the layers we come so near to the common origin of the two groups that we may well look for something directly comparable. The formation of the germ layers is not yet entirely understood in mammals. Selenka[1] described an immigration gastrula in Didelphys rirginiana, and Van Beneden came to the conclusion that in the mammals the material for the ectoblast and the entoblast or hypoblast was separated even at the first segmentation division. The descendants of the ectoblast cell grew around those of the hypoblast cell and so produced an epibolic gastrula, and the region where the epiblastic cells finally closed over the hypoblast was regarded as the blastopore; it was situated in the region at which the embryo later formed. Van Beneden has now given up this hypothesis for sufficient reasons, but it has been revived by Duval, who, however, places the closure of the gastrula at the anti-embryonic pole. I believe, with Van Beneden, whose conclusions rest on extensive observations, that Duval has been deceived by artefacts. Nor can I regard Selenka's observation as certain. The material was very scanty and its investigation does not seem to me to have been beyond criticism. If, for the present, we disregard theories, we can say that in mammals there is formed by segmentation a solid mass of cells, a morula, in which, in many cases, an outw layer can be distinguished at an early stage from an inner cell mass. If this outer layer is incomplete, the interruption occurs at the embryonic pole. At the opposite pole the outer layer separates itself from the inner cell mass. Van Beneden believes that this separation takes place by the vacuolation of the lower cells of the inner mass, but it may also be supposed to occur by a simple separation of the outer layer from the mass. There is thus formed, from a cell mass, a morula, a small vesicle, a blastula; to the embryonic pole of this blastula is attached a mass of cells, which, among other things, includes the actual embryonic anlage.
On the under surface of this mass of cells there arises, by a process which must be termed delamination, a layer of cells which will become the intestinal and yolk-sack epithelium, that is to say, the entoderm. It may be formed at a time when the cell mass still projects into the interior of the embryonic vesicle (as, for example, in the deer) or when it has spread out to form the embryonic shield (as in the dog), but these differences will be considered later. Here we must note that with the appearance of this cell layer, which grows around the inner wall of the embryonic vesicle and reaches the anti-embryonic pole sooner or later, except in some cases, the mammalian ovum has reached the gastrula stage; it consists of two layere or cell complexes, of which the inner one will become the intestinal and yolk-sack entoderm.
As has been already mentioned, the development may take place in different manners, even if those mammals which present a so-called inversion of the germ layers be disregarded. We will follow it through three different types.
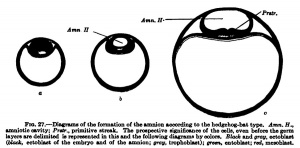
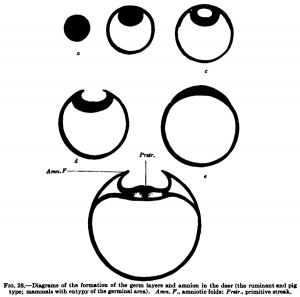
In the hedgehog, according to Hubrecht, and similarly in the bat, according to Van Beneden, there is formed from the inner cell mass in addition to the entoblast mentioned above (Van Beneden's lecithophore, the yolk layer of other authors) an outer germinal layer, from which the eetoblast of the embryo and of the amnion and the mesoblast are formed. This occurs, as is shown in Fig. 27, h, by the appearance of a cavity in the round mass of cells shown in Fig. 27, a, which cavity is directly transformed into the amniotic cavity; in its floor the embryonic shield is formed, in the region of the embryonic shield the primitive streak appears, and from the primitive streak the middle germ layer or mesoblast arises. The development of the mesoblast and primitive streak in the different times will be discussed later and we may now consider the second tjrpe, shown by the deer and also by the sheep and pig. In the deer, as in the hedgehog and hat, a cavity is formed in the inner cell fnass (Fig. 28, c), but this cavity does not remain closed and is not directly transformed into the amniotic cavity; it opens to the exterior, the embryonic shield flattens out upon the surface of the ovum, and the amnion is formed from a fold that rises around the embryonic shield.
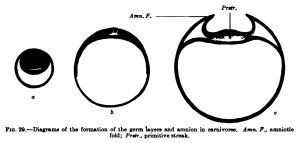
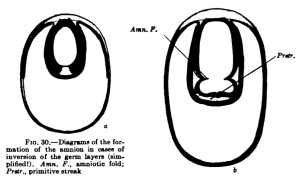
A third type (Fig. 29) is found, for example, iir-the dog and other mammals, and in it the inner cell mass flattens out to fonn th^ embryonic shield before the entoblast appears; from the beginning the embryonic shield is spread out upon the surface of the ovum, and in the rabbit it is at first covered by a thin layer of cells (Rauber's covering layer), which soon disappears. A fundamental difference between these three types does iiot exist, nof does it exist in the so-called inversion of the germ layers which may be observed in many rodents. The mode of development of mammals showing this inversion is readily intelligible if one takes as a starting point for it the first type of development described above (that of the hedgehog and bat, Fig. 27). In Fig. 30 are two diagrams. which will make clear the so-called inversion. In Fig. 30, a, the two-layered ovum has become invaginated into itself and the invagination opening has become closed by a trophoblast growth. Fig. 30, b, shows the formation of the mesoblast and the amnion.
