Book - Manual of Human Embryology 19-2
| Embryology - 27 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Felix W. The development of the urinogenital organs. In Keibel F. and Mall FP. Manual of Human Embryology II. (1912) J. B. Lippincott Company, Philadelphia. pp 752-979.
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
Cite this page: Hill, M.A. (2024, April 27) Embryology Book - Manual of Human Embryology 19-2. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Book_-_Manual_of_Human_Embryology_19-2
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G
II. The Development of the Reproductive Glands and their Ducts
Introduction
The ovary and testis are up to a certain period of development exactly alike. Every vertebrate embryo forms at first an indifferent reproductive gland from which, by the emphasis of certain characters, the sexually differentiated organ is formed. The sexual differentiation of the reproductive gland is associated with a second differentiation which earlier, simultaneously, or later effects other organs.
To the sexual differentiations of the reproductive glands belong :
- The transformation of special genital cells, differing from all other cells of the body, into ova or spermatozoa.
- The formation of ovarian follicles or seminal tubules.
- A series of small differences:
- The caudal pole of the female reproductive gland reaches the posterior wall of the genital cord, while that of the testis ends just above it.
- The cranial half or two- thirds of the ovary is rotated during development through 90° and so comes to lie at right angles to the rest.
- The tunica albuginea of the testis appears very early, that of the ovary very late.
- The a. spermatica interna has a quite different course in the two sexes.
- The caudal pole of the testis is united by the ligamentum testis to the chorda gubernaculi, the caudal pole of the ovary by the ligamentum ovarii proprium to the wall of the uterus.
To the sexual differentiations of other organs belong :
- The formation of different efferent ducts for the products of the two reproductive glands. It may be noted here that every embryo, whether it later manifests the male or the female type, develops efferent ducts as well for the ova as the spermatozoa ; the duct which remains functionless degenerates.
- The different formation of the external genital organs. Here also there is an indifferent shape through which both sexes pass before they acquire the special sex characters.
- A series of small differences:
- Accessorv tubes are onlv to be found in female embryos.
- The anlagen of the fimbriae or accessory ostia are present at an early period in the female embryo, but are wanting in the male.
- The excavatio vesico-uterina is present in the female embryo, but not in the male.
- At the beginning of the third month the clitoris hangs decidedly downwards, while the penis stands out horizontally (Herzog, 1904).
- The developing penis rises directly from the cloacal tubercle, while the clitoris is separated from it by a groove.
- The praeputium penis is formed by the ingrowth of one glandar lamella, which has the form of a cylindrical mantle. The prseputium clitoridis develops by the ingrowth of three glandar lamellae, an unpaired, middle, cylindrical one and two paired, straight ones, to the right and left of the unpaired one.
The Genital Cells
The most important cells of the reproductive glands are the genital cells. They are distinguished from all other cells of the body, the soma cells, by the size of their cell-bodies and nuclei; the content of the cell-body is about 27 times as great as that of the nucleus. This latter has usually the form of a round vesicle, frequently of a double vesicle (twin form), and contains a widemeshed delicate chromatin network (noyau leptotene (τατν ια thread, λεππδς slender)), von Winiwarter, 1900) with several nucleoli ; the cell-body is feebly granular, stains only with difficulty and contains in the young condition yolk granules and granules (mitochondria) of a special form.
Hitherto it has been supposed that the genital cells were specially differentiated coelom cells, derived from that portion of the coelom wall that forms the reproductive glands. But the more our knowledge of the origin of the genital cells in the vertebrates increases, the more probable does it become that we must modify this original belief. It has now been shown for all the classes of vertebrates with the exception of the mammalia (Rubaschkin, 1909), that the first (?) genital cells have a special origin, probably being derived directly from the segmentation cells. I, therefore, term these cells primary genital cells in contradistinction to those that are differentiated from the epithelial covering of the reproductive glands; these may be termed secondary genital cells.
The proof of the origin of the primary genital cells from the segmentation cells is based on the special form in which the mitrochondria occur in their protoplasm (Lams and Doorme (1907), Meves and Duesberg (1907, 1908), van der Stricht (1900, 1909), Rubaschkin (1910), Tschaschin (1910)). The mitochondria of all the cells of the segmented germ have the form of granules, and the various granules are completely separate from one another. With the formation of the germinal layers a differentiation takes place among the cells in such a way that in the cells of the entoderm of the caudal portion of the body the mitochondria retain their granular form, while in those of the entoderm of the anterior part of the body, and in those of the ectoderm and mesoderm the isolated granules are transformed first of all into chains of granules and finally into rods or filaments.
Subsequently also the majority of the cells of the posterior half of the body show the filament form, so that at the stage of the first segmentation of the mesoderm only a few cells still show the original granular form of the mitochondria; these few cells are the primary genital cells. It is possible that with the aid of mitochondrial staining it will be shown that the secondary genital cells are derived from the primary ones and then all grounds will be removed for contrasting them as special cells with the primary genital cells.
In holoblastic ova the primary genital cells are formed in the posterior wall of the gut, in meroblastic ova in the floor of the segmentation cavity and in the germinal wall. Since the reproductive glands develop in all vertebrate embryos beside the root of the mesentery, we must suppose that a wandering of the primary germinal cells occurs. By noting all the localities in which primary genital cells are found we may reconstruct the path which is followed by the individual cells from their place of origin to the genital fold. It leads from the mesenchyme between the wall of the intestine and the parietal mesoblast into the root of the mesentery, and from there into tbe urogenital fold, at first into its connective tissue and finally into its epithelial covering. We may distinguish, therefore, between regional and extraregional genital cells, understanding by regional cells those that lie among the epithelial ceils of the genital folds; all those that have not reached that position we group together as wandering or extraregional cells. All the primary genital cells do not migrate at the same time; whether all reach their goal is uncertain, but in any case the possibility of strayed genital cells must be admitted. The secondary genital cells occur only in the genital folds and they are, accordingly, regional from the beginning.
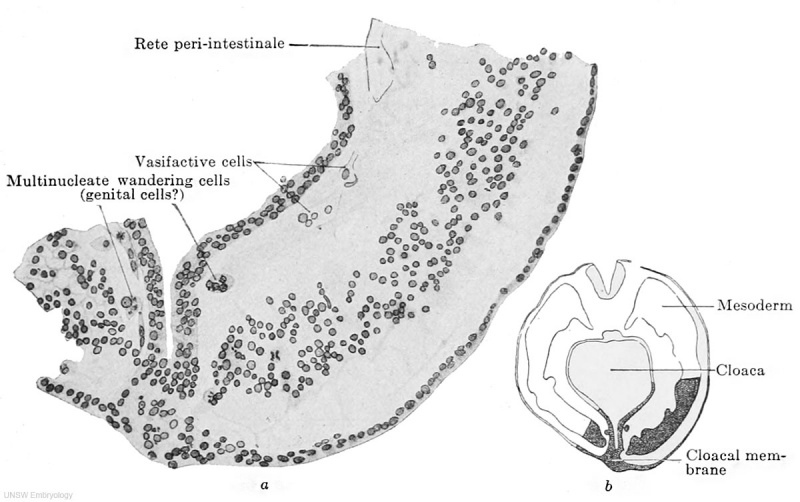
Fig. 609 a and b. — Part of a transverse section through the embryo Pfannenstiel III, 2.5 mm. greatest length and with 13-14 pairs of primitive segments. (From the collection of the late Professor Pfannenstiel, Kiel.) The section passes through the cloacal membrane and its exact position may be seen from the adjacent figure b. The mesoderm is still quite loosely aggregated. Between it and the entoderm there is at the upper edge of the figure a fully-formed portion of the rete peri-intestinale, and further down there are cell chains and masses (to the left of the cloaca) from which new networks of the rete are forming. The section shows at two places, to the right and left of the cloacal membrane, large cells partly multinucleate, partly filled with yolk granules and partly free from them, and lying free between the mesoderm and endoderm. These cells may be termed wandering cells and hypothetically may be interpreted as primary genital cells.