Opinions regarding the development of the mesoblast in mammals are still very divergent. Ortain it is that the primitive streak is its principal seat offormation. It arises from the primitive node, a thickening of the upper germ layer, from which the mesoblast cells grow out between the upper and lower germ layei*s. But whether this jDrimitive node appears within the limits of the embryonic shield and the primitive streak is formed by a growth from it directed caudally, or whether the primitive node appears at the caudal end of the embryonic shield and the primitive streak is formed by a forward growth from it — these are questions concerning whose answers there is still difference of opinion; this is true also as to the question whether the primitive streak is the only source of formation of the mesoblast. Bonnet, for example, recognizes two other sources. He believes that a mesoblast area surrounds the embryonic shield, the mesoblast in this area being formed from the inner germ layer. He described this area in the sheep, but could not find it in the dog; and it is certain that it does ti6t occur in a number of other mammals. A second source of the mesoblast from entoblast Bonnet finds in the " completing plate " of the primitive gut cord. It lies in the anterior region of the head and from it, in addition to a premandibular rudiment of the gut, there arise the chorda and the mesoderm of the head region.[2]
At the height of its development the mammalian primitive sireak extends throughout almost the entire length of the embryonic shield, beginning a short distance behind its cranial end and extending to the caudal end ; later it degenerates in a craniocaudal direction. At its cranial end a canal, the canalis neurentericus, which traverses the embryonic shield, may be formed; its caudal end gives rise to the cloacal membrane. From it there is formed a large portion of the body of the embryo, for, in my opinion, there can be no doubt but that it originally extends far into the head region; its anterior end and the neurenteric canal, when it is present, migrate, therefore, in a craniocaudal direction. From the axial mesoblast which is thus formed in front of the anterior end of the primitive streak the chorda arises, from the more lateral mesoblast the primitive somites and the mesoblast of the embryonic body situated still more peripherally.
The chorda anlage is transitorily enclosed within the entoderm, but later it again separates from it. For a time the growth of the primitive streak keeps pace with the amount of material separated off from it; but later the streak becomes gradually smaller, giving off more material than is replaced by growth, and, finally, it becomes transformed into a region of proliferation which is usually termed the tail bud, but which also gives rise to a portion of the body. This region of proliferation consists of an indifferent cell material into which the medullary tube, the chorda, intestine, and mesoderm pass and from which the caudal portions of these structures are differentiated.
A sharp distinction cannot be drawn between the primitive streak and the tail bud; but« since the tail bud is distinct as such even in mammalian embryos with less than twenty primitive somites and the degeneration of the primitive streak is in full swing in still younger embryos, it would perhaps be better to speak of a trunk bud than of a tail bud.
Having thus learned how the germ layers are formed in the mammals we may now inquire how their formation compares with what occurs in other animals; in other words, we now come to a consideration of the theories generally known as the gastrulation and the coelom theories. And here we must first agree upon the definition which is to be applied to the process of gastrulation. The inconsistent and frequently actually contradictory definitions that have been given of gastrulation have so obscured what is in itself a difficult question, that it is not easy to give an account of it. The anatomists and purely vertebrate zoologists are to blame for some of the difficulty, in that they have not perceived that the process of gastrulation is not something confined to the vertebrates, but, as the fundamental works of Ray Lankester and Haeckel have shown, is a process which occurs in the majority of metazoa. Hubrecht has rendered good sendee by energetically pointing out that it is not permissible to define gastrulation differently in the vertebrates and in the other metazoa; and I am thoroughly in agreement with him in this, even although I considered the question for many years entirely from the standpoint of a vertebrate zoologist. accordingly, I define gastrulation as the process by which the cells of the metazoan ovum are separated into an upper and a lower layer, into ectoblast (ectoderm) and entoblast (hypoblast, entoderm), and by entoblast I mean only the cells forming the intestinal epithelium.[3] That we must regard the final result as the most important thing in the idea of gastrulation and must disregard the manner in which this is brought about (delamination, immigration, invagination, epibole) I had already pointed out before Hubrecht, and had shown that the processes that had usually been designated as gastrulation in the vertebrates could not be directly compared with the gastrulation processes of the invertebrates. I must, therefore, consider the question here so far as is necessary for the understanding of the nomenclature and the older literature, and for further details would refer to my paper of 1901 already cited and to the explanations by Hubrecht,[4] myself,[5] and Brachet.[6]
The entire theory of the formation of the germ layers and gastrulation in the vertebrates is based upon the development of AmphioxtM. In this form the material for the formation of the intestinal entoderm, the mesoderm, and the chorda is laid down in the interior by a single process, and for a long time all investigators have incorrectly designated this entire process as gastrulation and have started their Consideration of gastrulation in the vertebrates from it. In the same way in 1901 I defined gastrulation, pointing out, it is true, that the definition could only apply to vertebrates, as the process by which the cell complexes for the intestinal entoderm (i,e,, intestinal and yolk-sack entoderm), the mesoderm, and the chorda were brought into the interior of the ovimi. I stated, further, that this definition could be extended to all vertebrates if two phases were recognized in it: a first phase in which the intestinal entoderm, and a second, in which the mesoderm and the chorda were formed. If one assumed that alterations in the true relations of these two phases occurred, all variations could be explained and the common basis for both processes could be recognized.
I had come to this conclusion in 1889[7] as the result of studies in the development of manmialia, and at about the same time Hubrecht[8] also reached it independently. I have since carried the idea further and will here refer to some of the points which I brought together in my observations and conclusions in 1893.[9]
I said then:
- " In the mammals gastrulation takes place in two phases. In the first phase the lower germ layer, the entoderm of authors, is formed; in the second phase the chorda and mesoderm. From the so-called entoderm of the mammals there is formed essentially only the intestinal epithelium and that of the yolk sack. That additions are made to the intestinal epithelium from the cell mass which is formed in the interior of the ovimi during the second phase of gastrulation cannot be absolutely denied ; but in any event these additions, * if they actually occur,' are insignificant and are limited to a small region of the intestine. In the other anmiotes gastrulation also occurs in two phases, yet the cell complexes formed in the interior of the ovum by the two gastrulation phases do not exactly correspond either qualitatively or quantitatively in different forms: thus in the Reptilia, and especially in the Chelonia, the second phase of gastrulation carries into the interior of the ovum the whole, or at least a considerable portion, of the intestinal epithelium; while in the mammals the intestinal epithelium, together with that of the yolk sack, gaijis its definitive position during the first phase.'* Later observations, however, show that reptiles more closely approximate the mammals in this respect, and it is becoming more probable that in the Reptilia also the lower layer, usually termed the paraderm, yolk layer, or secondary entoderm, gives origin to the intestinal epithelium.[10]
Concerning the gastrula cavity I said:
- " The gastrulation cavity of the first phase of gastrulation, if it is formed at all, fuses very early, or immediately, with the segmentation cavity in mammals; but probably it does not occur at all.
- " The cavity of the mammalian ovimi in the two-layered stage is, consequently, to be regarded as the sum of a portion of the gastrula cavity and a portion of the segmentation cavity. Another portion of it is to be found in the cleft between the two primary germ layers. Corresponding conditions, which I shall for the present regard as merely analogies, occur in the Amphibia. In birds and reptiles the germ, or subgerminal, cavity is also to be considered a portion of the gastrula cavity, and a portion of the segmentation cavity. A second portion of this latter cavity in the Sauropsida is also the cleft between the upper and lower layers of the two-layered germ.
- " The chorda cavity of mammals is to be regarded as a part of the gastrula cavity and belongs to the second phase of gastrulation. Only exceptionally are cavities found in the second phase which can be considered as remains of the Coelomic diverticula of the archenteric cavity.
- " In the mammals the primitive streak is to be regarded as equivalent to the blastopore. In the germinal ring of the germinal disk of the birds and reptiles I also see a homologue (morphologically equivalent part) of a portion of the blastopore; it has, of course, been extremely modified."