A series of transitional forms may be found between the secondary genital cells and the ordinary coelom cells (cells with noyaux protobroques a and b, von Winiwarter, 1900). I denote these transitional forms as genitaloid cells (cells with noyanx deutobroques, von Winiwarter, 1900). They agree with the genital cells in the structure of their nuclei, which are vesicular with a wide-meshed, delicate chromatin network, with the eoelom cells in the smallness of their cell bodies, which closely surround the nuclei. So soon as genital cells — whether they be primary or secondary — occur in the epithelium of the genital fold we speak of a germinal epithelium (Waldeyer, 1S70) ; this, then, denotes a mingling of ordinary ccelomic and genital cells. Just as a region of eoelom epithelium may become germinal epithelium, so also it may lose its character of germinal epithelium should its genital cells migrate out of it or otherwise disappear. The ordinary eoelom cells react to the immigration of genital cells by increasing in volume, passing from a flattened into a cubical form. But such a modification of flattened eoelom cells must not, without the presence of genital cells, be taken as evidence of the formation of a germinal epithelium. For wherever, as the result of a folding process, two ccelomic epithelial surfaces come into contact, an increase in the height of the flattened epithelium occurs, apparently as the result of a mutual formative stimulus.
Fig. 610. Transverse section through an embryo of 4.7 mm. vertex-breech length and 4.9 mm. nape length and with 33-35 pairs of primitive segments. (Embryo 137, G. 31, from the collection of the II Anatomical Institute, Berlin, Professor O. Hertwig; slide 9, row 3, section 1.) The section passes through the 11th primitive segment and the 5th mesonephric tubule. The urogenital fold is shown passing on the right into the root of the mesentery, on the left into the lateral wall of the body. A primary genital cell is to be seen in the root of the mesentery.
All the primary genital cells disappear in amniotes ; whether they pass through a latent period to become manifest later as secondary genital cells, though possible, has not been proved.
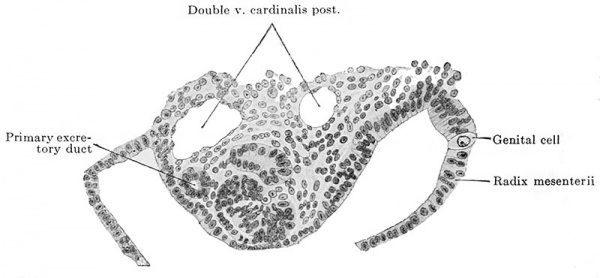
And now, as regards man it is probable that he also possesses genital cells. The following facts are in favor of such a supposition : in an embryo of 2.6 mm. greatest length and with 13-14 pairs of primitive segments there were in the neighborhood of the cloaca, that is to say, in the region of what was originally the primitive streak, between it and the visceral mesoblast, large, free cells similar to the genital cells of other vertebrates and distinguished from the surrounding cells by the size of their cell-bodies and by possessing yolk granules. In the entire embryo seven such cells were recognizable, all in the immediate vicinity of the cloaca and all between it and the visceral mesoblast, i.e., extraregionai. Id Fig. 609 a two of the primary genital cells of this embryo are shown, one to the right and the other to the left of the cloaca. An embryo of 2.5 mm. greatest length and with 23 pairs of primitive segments had twelve primary genital cells, ail extraregional, situated in the vicinity of the cloaca and the adjoining regions of the body; one of these cells is shown in Fig. 532 6. Finally an embryo of 4.9 mm. nape length and with 33-35 pairs of primitive segments showed typical genital cells in the first to the fifth and in the eleventh to the twelfth body segments, the cells lying partly in and partly below the ccelomic epithelium near the root of the mesentery or in the medial slope of the urogenital fold. I show in Fig. 610 one of these cells in the root of the mesentery.
The smallness of the number of these observations allows of no final conclusion, but they speak in favor of the view that man also possesses primary genital cells.
But even if wandering genital cells are to be recognized in human embryos, there is still the possibility that they may be " strays." Such strayed genital cells do not, perhaps, degenerate, but may develop further, and, above all, divide, forming, perhaps, parent cells for tumors, and especially for teratomata.
What has been said above for the amniotes in general, namely, that all the primary genital cells disappear, holds also for man. In any case, at the time at which the indifferent reproductive glands are formed there are neither extraregional nor regional genital cells.
Development of the Indifferent Reproductive Glands
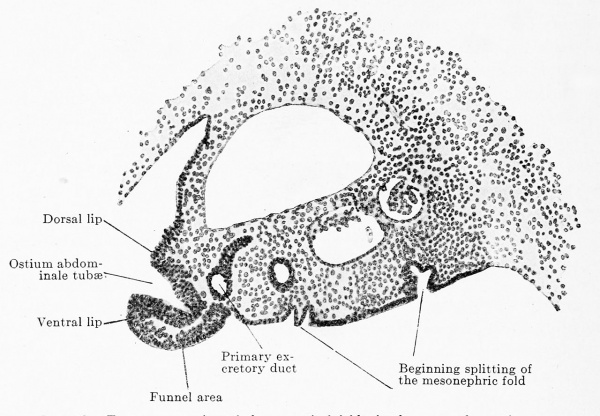
The anlagen of the reproductive glands appear within the urogenital folds, whose development and fate have been described above (p. 783-787). A small strip of the epithelium of the genital fold and this alone, forms the parent tissue of the reproductive gland. The epithelium of the urogenital fold usually consists of two layers of cells (Figs. 558, 559, 611) and is spread out uniformly over the entire surface of the fold in embryos up to 4.7 mm. vertex-breech length. In embryos of 5.3 mm. greatest length the epithelium over the summit and the medial slope of the fold, as far as the root of the mesentery, commences to become many layered, that on the lateral slope remaining two-layered or even becoming single-layered (Figs. 563, 612). The region of the manylayered epithelium represents the reproductive gland area, which, as a broad strip, extends throughout the entire length of the medial half of the urogenital fold. Since the thickened epithelium passes over quite gradually into the non-thickened portion, the reproductive gland has no sharp boundaries either medially, laterally, cranially or caudally. To tlio stage of the thickening there follows the stage of the ingrowth of the epithelium into the interior of the urogenital fold, and as the epithelium grows in it compresses the loose mesenchyme tissue of the fold and there is thus formed in the zone of ingrowth a strip of denser mesenchyme tissue, that is everywhere sharply defined from the ingrowing epithelium; in the epithelium itself there are numerous mitoses (Figs. 563, 612). That the epithelium actually grows inwards and not outwards towards the body cavity is shown, in the first place, by the permanent dorsoventral diameter of the urogenital fold and, in the second place, by the displacement of the Malpighian corpuscles of the mesonephros; the ingrowing epithelium pushes the various corpuscles before it ; while the corpuscle in Fig. 548 is still at the surface of the fold, in Fig. 549 it is displaced quite dorsally. The growth of the epithelium of the reproductive gland area forms a solid mass, which has, however, a wavy boundary towards the mesenchyme (Fig. 613). As soon as the growth has reached about one-third of the dorsoventral diameter of the urogenital fold, the formation of the genital fold, described above (p. 785), begins. The lateral and medial grooves, which produce it, press inwards just at the boundary between the mesenchyme and the epithelial growth, and thus the whole genital fold is filled by a perfectly homogeneous mass, composed entirely of derivatives of the coelomic epithelium. We must, consequently, note at this point that everything that is later developed within the genital fold has a common origin from the coelomic epithelium.
Fig. 611 a and b. Transverse section of the urogenital fold of a human embryo of 4.9 mm. nape length, at the level of the 14th primitive segment. (Embryo 137, G. 31, from the collection of the II Anatomical Institute, Berlin, Professor O. Hertwig; slide 13, row 1, section 3.) X 150. The urogenital fold is uniformly covered over its entire surface by a one- to two-layered epithelium.
Fig. 612. Transverse section of the urogenital fold of a human embryo of 7 mm. greatest length. (Embryo Chr. I, from the collection of Professor Hochstetter, Vienna; slide 8, row 10, section 6.) The epithelium on the medial side of the urogenital fold has thickened and forms the anlage of the reproductive gland.
Since the ingrowing epithelial mass displaces the mesonephric tubules, it is from the beginning in intimate relations with these, in the first place, with the medial surfaces of the Bowman's capsules and, in the second place, with the points where the tubuli collectivi bend into the tubuli secretorii ; there is always, however, a sharp boundary between the mesonephric structures and the germinal epithelial mass.