These views have, it is true, not been unopposed, but since they have been reproduced in O. Hertwig's " Handbuch " and in his " Lehrbuch " and his " Elements," they may be regarded as the prevailing ones. How they must be modified if we are to make use of the definition given above, which applies to all metazoa that have an intestine, must now be considered. In the first place, it may be said that the separation of the gastrulation process into two phases has not been without results. What I have hitherto termed the first phase of gastrulation in the vertebrates, and especially in the mammals, must now alone be regarded as gastrulation, and the process which I formerly termed the second phase of gastrulation, that is to say, the formation of the mesoderm and chorda, since there is nothing directly comparable to it in the invertebrates, or, at all events, nothing comparable that can be regarded as belonging to gastrulation, must be separated from that process and regarded as a process by itself, even although it has become intimately associated with or even, so to speak, included in gastrulation as the result of a shifting in the time relations. But with this formal separation of the two processes and the simple modification of the nomenclature we have not yet reached perfect clearness, difficulties still remaining as to the homologue of the blastopore. In my earlier definition I compared directly the primitive streak of the amniotes with the blastopore; but this will not now hold, since the characteristic of the invertebrate blastopore, the direct transition from ectoblast to entoblast, no longer obtains in the amniotes. Between the ectoblast and entoblast there is interposed in the primitive streak the mesoblast, derived from the upper germ layer, and the streak also gives rise to the chorda; indeed, we may say that for a time, in early stages, the chorda arises directly from the upper layer. We can compare with the blastopore only the line of junction of the entoderm with the mesoblast and chorda, where such a line exists; and if we are to make comparisons with the invertebrates we must regard the mesoderm and chorda as products of the upper genn layer, the ectoblast.[11]
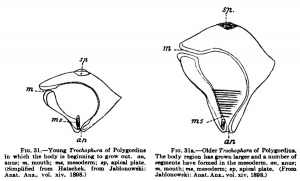
What has hitherto been designated as the blastopore in the vertebrates, Amphioxus included, lies in the territory of the ectoblast and its derivatives and not at the boundary between ectoblast and entoblast; this structure, which undoubtedly resembles the blastopore most closely, but can only be compared with it at its first formation, is comparable to the primitive streak. Furthermore, the vertebrate body is formed not by the simple conversion of the gastrula into it, but a budding zone is formed in the region of the blastopore from which the segments of the vertebrate body are budded off. At this stage a comparison with the Trochophora (Fig. 31), a widely distributed lan^a among the annelids and molluscs, is quite possible, as Kopsch[12] and J. Jablonowski[13] have shown. Just as we can distinguish in the body of the larva an anterior unsegmented portion in which the body is beginning to grow out. an. The body region has grown larger and a number of which has been formed by the gastnilatioa process, and a posterior segmented portion which owes its existence to a budding process that succeeds gastnilation, so too is it possible in the vertebrate embryo. Hubrecht consequently distinguishes between cephalogenesis and notogenesis in the development of vertebrates. By cephalogenesis the anterior unsegmented portion of the vertebrate body is formed as a result of gastrulatton ; by notogenesis, a process of budding, the suCoeeding portion is formed. One must not, however, misunderstand these expressions. The limits of the two portions of the body must not be sought where the head now joins the trunk; trunk segments in unknown number have been taken up into the head and " the question concerns, on the one hand, only the most anterior region of the head, to which the olfactorius and opticus belong; and, on the other hand, the remaining portion of the brain, together with the base of the skull with the remains of the chorda and the visceral arches, and the entire trunk."
anus; m, mouth; ms, mesoderm; «p, apical plate. segments have formed in the mesoderm, an, anus; (Simplified from Hatschek, from Jablonowski: m, mouth; ma, mesoderm; «p, apical plate. (From Anat. Anz., vol. xiv, 1898.) Jablonowski: Anat.Ans., vol. xiv, 1898.)
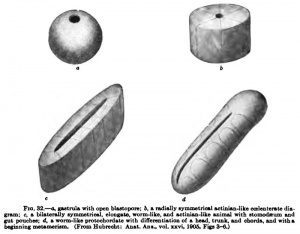
While it is far from my intention to attempt a derivation of the vertebrates from the actinite at all directly, yet it may be well to consider the possibility of derivation from such simple forms. With this object I give here Hubrecht's speculations (Quart. Joum. Micr, Sci., vol. xHx, p. 410) (Fig, 32) ; " Once the didermic gastmla-stage reached, a second phase of ontogenetic development is inaugurated which is also of higli phylogenetic importance. In this phase the bilaterally symmetric metameric animal gradually appears which we have to compare with possible phylogenetic transition forms that have connected the Vertebrates with radially symmetrical ancestors. This attempt at a plausible and rational reconstruction of the Vertebrate ancestry is, of course, hampered by the circumstance that no trace of those forms is any longer in existence. Still, an actinia-like, vermiform being, elongated in the direction of the mouth slit, imposes itself upon our imagination, such as has served for the theoretical speculations of Sedgwick on this same subject, and has once been accepted by van Beneden for the precursors of the Chordata." Hubrecht has pointed out that " the processes of growth by which a Coelenterate gastrula becomes fixed and gradually changes into a sessile Actinian can hardly be looked upon as protracted phases of gastrulation. This will be more difficult yet when the animal has already acquired a higher degree of complication than that of the Coelenterates, and swims about in the shape of a worm-like, lower chordate animal. We know of Polygordius and of other primitive worm types that to the radial, didermic larval stage — the Trochophora — another developmental phase suCoeeds, during which we obsen^e proliferation in the anal region, leading to an increase in the distance between the anus and the apex of the metamerical worm, the latter budding off, so to say, from the radial trochophora.
" We find similar processes in the Vertebrates, but without a free trochophora larva, and to this latter radial and didermic primitive stage corresponds in the Craniata the rapidly passing earliest phase in which delamination calls forth two germinal layers. Both in Elasmobranchs and in mammals we notice that the cellular material which is present in those very earliest stages contributes especially — as it does in the trochophora — towards the formation of the anterior part, the head, and that, following upon this, a proliferation-process is inaugurated (comparable to the origin of the metamerical worm out of the trochophora larva) by which the notochord and the somites, i.e,, the bilaterally symmetrical metameric animal, are called into existence."
Hubrecht, going far back in the phylogeny, compares this proliferation with the growth of an elongated actinian. He imagines the coBlom pouches of the actinian, still in communication with the intestine, to represent the somites; the nerve ring on the oral disk would represent the spinal cord; the stomodseum, the chorda; and the actinian mouth, which is not to be regarded as its primitive mouth or blastopore, but is a secondary formation, represents the primitive streak, that stands in such intimate relation to the chorda (that is, to the actinian stomodseum).
The ingerent opening of the actiniaa gastrula elongates to form the actinian mouth that leads into the stomodaeum; the blastopore of the mammalian gastrula, which never opens, elongates posteriorly to form the primitive groove, whose floor, the primitive streak, furnishes the material for the chorda.
" There is, then, during ontogeny an unbroken continuity between the blastopore of the Actinian and its oral slit, between the blastopore of the Vertebrate (often only potential in mammals and not identical with the opening that is called by that name in Sauropsids) and its primitive groove. A phylogenetic continuity has to be statuated between this oral slit of the Actinia and the peculiar spot (behind the so-called anterior lip of the blastopore) which on the Vertebrate embryonic shield gradually moves backwards and establishes in many cases an open communication between a portion of the Vertebrate intestine and the exterior. The primitive streak, however, the solid material that proliferates downwards from the ectoderm, coalesces with the entoderm, and brings forth the notochord from its median (though really paired) portion and the somites from its lateral wings — this primitive streak can never be identified with a blastopore. For we have above attempted to demonstrate that in this primitive streak we encounter the material which, also in the Actinia, (1) proliferates downwards from the ectoderm and produces the stomodaBum, (2) coalesces with the entoderm, (3) is in direct continuity with those parts which are preparing to give rise to coelomic pouches but are yet continuous with the primitive enteron." Hubrecht proposes a modification of the nomenclature in that for that portion of the vertebrate embryonic disk which he has compared with the actinian mouth and stomodaBum he suggests the use of the name " dorsal mouth " instead of primitive mouth, gastrula mouth, or blastopore. In this manner the contrast with the phyla of the annelids and molluscs will be more plainly brought out.
If now we summarize what has been stated above and apply it to the formation of the germ layers in the mammals, we must say :
1. In the TnRmmRls the entoderm (yolk and intestinal entoderm) is formed by delamination ; and it is only this delamination process that one can term gastrulation. The two cell-complexes which are formed as the result of gastrulation are to be termed ectoblast (ectoderm) and entoblast (hypoblast, entoderm). That the superficial layer of the wall of the embryonic vesicle, for a time the only layer that is present, must also be regarded as a portion of the ectoblast seems selfevident; the so-called covering layer belongs to it also. In so far as it is concerned in the nourishment of the ovum it may be termed, following Hubrecht, the trophoblast. As trophoderm — in the sense in which that word is used by Minot — a portion of the trophoblast is again to be distinguished, which in certain cases produces the penetration of the ovum into the wall of the uterus. Morphologically the trophoblast may be divided into the cytotrophoblast, from which the Langhans layer of cells is formed in the human ovum, and the spongiotrophoblast (plasmoditrophoblast), which gives rise to the syncytium.
2. A typical blastopore has not yet been certainly observed in the mammals. An invagination blastopore need not be expected to occur; some observations by which a connection of the ectoderm and entoderm was shown in early stages, before the formation of the mesoblast, have suggested the occurrence of a rudimentary blastopore. The primitive node and primitive streak — whether or not a primitive groove forms in the primitive streak is a matter of subordinate importance — are certainly associated with the blastopore, but cannot be directly homologized with it.
3. The anterior end of the primitive streak, at the time when the streak is at its greatest extension anteriorly, but not always its most anterior part, must be regarded as indicating the position of the blastopore.
4. The invagination processes by which in the mammals the formation of the chorda and mesoblast is initiated, are comparable to the corresponding processes in Amphioxus, but can be regarded as the gastrulation process neither in Amphioxus nor the vertebrates. We must accordingly say that the chorda and mesoblast arise from the ectoblast, the chorda partly directly and partly after it has belonged to the mesoblast for some time. The enclosure of the chorda in the entoblast is an entirely secondary phenomenon.