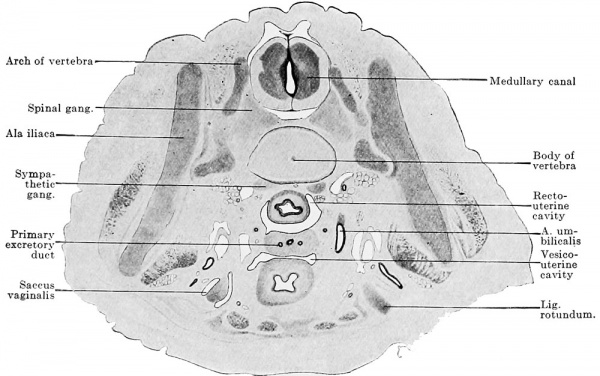
The reproductive gland anlage accompanies the urogenital fold in its gradual growth caudally, but its definitive extent is reached only after sexual differentiation. In its maximal extent it reaches from the sixth thoracic to the second sacral segment. This extent, however, occurs in no one embryo, since degeneration begins at the cranial end before the addition of new reproductive gland elements at the caudal pole is completed. Since an epithelial thickening is present cranial to the sixth thoracic segment in the line of the anlage of the reproductive gland, the anlage may be regarded as reaching almost to the cranial end of the definitive body cavity. But no matter how far cranially we place the cranial end of the reproductive gland area, its cranial pole never reaches the cranial end of the urogenital fold. The caudal pole comports itself differently in the two sexes ; while it reaches the dorsal surface of the genital cord in the female and so comes to lie within the excavatio recto-uterina, in the male it remains above the horizontal portion of the urogenital fold.
In the following table I give determinations of the position of the upper and lower reproductive gland poles in a series of embryos and, when possible, the length of the entire anlage.
Table showing the Growth in Length and the Degeneration of the Reproductive Gland
| Table showing the Growth in Length and the Degeneration of the Reproductive Gland | |||||||
|---|---|---|---|---|---|---|---|
| Embryo | Direction of section | Right gland | Left gland | Length of gland in micra | |||
| Cranial | Caudal | Cranial | Caudal | Right | Left | ||
| 10 mm | Transverse | 6 Th | 12 Th | 7 Th | 12 Th | ... | ... |
| 11 mm | Transverse | 7 Th | 1 L | 7 Th | 1 L | ... | ... |
| 12.5 mm | Transverse | 8 Th | 1 L | 8 Th | 1 L | ... | ... |
| 13 mm | Sagittal | 9 Th | 2 L | 9 Th | 2 L | ... | ... |
| 13 mm | Transverse | 9 Th | 3/4 L | 10 Th | 3/4 L | 2805 | 2325 |
| 14.75 mm | Sagittal | ... | 4 L | 9 Th | ... | ... | ... |
| 17 mm | Transverse | 10 Th | 3 L | 10/11 Th | 3 L | ... | ... |
| 18 mm | Transverse | 10 Th | 3 L | 10/11 Th | 3 L | 1720 | 1550 |
| 19.4 mm | Transverse | 11 Th | 3/4 L | 10/11 Th | 3 L | 2100 | 2010 |
| 21 mm | Transverse | 1 L | 4/5 L | 12/1 Th/L | 5 L | 1270 | 1550 |
| 22 mm | Sagittal | ... | ... | 2 L | 5 Th | ... | ... |
| 22 mm | Sagittal | ... | ... | 12/1 Th/L | 5 L | ... | ... |
| 22.5 mm | Transverse | 12 Th | 3/4 L | 12 Th | 3/4 L | ... | ... |
| 26 mm | Transverse | 1 L | 3/4 L | 12/1 Th/L | 3/4 L | 1290 | 1575 |
| 28 mm | Transverse | 12/1 Th/L | 4 L | ... | 4 L | 1540 | ... |
| 28 mm | Transverse | 3 L | 5 Th | 2 L | 5 Th | ... | ... |
| 28.5 mm | Transverse | 1 L | 3 Th | 1 L | 3/4 Th | ... | ... |
| 29 mm | Sagittal | 1 L | 5 L | ... | ... | ... | ... |
| 30 mm | Transverse | 3 L | 5 L | ... | ... | 1500 | 1800 |
| 30 mm | Transverse | 2/3 L | 5 Th | 3 L | 5 Th | 2490 | 1980 |
| 35 mm | Transverse | 3 L | 5 L | 4/5 L | ... | 1740 | ... |
| 50 mm | Transverse | 4 L | 2 Sa | 4/5 L | ... | ... | ... |
| 60 mm | Transverse | 3/4 L | 5/1 L/Sa | 5 L | 5/1 L/Sa | ... | ... |
| 60 mm | Transverse | 5 L | 1/2 Sa | 5 L | 1/2 Sa | ... | ... |
| 70 mm | Transverse | 4 L | 1/2 Sa | 5 L | 1/2 Sa | ... | ... |
| Explanation of Table: The table represents measurements from transverse and sagittal sections. The determinations of the position from sagittal sections are certain and their figures are printed in heavy type; the direction of the transverse sections varied in the various series and according as they were craniodorsal — caudoventral or caudodorsal— cranioventral they might yield quite different results. | |||||||
| Links: 1912 Length Growth and Reproductive Gland Degeneration | Reproductive Gland Growth Table | Collapsible Table | Ovary Development | Testis Development | |||||||
The table shows the gradual extension of the anlage of the reproductive gland; it reaches in maximo from the sixth thoracic to the second sacral segment, that is to say, over fourteen segments, and, eventually, it extends over only three or four, so that it degenerates in ten to eleven segments.
The table also shows the caudally directed growth of the anlage. The caudal pole gradually descends from the 12th thoracic to the 2nd sacral segment (at least in female embryos). The caudal pole — whether the embryo be male or female — lies from the beginning as low as or very frequently lower than in the adult and, accordingly, the so-called internal descent of the testis and ovary vanishes, neither really exists. The cranial pole of the gland does, indeed, change its position, but it changes not because it descends, but because the upper three-fourths of the gland degenerates. What seems to be a descent reveals itself to be a shortening, and we may see from the table that the absolute length of the gland diminishes in spite of its progressive growth in length along with the growth of the entire body. That the ovary eventually becomes rotated so that the cranial pole becomes the lateral and the caudal the medial, this has nothing to do with a descent, for during the rotation the caudal pole does not come to lie at a lower level. As regards the conus inguinalis (see p. 793), the mark for the later abdominal opening of the inguinal canal, the caudal pole of the reproductive gland lies lower than it does in both sexes.
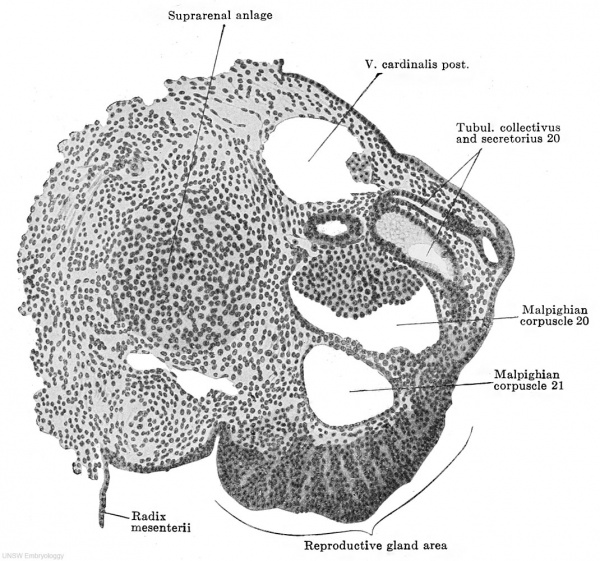
Fig. 613. Transverse section of the urogenital fold of a human embryo of 11.0 mm. greatest length, 9.0 mm. head-foot length. (Embryo P. 1, from the collection of Professor Hochstetter, Vienna.) X 150. Almost all the parts of a mesonephric tubule are cut. Medial to the tubule is the suprarenal anlage; the mesonephric fold lies in the frontal plane, its summit is marked by the primary excretory duct and a dorsolateral and a ventral surface may be distinguished. At about the middle of the ventral surface is the thickening of the peritoneal epithelium which forms the reproductive gland area. ./This consists solely of ccelomic epithelial cells, which are becoming somewhat loosely arranged. No differentiation whatever of the epithelial mass is to be seen.