5. Even although the region of the primitive streak, with or without a primitive groove, cannot be compared directly with the fused lips of the blastopore of a gastrula, nevertheless they must be regarded as modified structures that have arisen in association with a typical blastopore and must be compared with what it has been the custom to call the lips of the blastopore in the vertebrates, from Amphioxus upwards. The processes which are here concerned are comparable to the budding processes which in the Trochophora larva succeed the gastrulation processes. This becomes especially clear when one considers the later transformation that the primitive streak undergoes and by which it becomes converted into the so-called " tail bud," a structure from which (as has already been pointed out) not only the tail arises, since the line between the tail and the trunk is merely conventional. The principal limit that concerns us here lies far forward in the head region, between those portions of the body which are formed by the gastrulation process and those that owe their existence to the succeeding proliferation process. The anterior end of the primitive streak, at the time of its greatest anterior extension, marks this limit.
There result from these theoretical considerations certain conclusions that are of interest from the more practical side. A longitudinal splitting of the embryo, which may extend into the head region, may, under some circumstances, be regarded as produced by inhibition of growth, which can be referred to the primitive streak and the processes which take place in it.
Roux's hemitheria anteriora such as the calf studied by Roux's pupil Eckhardt[14] which Roux regarded as due to the early degeneration of the two segmentation cells which contained the anlagen of the caudal half of the body, are much more probably due to disturbances in the territory of the primitive streak. according as these occur early or late, a greater or smaller portion of the posterior extremity of the body will be wanting. Bob-tailed cats and dogs also belong to this class of developmental inhibitions; in these the inhibition first occurred after a portion of the tail had developed from the tail bud.
Embryology throws light upon the occurrence of coccygeal tumors and their varied structure by the fact that in the " tail bud " an indifferent cell material is present from which all the germ layers may be produced.
In conclusion, we may now return to man and endeavor, from what we at present know concerning his development and that of animals, to form a picture of the formation of his germ layers. We must assume that the human ovum, similarly to that of the guinea-pig, burrows into the mucous membrane of the uterus, destroys the maternal tissues, and so makes a cavity for itself. At the time of the penetration into the mucous membrane the diameter of the ovum can scarcely amount to 0.5 mm. Very early, but probably only after the ovum has burrowed into the mucous membrane, the formation of the coelom and of the germ layers begins. I assume that this formation can begin only at that time because the burrowing process would otherwise encounter difficulties from the enlargement of the ovum made necessary by the formation of the coelom and mesoblast ; and it may also be supposed that those cells would first be formed which are intended for the destruction and absorption of the maternal tissue, the trophoblast cells, which belong to the later ectoblast complex. At all events, one must assume, as Spee pointed out as long ago as 1896, and as is now rendered almost certain by the Peters ovum, that at the time of the first formation of the mesoblast the diameter of the ovum does not exceed 0.5 mm.
The formation of the mesoblast follows immediately upon that of the amniotic (medullo-amniotic) cavity and that of the cavity of the yolk sack and intestine. That the coelom in the human ovum is formed by a splitting process is indicated by the scattered mesoblastic strands which stretch between the yolk sack and the chorionic mesoblast, as well as by observations in many other mammals.[15]
The human ovum is, consequently, to be assigned to the category of schizocoel ova. It is, however, not yet quite clear how the cavity traversed by scattered strands of mesoblast and lying between the yolk sack and the chorion in the Peters ovum is to be interpreted. It may be supposed to represent the extraembryonic coelom ; but it may also be imagined that it has arisen from an extensive loosening up of the tissue and not by a splitting of the mesoderm, and that the triangular space beside the caudal extremity of the embryo (see Fig. 96, p. 108, in the chapter on the development of the egg membranes and the placenta tion), which is lined with flat cells having an epithelial arrangement, is the first anlage of the eoelom.
As to the amniotic (medullo-amniotic) cavity, it may be said with a probability bordering upon certainty that it arises by a splitting of a solid cell mass; consequently, amniotic folds never occur in man. The amniotic duct or cord — a connection between the epithelium of the amniotic cavity and the surface of the chorion, indications of which have been observed by Eternod and Marchand and more distinct evidence by Beneke in himian ova and by Selenka in apes — appears to arise later, and, indeed, it is questionable if it is of regular occurrence ; it is a phylogenetic memory from dim ancestral times. Selenka (1903) believes that this amniotic umbilicus, as he calls it, following Bonnet, does not reach complete development in apes; that may also be the case in man; at all events it is wanting in the youngest stages yet observed, and, if it is of constant occurrence, its existence must be limited to a very short period of time.
As the amniotic (medullo-amniotic) cavity, so also the cavity of the yolk sack is formed by the splitting of an originally solid mass of cells; in the Peters ovum it is still so small — smaller than the ripe ovarian ovum — that one can hardly imagine it to be formed by being enclosed by a surrounding epithelial lamella of entoblast. No important difficulties stand in the way of its origin by a splitting process.