The indifferent stage of the reproductive gland lasts only a short time. During it the uniform epithelial mass of the genital fold separates into a superficial epithelium and an epithelial nucleus, a sharp boundary existing between the two. The surface epithelium retains its closed epithelial structure and may consist of from one to at most two layers. The nucleus becomes looser in texture, so that at the end of the indifferent stage its epithelial origin is no longer evident.
The Differentiation of the Reproductive Glands
In the differentiation of sex we must distinguish between the differentiation of the genital cells into spermatogonia or oogonia and the differentiation of the genital cell mass, the actual reproductive gland, into testis or ovary. This distinction finds its justification, in the first place, in the fact that a reproducive gland may assume the characters of a testis without forming spermatogonia, as is the case with cryptorchid testes, and, in the second place, in the occurrence of malformations, scattered ova in testicular tissue or testicular ampullae in the ovary.
Whether or not a differentiation of genital cells into spermatogonia or oogonia really occurs is yet an open question. It is possible that the future sex of all the genital cells is already determined at fertilization. Every sex-cell, no matter whether it is male or female, possesses a definite force of heredity, which, during the course of development, becomes increased, or perhaps is only made more active, and reaches an optimum stage, after which it perhaps diminishes or become less active. In the optimum stage each sex-cell may possess the power of reproducing the other sex, the ovum males and the spermatozoa females (crossed inheritance). If now the ovum and spermatozoon unite during fertilization, differences in the immaturity, maturity or over-maturity of the two bring about numerous combinations, the stronger partner, that is to say the one which is nearest the stage of maturity, whether it be on the side of immaturity or overmaturity, will dominate in the determination of the sex. And the further possibility is not to be dismissed off hand, namely, that there are sex-cells of different types, male and female ova, etc. All these questions are not as yet ready for settlement, but will play an important part in the immediate future and therefore must at least be mentioned here.
The differentiation of the reproductive gland into testis or ovary is actually to be observed. The opponents of the theory that the sex of the ovum is already determined, overstep the mark when they deny a differentiation of the reproductive gland.
This differentiation consists in the characters of the male gland being developed in embryos of 13 mm. greatest length, at the earliest, while the female reproductive gland of the same age still lingers in the indifferent stage. The two characters of the male gland are: 1. The occurrence of branched, anastomosing epithelial cords, the testis cords. 2. The occurrence of a broad tunica albuginea between the superficial epithelium and the testis cords. It is therefore possible in young stages to identify the male individuals positively, and the females per exclusionem. One may say that the embryo has reached the age when testis cords should be present; they are not present and therefore the embryo must be a female. Such a determination has always chances of error and the doubts of the observer increase the more the embryo reaches the limits of the indifferent stage. Probably no observer will venture to label an embryo of from 13 to 15 mm. greatest length as a female, if he finds in it no testis cords ; the possibility that it is a case of retarded differentiation of a male, can never be excluded. Under these circumstances the small sexual differences in other organs acquire increased importance, because they are positive for the female sex. The formation of accessory funnels on the Müllerian duct seems to occur only in the female sex. Since the principal funnel occurs in embryos of 11 mm. and accessory funnels certainly in those of 13 mm., these latter will supply a valuable means for sex diagnosis, provided that further observations on a larger amount of material show that accessory ostia actually occur only in female embryos and that all female embryos develop them. Accessory tubes are too rare to be of use for sex diagnosis, but, on the other hand again, the presence or absence of the excavatio vesico-uterina is an important sign. The genital cord, by whose fusion with the dorsal wall of the bladder the excavatio is formed, is, indeed, first seen only in embryos of 20 mm., but the fusion of the urogenital folds (by whose union the genital cord is formed) with the posterior wall of the bladder takes place in male embryos before their union. The formation of the excavation in female embryos and its absence in males is a regularly occurring phenomenon.
By employing all these means the beginning of the sexual differentiation may be determined in female embryos at a stage of 18 to 20 mm. in length.
Transformation of the Indifferent Reproductive Gland into the Testis
The differentiation of the sexless reproductive gland into the testis will be considered first, because in this case the relations are much simpler than in the differentiation into the ovary.
We left the indifferent reproductive gland at the stage of development in which the epithelial mass had separated into a superficial epithelium and the epithelial nucleus. This separation is preserved throughout the eutire developmeut of the testis. Consequently the epithelial nucleus alone is the active layer, it forms the tunica albuginea, the testis tubules and the rete testis; the superficial epithelium remains passive and grows only in proportion with the enlargement of the entire organ, it is for the most part one-layered, several cell layers being present only on the surface opposite the attachment of the mesorchium; the greater portion of its cells are indifferent epithelial cells, among which genitaloid cells are scattered here and there. The superficial epithelium has, therefore, the character of a germinal epithelium formally but never functionally, and since it very early loses the genitaloid cells it is better not to speak of a germinal epithelium, but to adhere to the indefinite name "superficial epithelium." The epithelial nucleus becomes very loose and develops quite suddenly the testis cords, the loosely arranged cells concentrating at certain places, separating incompletely from their surroundings and arranging themselves to form anastomosing cords. It seems as if all the testis cords are formed at once, and the subsequent enlargement of the organ depends entirely on the elongation of the tubules, on their coiling, which is associated with their elongation, and on their increase in diameter. The cords are arranged transversely to the long axis of the testis, their inner ends, being arranged around the point of insertion of the mesorchium — indicating at once, therefore, the future hilus — while the outer ones radiate towards the surface of the testis (Fig. 614). The outer end of each cord is thickened, the inner one is pointed, and all the inner ends unite to form a closely aggregated epithelial mass (Fig. 614), which extends along the insertion of the mesorchium throughout the entire length of the embryonic testis ; this epithelial mass contains the cells that will form the anlage of the rete testis and may therefore be termed the rete blastema. This blastema stands in no relation to the parts of the mesonephros, except for its association with the epididymis tubules. The outer ends of the cords never reach the superficial epithelium, but a layer of at least two or three or usually more cells intervenes, from which the tunica albuginea develops. At first the testis cords have an irregular form on cross section, but later they become distinctly round. Since they all converge to the narrow line of insertion of the mesorchium, they cannot all reach the rete blastema, and therefore they unite by twos or threes in order to attain this connection in common. They are united together by anastomoses which are distinctly arranged in two groups; the one group lies about midway between the periphery and the hilus, the other unites their peripheral ends. These peripheral anastomoses are so constantly arranged in the same direction that they appear like large arches into which the testis tubules open (Fig. 614). The testis cords are so arranged that they leave intervals or intermediate cords between them, of the same width as themselves (Figs. 614 and 615). At the boundaries between the two there is even in an embryo of 21 mm. a distinct formation of connective tissue, consisting of spindle-shaped cells arranged one behind the other and alongside each other, all with their long axes parallel to the long axes of the testis cords (Figs. 614 and 615). Thus there are formed around the individual testis cords and their anastomoses actual connective tissue sheaths; at the hilus all the sheaths unite to form a boundary around the rete blastema, surrounding it in a zig-zag line (Fig. 614) ; this is the anlage. of the mediastinum testis.
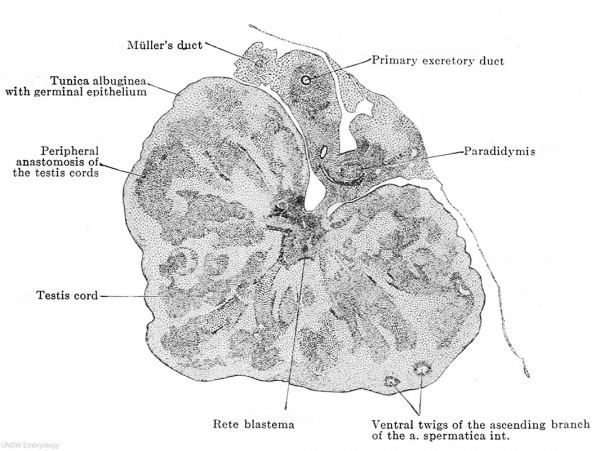
Fig. 614. Transverse section of the testis of a human embryo of 70 mm. head-foot length. (Embryo R. Meyer 267; slide 35, row 3, section 3, from the collection of Professor R. Meyer, Berlin.) X50. Solid testis cords have appeared in the testis and are connected by anastomoses at their middle and at their outer ends. The majority of them have already acquired an investment of young connective tissue. Their inner ends unite at the mesorchium to form the rete blastema and their connective-tissue sheaths form a connective-tissue boundary around the blastema, the first indication of the mediastinum testis. Two distinct layers may be recognized in the tunica albuginea, an outer one of connective tissue and an inner one composed of what are still indifferent epithelial cells. At the boundary between the two are twigs of the ascending branch of the a. spermatica int.
The testis cords consist at first of numerous indifferent epithelial cells with dark, homogeneous nuclei. Between these lie scattered genitaloid cells, which very soon develop to genital cells (Fig. 615). These are usually present in embryos of 70 mm. headfoot length, while the genitaloid cells appear simultaneously with the testis cords. The narrow inner ends of the cords contain only indifferent cells ; the tubuli recti are formed from them. The testis cords once formed alter little during the fetal period. The indifferent cells, that at first are altogether without arrangement, gradually acquire one, their nuclei become oval and place themselves radially to the future lumen. Thus there is formed a manylayered epithelium, into which the genital cells enter as spermatogonia (Fig. 616 a). The first lumina appear quite irregularly at the outer ends of the solid testis cords in the seventh fetal month. They arise partly by the migration of the cells from the centre to the periphery, partly by the resorption of the inner cells. From the outer ends the lumen gradually extends towards the hilus, and a second lumen extends from the rete testis "along the tubuli recti towards the first one, the two meet and thus the testis tubules, the tubuli recti and the rete tubules become hollow. All the testis cords are not transformed into tubules at birth. According to the observations of Branca and Basseta (1907) the number of genital cells increases progressively from the fifth month up to birth; after birth all the genital cells disappear and the testis tubules are lined by indifferent cells alone. With the onset of puberty a new generation of genital cells is formed, which then enter upon the formation of spermatozoa. The results of these two authors have been confirmed by Popotf (1909). I have no personal experience on this question and can only point out that in a human embryo of the seventh month obtained by laparotomy the number of genital cells had not diminished and that a well-preserved testis from a newborn child showed a remarkable number of genital cells.
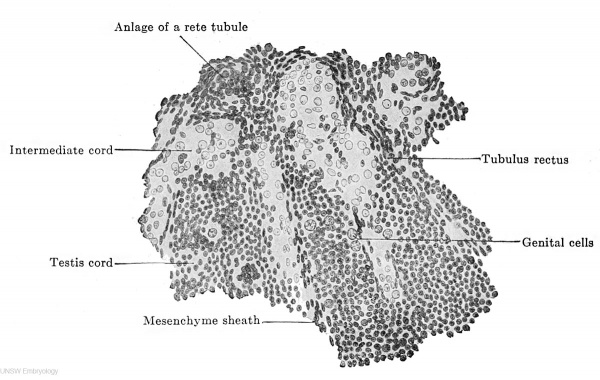
Fig. 615. Part of a transverse section through the right testis of a human embryo of 70 mm. headfoot length. (Embryo R. Meyer 267; slide 35, row 1, section 2, from the collection of Professor R. Meyer, Berlin.) The testis cords are shown as they pass into the rete blastema. One sees the structure of the testis cords from indifferent epithelial cells and genital cells, and, further, their passage into tubuli recti. Betweenjthe testis cords are the intermediate cords, filled with cells whose nuclei are vesicular and remarkably poor in chromatin.
The intermediate cords are at first composed of indifferent and genitaloid cells, but later the indifferent cells disappear almost completely; I assume that they are employed in the formation of the connective tissue sheaths. In embryos of 45 mm. greatest length the genitaloid cells are transformed into large pale cells (interstitial cells), which resemble genital cells in shape and size, but their nuclei contain only little or even no chromatin (Fig. 615). These cells gradually disappear after the fifth month, and in fetuses of the seventh month (Fig. 616) they are still present only here and there beside the vessels in the spaces between the testis tubules. After birth an abundant connective tissue develops between the tubules, and, as a result, the number of interstitial cells diminishes still further; after puberty (in the 33rd, 37th and 40th year) they again appear to occur in increased numbers (Popoff 1909).
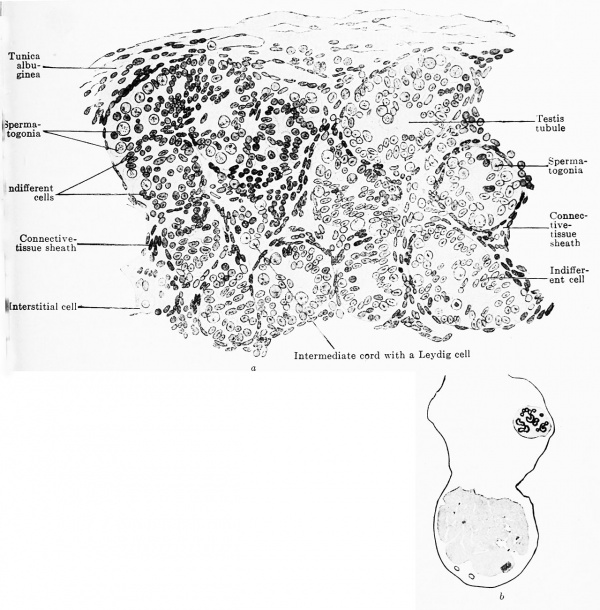
Fig. 616 a and b. Transverse section through the testis of a human embryo of the 7th month. (Embryo obtained by operation. From the collection of the Anatomical Institute, Zurich.) The testis cords have a rounded form; their cells are partly indifferent epithelial cells that are beginning to arrange themselves like an epithelium, and partly genital cells, scattered as spermatogonia among the indifferent cells; in the centre is the remains of an intermediate cord with Leydig cells. The tunica albuginea is already formed of a distinctly fibrous connective tissue.
The septula testis are formed by the thickening of the connective tissue sheaths of the testis cords. After the resorption of the intermediate cords the sheaths of adjacent testis cords come into apposition and then appear as a single structure, which extends radially from the hilus to the periphery. True septula testis are present from the sixth fetal month onwards.
It has been stated that the rete blastema is derived from a cell mass formed by the union of the thin inner ends of the testis cords. It extends throughout the entire length of the testis, leaving only the cranial and caudal poles free. It is certainly a derivative of the epithelial nucleus and is always, in young stages, sharply defined from the mesonephros and its mesenchyme. One sees, indeed, sharply defined mesonephric tubules passing in the region of the epididymis as far as the rete blastema and even producing depressions in it, but one never sees a proliferation arising at their blind ends, from which the rete blastema might be derived. A union between them and the rete occurs later as the urogenital union and will be discussed under this title in a special section. The rete blastema is surrounded by a mantle of young connective tissue on the surface towards the testis (Fig. 614). In the blastema there now occurs at definite points a concentration of the cells and there are formed irregular solid spheres of cells, imperfectly bounded externally. The individual spheres are connected by straight cords and there is thus formed a solid network (rete testis) which gradually becomes more distinct by the remaining cells of the blastema assuming a spindle shape and becoming connective tissue cells (Fig. 615). From these spindle-shaped cells of the rete and from the connective tissue boundary that separates them from the testis tubules the mediastinum testis is developed. Since it is derived from the rete blastema, like this it must extend throughout the entire length of the testis, as it actually does in the testes of older fetuses and in those of new-born children. When, at puberty, an unequal growth of the testis occurs, the mediastinum comes to lie more in the cranial two-thirds of the organ.
The tunica albuginea appears simultaneously with the formation of the testis cords and represents simply the cortical portion of the epithelial nucleus that is not traversed by the cords; it is accordingly formed at first only of scattered, loosely arranged, round cells. Then the cells lying immediately below the superficial epithelium begin to be transformed into connective tissue, whose spindle-shaped nuclei are all parallel to the surface (Fig. 614) . The remains of the epithelial bounding layer that is not converted into connective tissue increases very greatly; thus there is gradually formed an exceedingly thick bounding layer, which step by step from the periphery is transformed into connective tissue. In fetuses of the fifth month there is already a broad connective tissue tunica albuginea.
Malformations of the Testis
Only those malformations that are due to inhibition- of development will be considered, and no mention will lie made of those resulting from pathological processes.