Graf Spee in his paper of 1889 makes use of ova which present an inversion of the germ layers for an explanation of the conditions which obtain in the human ovum.
On the other hand, I maintained (1890) that the peculiarities of the himian ovum were to be explained by the early formation of the extra-embryonic eoelom and of the amnion, and that a sinking of the human embryonic anlage deep into the yolk sack, as in ova with inversion of the germ layers, could not be imagined.
In the endeavor to obtain a clear picture of the processes just discussed, the extraordinary minuteness of the embryonic structures must constantly be borne in mind. The diameter of the yolk sack in the Peters ovum is 0.19 mm., that is to say, it is not quite the size of a ripe human ovum.[16]
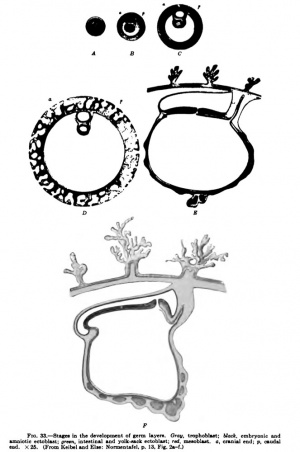
Elze and I have endeavored in our "Normentafel zur Entwicklungsgeschichte" to embody the results of the above considerations in a series of diagrams. Fig. 33, Ay represents an ovum towards the end of segmentation as a whole object. Fig. 33, B-Fy are sections, and it is assumed that all sections pass through the median sagittal plane of the future embryo. The figures are of such a size that they may be regarded as enlarged twenty-five times. The ovum shown in Fig. 33, A, is still surrounded by the zona pellucida; it may have just reached the uterus. B represents an ovum which has already eaten its way into the mucous membrane of the uterus. Four groups of cells are to be recognized in it. The periphery consists throughout of ectoblast cells — the trophoblast, represented in gray. Inside this trophoblast mantle are three cell-complexes. That which gives rise to the ectoderm of the embryo and of the amnion is represented in black; to the right, corresponding to the caudal end of the later embryo, we have left indistinct the boundary between it and the mesoblast complex, represented in red, in order to indicate that in this region there is perhaps a transition between the two complexes. We have represented this connection in the diagram, because wherever the development of the mesoblast has been sufficiently studied, the greater portion of it, at least, is found to arise from the ectoblast complex, and we find later, even in human ova, a primitive streak. It is extraordinarily difficult to picture to oneself how the processes actually take place in man, owing to the minuteness of the human ovum at this stage. The mesoblast complex is everywhere forcing the entoblast complex, represented in green, away from the trophoblast shell.
Fig. 33, C, shows an older stage. By a process of splitting in the ectoblast and entoblast complexes, the amniotic (medulloamniotic) cavity in the one case and the cavity of the intestine and yolk sack in the other have been formed, and in a similar manner the extra-embryonic coelom has formed in the mesoblast complex. We have already pointed out that another mode of formation of the coelom is possible. In this case we must suppose that in this stage the extra-embryonic coelom is not yet formed and we must imagine the cavity inside the red, which is left white in Cy to have a pale reddish tinge and to represent solid but very loosely compacted mesoblast. Around the amnion, the yolk sack, and at the periphery next the trophoblast, the mesoblast, which as a whole is still solid, is somewhat denser. The amnion at this stage may still be in contact with the trophoblast shell.
Diagram D, Fig. 33, shows the conditions which obtain in the Peters ovum. The trophoblast mantle surrounding the ovum has developed lacunae which are filled with maternal blood, and the mesoblastic axes of the villi have begun to grow out into the trophoblast. At the caudal extremity the belly stalk has become distinct, but an allantoic duct is not yet formed. Whether or not a very small primitive sireak waspresent in the Peters ovum must remain doubtful; we assume that the delimitation of the ectoblast from the mesoblast was not quite sharp at the caudal end, and this we take to be the anlage of a primitive streak. The embryonic coelom is represented as completely formed, although a somewhat different interpretation of the conditions in the Peters ovum has been mentioned. The ectoblastic covering of the embryo and the amnion are everywhere being forced away from the trophoblast by mesoblast cells.
Diagram E, Fig. 33, represents a median sagittal section through an ovum of the stage seen in the Frassi ovum. The anterior half of the section, in the region of the embryonic shield, is oCoupied by the floor of the medullary groove; behind it is the neurenteric canal; and then, lying in the same plane as the anterior half of the embryonic ^nield, the region of the primitive streak, which occupies about b&lf the shield. At the caudal end of the priim4;iye streak th^ feioacal membrane is already recognizable. The chorda-ts' enclosed within the entoblast; anlagen of blood and blood-vessels occur in the yolk sack. An allantoic duct is present.