A testis may be lacking, in which case the entire urogenital fold has usually failed to form, and therefore the kidney, ureter, ductus deferens, and suprarenal body of the same side will also be lacking.
The testis may be doubled. Ahlfeld (1880) records only one autopsy in which the duplication was observed; all other cases were determined during life and are therefore not certain. One must guard against errors in diagnosing a duplication; Romanovsky and von Winiwarter (1905) have described a case in which the right and left testes were both situated in the left scrotal sack.
Both testes may come into contact in the median line and may be united together; in such a case they will lie between the bladder and the anterior abdominal wall. In one embryo (R. Meyer, 270) of 60 mm. head-foot length the two testes had their hilus sides turned towards each other, the retia testis being well developed and fused.
The form of the testis may be completely normal macroscopically, while the microscopic examination shows that spermatogonia are completely lacking; the testis was accordingly sterile. This sterility is usually associated with cryptorchism.
The Transformation of the Reproductive Gland into the Ovary
The indifferent reproductive gland consists of an epithelial nucleus and a superficial epithelium, both sharply separated from one another. But while in the testis this first separation is a permanent one and the. superficial epithelium is reduced to an insignificant investment, this is not the case in the ovary. Between embryos of 70 mm. head-foot length (this limit may perhaps be placed somewhat earlier) and those of 180 mm. head-foot length there is a development period in which the superficial epithelium is sometimes only indistinctly and sometimes not at all marked off from the subjacent epithelial nucleus. Whether this undeniable new union between the two parts is to be regarded as a new impulse by which a second epithelial nucleus is formed from the superficial epithelium, cannot be determined with certainty. The arguments pro and con will be considered later on and it will suffice at this point to indicate the possibility of such an impulse ; for by it the superficial epithelium of the female reproductive gland acquires the significance of a germinal epithelium, and even although it contains no genital cells, yet it has cells which are able to transform themselves into these and into young ova. The superficial epithelium possesses the significance of a germinal epithelium, however, only during the period mentioned above. In embryos of 180 mm. trunk-length, a connective tissue layer, the tunica albuginea, develops between it and the epithelial nucleus, and with this a sharp delimitation of the epithelial nucleus occurs, the tunica forming a partition which excludes any further participation of the germinal epithelium in the formation of young ova ; the germinal epithelium thus again becomes a simple superficial epithelium.
In older fetuses and in new-born children foldings of the surface epithelium occur, but they have nothing to do with the formation of ova, indicating only the completion of the lobed form of the ovary. Solid, hair follicle-like, downwardly growing epithelial cords are formed from the superficial epithelium, but they never extend beyond the limits of the albuginea and again degenerate without effect.
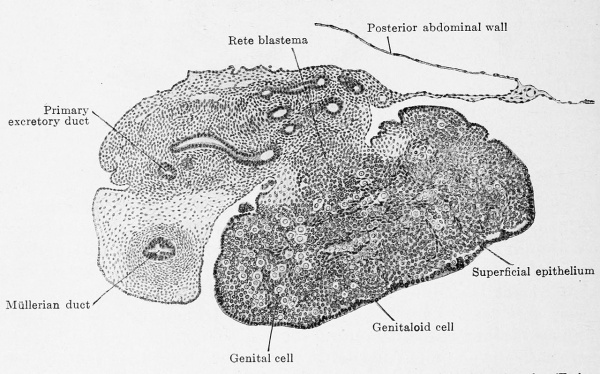
The actual reproductive gland producer is the epithelial nucleus. In embryos of 22 mm. greatest length it is composed of indifferent cells with sparingly scattered genitaloid and genital cells and it shows this composition up to embryos of 50 mm. headfoot length (Fig. 617). The nucleus fills the entire space enclosed by the superficial epithelium and also projects like a knob into the mesovarium. This knob consists of indifferent cells and very few genitaloid cells; it forms the rete ovarii and therefore deserves the name of rete blastema. Between the stages of 50 mm. and 80 mm. head-foot length the epithelial nucleus begins to become looser, starting from the rete blastema which remains unaltered, and one can distinguish a clear medullary zone from a denser cortical zone (Fig. 618). The loosening may occur under most varied forms ; the special example represented in Fig. 618 shows a structureless medullary zone and a cortical zone that is incompletely broken up into anastomosing cords.
Fig. 617. Transverse section of the ovary of a human embryo of 50 mm. head-foot length. (Embryo R. Meyer 272; slide 2, row 1, section 2, from the collection of Professor R. Meyer, Berlin.) X ca. 75. The section shows the triple division of the mesonephric fold into a tubal portion with the Müllerian duct, a gland portion with the primary excretory duct and mesonephric tubules and, finally, a very thin mesentery portion. In the ovary the superficial epithelium is distinctly separated from the epithelial nucleus. The nucleus is indistinctly dividing into a medullary layer, with numerous genital cells, and a cortical layer, poor in genital cells. Numerous trabecular of young connective tissue occur in the epithelial nucleus. The rete blastema is partly in the mesovarium and contains genitaloid cells.
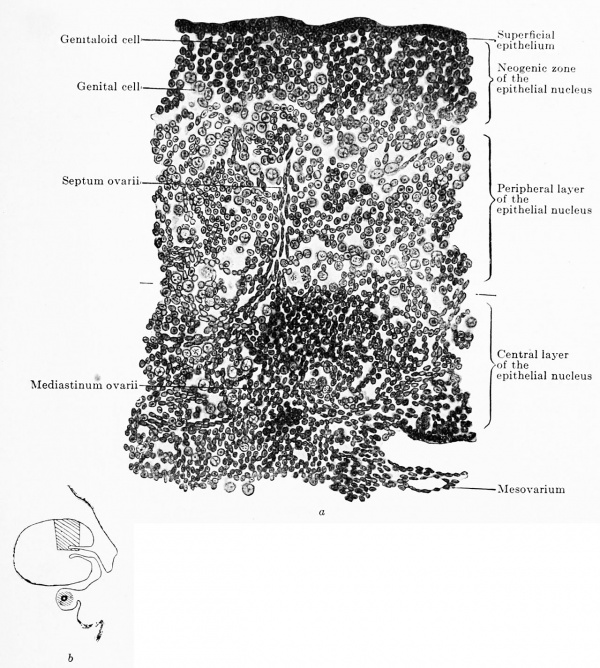
In the epithelial nucleus three concomitant but independent processes occur: (1) the ingrowth of connective tissue and vessels from the hilus towards the periphery; (2) the conversion of most of the genitaloid and indifferent cells into young ova, and (3) the new formation of the epithelial nucleus at the periphery, that has already been mentioned. While in the epithelial nucleus of the testis active testis cords appear, tins is never the case in the ovary, its epithelial nucleus forming no cords, but becoming split up into portions of the most varied forms by ingrowing connective tissue. The first traces of the connective tissue are seen in embryos of 28 mm. and are at first without regularity (Fig. 617). Then they gradually arrange themselves so that one can recognize a central investment around the rete blastema, a sort of mediastinum ovarii, and bands radiating out from this, the septa ovarii (Fig. 619). The septa traverse the central two-thirds of the transverse section and then begin to develop lateral branches and send out several apical shoots. These branches and shoots unite with those of neighboring septa so that a network is formed, which has wide and coarse meshes in the so-called medullary zone and narrow and fine ones in the so-called cortical zone; the fine cortical network eventually extends to the under surface of the superficial epithelium. The form of the meshes is exceedingly variable, the one extreme — very rare and only observed in one case — shows long rectangular meshes containing long, slender epithelial cords, such as are seen in the ovaries of other mammals, which unite to form a trabecular network like that occurring in the medullary cords of lymph-nodes; the other extreme — very frequent — shows polygonal meshes containing plump masses of the epithelial nucleus of most varied forms and all, of course, in connection. Between the two extremes are all sorts of intermediate forms, one of which is shown in Fig. 620.
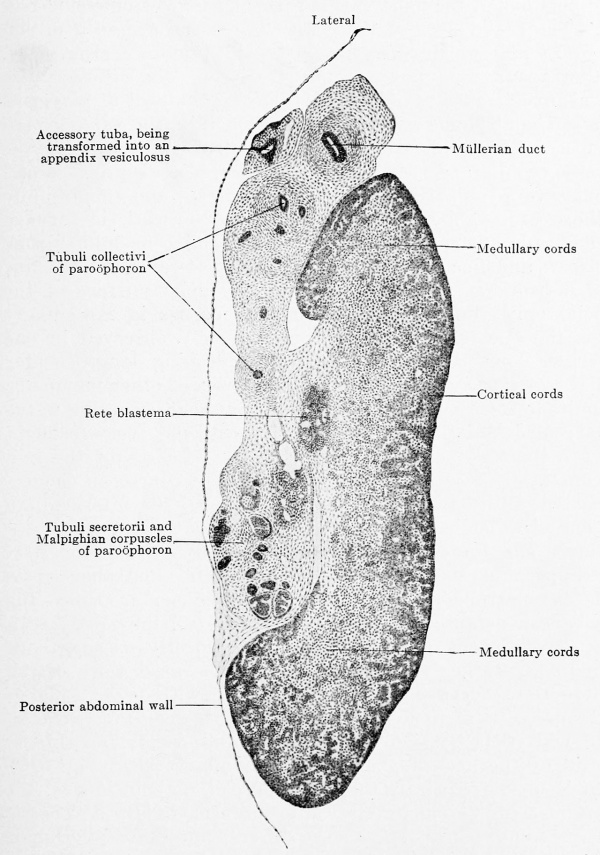
Fig. 618. Transverse section of the left ovary of a human embryo of 80 mm head-foot length. Embryo 266; slide 31, row 1, section 2; from the collection of Professor R.Meyer, Berlin.) X40. The section passes longitudinally through the upper horizontally bent part of the ovary and hence its apparent breadth. The epithelial nucleus has divided into medullary and cortical zones and the rete blastema is distinctly at the attachment of the mesovarium. Resides the Mullerian duct there is an accessory tuba, the portion of the mesonephric fold that contains it has already separated and we have before us the anlage of the appendix vesiculosus. The mesonephros is in complete degeneration; the section cuts it in the region of the paroophoron, and one may see how far the tubuli collectivi have already separated from their tubuli secretorii and Malpighian corpuscles.
Almost simultaneously with the connective tissue the first vessels appear at the hilus— probably in situ — and they grow towards the periphery more slowly than the septa. Since the vessels have a definite diameter and since, also, they produce a stronger development of the surrounding connective tissue, there is for a time a rather sharp distinction in the mesh-work of the connective tissue between a medullary zone with vessels and a cortical zone without them. The boundary between the two may coincide with that between the medulla and cortex, as this is drawn out by the loosening up of the medullary cells (Fig. 618) or, what is most frequently the case, there is no coincidence. It follows that a precocious differentiation into medullary and cortical zones is somewhat doubtful, since different processes — we shall hear of others — cause a separation into different cortical and medullary zones.
The tunica albuginea is also a formation of the septa ovarii. One sees (Fig. 620 a) how prolongations of the septa reach the superficial epithelium and then begin to creep under it. The more prolongations reach the surface the more compact becomes the layer formed by them beneath the superficial epithelium and the more distinct does the tunica become. It is difficult to assign a definite time for the beginning of its development. I have chosen the stage of embryos of 180 mm. trunk length, because in these the connective tissue prolongations first reach the superficial epithelium. An actually closed layer is formed only later. Fig. 621 shows the tunica of an embryo of eight months ; one sees the connective tissue septa entering it perpendicularly and then bending in arches to a horizontal direction. It is noteworthy that the folds of the ovarial surface, mentioned above, occur at the places where a perpendicular connective tissue strand enters the tunica.
Fig. 619 a and b. Transverse section of the ovary of a human embryo of 80 mm. trunk length, 100 mm. head-foot length. (Embryo R. Meyer 151, from the collection of Professor R. Meyer, Berlin.) • 230. In Fig. 619 b the portion drawn is shown in the entire transverse section. The ovary has divided into the superficial epithelium, the neogenic zone, the peripheral and the central portions of the epithelial nucleus. Around the rete ovarii a kind of mediastinum has formed, from which a septum ovarii radiates towards the periphery. The neogenic zone consists of indifferent, genitaloid and genital cells, closely packed. The peripheral zone of the epithelial nucleus is composed of the same kinds of colls as the neogenic zone, but the cells are much more loosely arranged. In the rete blastema there are b still genitaloid cells.
The transformation of the indifferent and genitaloid cells into genital cells and young ova begins in the immediate vicinity of the rete blastema and proceeds thence towards the periphery. This transformation process enlarges the poorly staining cell bodies and makes the nuclei paler, so that the central portion of the medulla appears very pale in comparison with the peripheral portions and with the cortex. If the transformation of the indifferent cells begins with the development of the connective tissue and vessels and progresses synchronously with these towards the surface, a marked distinction is established between medulla and cortex: the "medulla' is traversed by a broad, vascular connective tissue network, in whose coarse meshes are pale, young ova; the "cortex" is traversed by fine connective tissue trabecule, whose narrow meshes contain dark indifferent cells. This synchronous progress is, however, of rare occurrence. The two processes are usually not only unequally advanced, but one of the processes may show very different degrees of progress at different parts of the same ovary; there is thus brought about so much confusion that the determination of a boundary between the medulla and the cortex becomes impossible.
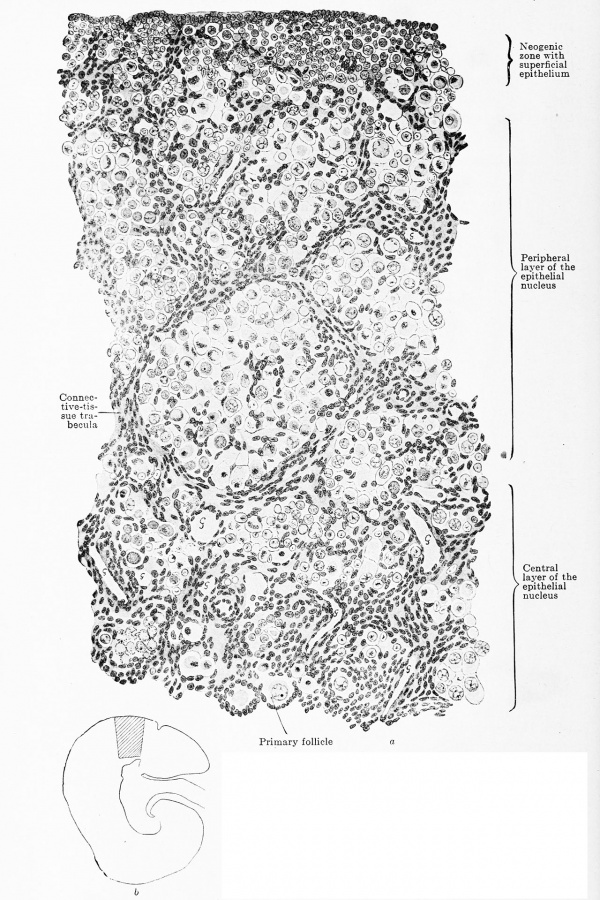
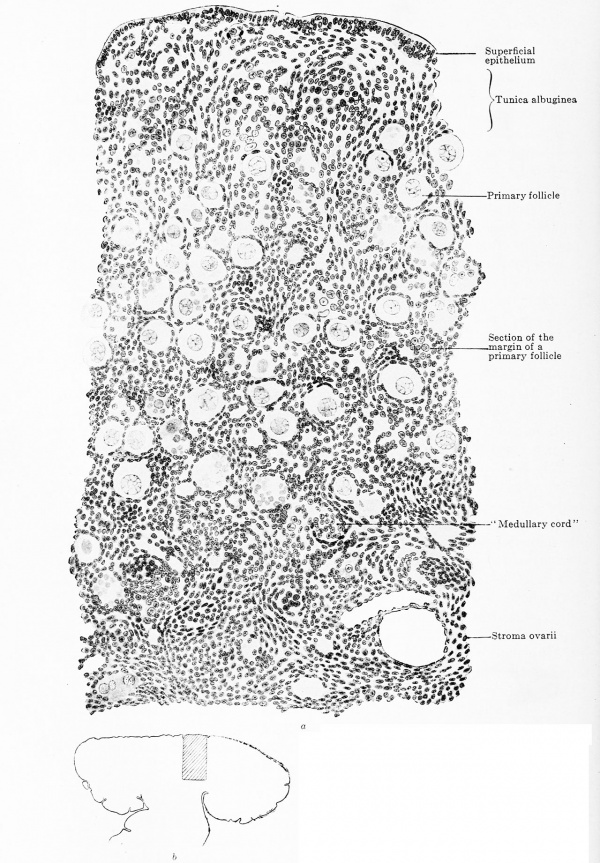
Fig. 620 a and b. Transverse section through the ovary of a human embryo of 180 mm. trunk length, 270 mm. head-foot length. (Embryo R. Meyer 152, from the collection of Professor R. Meyer, Berlin.) X 230. The trabecular of connective tissue have grown throughout the entire ovary and have reached the superficial epithelium. Towards the centre the meshes are wide, towards the periphery narrow. The three zones — neogenic, peripheral, and central — can still be recognized, but the superficial epithelium is indistinctly marked off from the neogenic zone. As a glance at the orientation figure shows, only the outermost layer of the central zone is drawn. In the meshes of the connective-tissue strands are ova spheres, the most of whose ova are degenerating. In the neogenic zone are numerous genitaloid cells, in the central zone already circumscribed primary follicles. In all lumina of blood-vessels a "G " is inscribed.