Diagram F shows a median sagittal section through the stage seen in Spee's Glaevecke embryo. Especially to be noted is the recession of tTie primitive streak and the fact that the now quite short primitive streak is bent down at an angle to the plane of the cranial extremity of the embryonic disk. A cloacal membrane must have been present at the caudal end of the primitive streak, but it is not represented in the diagram because it was not observed in the Spee embryo, probably on account of the direction in which the sections were made.
- ↑ For the literature, other than that which is directly cited here, see Keibel: Die Gastrulation und die Keimblattbildung der Wirbeltiere Ergebnisse d. Anat. u. Entwicklungsgesch., vol. x (literature to 1900), 1901.
- ↑ Although it is impossible to consider here the various individual opinions regarding the formation of the mesoblast in manmoals, nevertheless the observations of Wilson and Hill (J. T. Wilson and J. P. Hill: Observations on the Development of Omithorhynchus, Philosph. Transactions, Ser. B. vol. cxcix, 1904) on Omithorhynchus may be considered. In their opinion the primitive node, which marks the position of the blastopore, comes into relation with the primitive streak only secondarily; originally it lies outside the embryonic shield. Yet it seems to me questionable if the structure which the authors regard as the primitive node in early stages is the same structure which they so designate in later stages; I have wondered whether the primitive node of younger stages may not be the yolk navel.
- ↑ The intestine here includes its appendages, the yolk sack and the intestinal glands.
- ↑ A. A. W. Hubrecht : Die Qustrulation der Wirbeltiere, Anat. Anz., vol. xxvi, 1905.
- ↑ F. Keibel: Zur Gastrulationsfrage, ibid.
- ↑ A. Brachet : Gastrulation et formation de Fembryon chez les chords, ibid., vol. xxvii, 1905.
- ↑ F. Keibel: Zur Entwicklungsgeschichte der Chorda, Arch. f. -Anat. u. Physiol., Anat. Abt., 1889.
- ↑ A. A. W. Hubrecht: Die erste Anlage des Hypoblast bei den Saugetieren, Anat. Anz., 1888, vol. iii, pp. 906-912; and The Development of the Germinal Layers in Sorex vulgaris , Quart. Joum. Micr. Sci., vol. xxxi, 1890.
- ↑ F. Keibel: Studien zur Entwicklungsgeschichte des Schweines, Schwalbe's Morphol. Arb., vol. iii, 1893.
- ↑ Greil (Ueber die erste Anlage der Gefasse und des Blutes bei Holound Meroblastiem [speziell bei Ceratodus forsteri], Verb. Anat. Ges., 1908) states (p. 58) that it "must be recognized that in the first phase of Hertwig and other authors there is formed not the entoderm of the entire embryonic anlage but only the abortive entoderm of the yolk sack." This is certainly incorrect for mammals and birds, and probably for the reptiles also.
- ↑ This has already been done quite logically by Lwoff (B. Lwoff: Ueber einige wichtige Punkte in der Entwicklung des Amphioxus, Biol. Zentralbl. vol. xii, 1892; and Die Bildung der primaren Keimblatter und die Entstehung der Chorda und des Mesoderm bei den Wirbeltieren, Bull. Soc. Imper. des Natural, de Moscou, 1894) in the case of Amphioxus, He regards as the principal result of his investigations the conclusion that " in Amphioxus the invagination is in no wise to be regarded as a simple gastrulation, as has hitherto been done. There are, rather two different phases to be distinguished in it: in the first place, the invagination of the entoderm cells, from which the intestine is formed; and, in the second place, the invagination of ectoderm cells from the dorsal transition border, which form the ectoblastogenic anlage of the chorda and mesoderm.
- ↑ Kopsch : Gemeinsame Entwicklungsf ormen bei Wirbeltieren und Wirbellosen, Verb. Anat. Ges., 1898.
- ↑ J. Jablonowski : Ueber einige Vorgange in der Entwicklung des Salmonidenembryos und ihre Bedeutung f iir die Beurteilung der Bildung des Wirbeltierkorpers, Anat. Anz., vol. xiv, 1898.
- ↑ Eckhardt: Ueber Hemitheria anteriora (Roux), Dissert., Breslau, 1889.
- ↑ This process may now be regarded as certain as a result of the observations of Bryce and Teacher (he), which were published only after the first chapters of this " Handbook " had already been written and which must be noticed subsequently.
- ↑ According: to Kolliker and Ebner (" Handbuch der Gkwebelehre," 6 Aufl., vol. iii, 1902), the diameter of the ripe human ovum is 0.22-0.32 mm.; and Waldeyer remarks in Hertwigr's "Handbuch." concerning this statement, that he has never seen human ova measuring more than 0.25 mm.
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2024, April 28) Embryology Book - Manual of Human Embryology 5. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Book_-_Manual_of_Human_Embryology_5
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G