At first the ovary grows rapidly. A comparison of the transverse sections 619 and 620, both drawn under the same magnification, shows this at once; so much of Pig. 619 as extends from the surface to the transverse line corresponds to the entire Fig. 620. While the length of the body has increased in the proportion of 4:9, the ovary has grown in the proportion of 7 : 22. The growth of the testis is quite regular, the tubules elongating and enlarging and also the intermediate cords and the tunica albuginea. The ovary grows irregularly, the cortical portion of the epithelial nucleus alone undertakes the new formation of indifferent and genitaloid cells, the medullary portion remaining unaltered; a young cortical zone is thus developed over the old epithelial nucleus. Whence this young cortical zone comes I cannot say with certainty ; there are, however, two possibilities which may act singly or together. On the one hand, the indifferent and genitaloid cells of the cortical zone of the epithelial nucleus divide and thus, notwithstanding the progressive transformation of the cells of the nucleus into young ova, a new neogenic cortical zone may be continually reformed, just as the neogenic zone of the growing metanephros continually regenerates, notwithstanding its transformation into uriniferous tubules. In favor of this mode of development are the mitotic figures and the gradual transition of the cortical zone of the epithelial nucleus into the neogenic zone. The second po>-i bility has been referred to above; just as the ccelom epithelium grows in to form the epithelial nucleus at the beginning of the development of the reproductive gland, so the process may repeat itself, the superficial epithelium alone forming the neogenic zone. In favor of this mode is the temporary impossibility of distinguishing the epithelium from the neogenic zone ; against it is the striking absence of mitoses in the epithelium. I assume, therefore, that the principal growth takes place in the cortical layer of the epithelial nucleus and that only a very slight addition, if any, takes place from the superficial epithelium. But no matter how the development of the neogenic zone occurs, cords are never formed, the zone always forms a single mass. I come to the conclusion, therefore, that Pfliiger's cords do not occur in man.
As soon as the neogenic zone has appeared it becomes modified by the two processes described above ; the connective tissue divides it into irregular masses of cells connected with each other and the cells for the most part become transformed into ova. According as the two processes develop quickly or slowly and according as they proceed continuously or are interrupted by pauses, we find a broad or a narrow neogenic zone.
While epithelial material is being newly formed at the periphery, a degeneration of genital cells and young ova takes place at the centre from the third month onwards. In the destruction of both kinds of cells the nucleus is first broken down, its chromatin becomes massed and the nuclear membrane disappears (Fig. 620). The first sight of such a. picture as is shown in Fig. 620, when whole meshes are filled only with degenerating cells, suggests that the process is not a normal one ; it is only when one finds such pictures over and over again and finds no ovary of the last fetal months without degenerating cells that one becomes convinced that the destruction is a normal process. It progresses from within outwards and produces decided differences between the medulla and cortex, since with the destruction of the ova in the medulla there is a formation of the stroma ovarii. When, that is to say, all the epithelial cells in a mesh have been destroyed, the connective tissue grows strongly and fills the entire space, and when adjacent meshes have become transformed into connective tissue there is formed a closed connective tissue nucleus, the stroma ovarii. Gradually the connective tissue consumes in this manner so much of the epithelial nucleus that the old one (medulla and cortex) completely vanishes, as is shown in Figs. 617 and 618 ; layers of the neogenic zone probably undergo the same fate; the cortex of the mature ovary is, accordingly, a product of the neogenic zone alone. In different embryos cortex should not be compared with cortex or medulla with medulla off hand. When statements occur in the literature to the effect that "the medulla degenerates," "the medulla persists in certain cords," "the medulla forms Graafian follicles," they can be confirmed, with an addition, however, to the effect that quite different generations of medulla are under consideration and that in the "medullary portions" cortical portions of younger embryos are included.
All the cell masses and cords in the region of the stroma ovarii are not destroyed *by this tissue: some always persist and deserve the name of "cords," since they are completely closed, elongated, sometimes straight and sometimes curved structures. They are always situated close below the rete and therefore are derived from the central portions of the epithelial nucleus; accordingly they are correctly termed "medullary cords." These eventually degenerate, the last disappearing usually towards the close of fetal life, although scattered cords are frequently still to be found in the first year of life and very rarely in the adult ovary.
The extension of the destruction process to young ova varies, so that one finds ovaries that are rich in ova and others that are poor, the number of ova showing very decided differences.
When the formation of cortical substance ceases and the process of destruction, whose final result is the formation of the stroma ovarii, comes to a standstill, the remaining cortical layer becomes broken up by outgrowing connective tissue. In this process also a great variability occurs. The connective tissue may divide the cortex into individual ova (Fig. 621) or into larger masses of ova. Each ovum surrounds itself with an investment of cells, the follicle cells, which are in no way to be distinguished from the surrounding connective tissue cells. The ovum together with its follicle cells form what is termed a primary follicle.
In the first year a part of the primary follicles begin to grow and to be- converted into Graafian follicles ; scattered Graafian follicles are to be found even in the ninth month. In the second year some of the follicles are fully formed and contain apparently mature ova. In the third year all the characters of the adult ovary are present and from that time onwards there is no further histological differentiation but merely an increase in size (Runge 1906). The question arises as to the fate of these precociously mature follicles. Runge found in a new-born child (in one case only) a true corpus luteum ; it is accordingly possible that a newborn child may extrude sexually mature ova in the normal maimer, but the usual fate of these precociously formed Graafian follicles is that they become transformed into atretic follicles or undergo cystic degeneration.
The rete blastema, whose formation was described above, remains unaltered for a long time. It always lies partly in the ovary and partly in the mesovarium. Genital and genitaloid cells gradually disappear from it completely and it then consists only of closely packed indifferent cells; the last genital cells in the rete were seen in an embryo of 55 mm. head-foot length. While the rest of the epithelial nucleus is divided up by strands of connective tissue, this is not the case with the rete blastema. It is, indeed, traversed by some blood-vessels, but it always remains a remarkably compact mass and is always delimited, though not sharply, from the surrounding tissue. Only towards the medulla of the ovary is it connected with the medullary cords (Fig. 618). In it there arise, in embryos of 60 mm. head-foot length at the earliest, net-like branched areas, in which the nuclei are even more closely packed than in the surrounding tissue; these are the rete cords, which are completely solid and are never sharply defined from the surrounding rete blastema. These cords are connected both with the medullary cords and with the tubules of the epoophoron (see under Urogenital Union). Towards the end of the fetal period distinct lumina appear in the solid rete and tubules lined with a single layered epithelium are formed. These tubules may persist throughout life (von Franque, 1896), but they always show a tendency towards cystic enlargement.
Fig. 621 a and b. Transverse section of the ovary of a human embryo of the 8th month. (From the collection of Professor R. Meyer, Berlin.) X 230. The superficial epithelium, tunica albuginea, cortical layer, and medullary layer are all well marked; of the last only the outer third is shown, : as may be seen from the orienting figure. In the cortical layer are isolated primary follicles, whose epithelium is'formed by genitaloid and indifferent cells and, on account of the latter, cannot be separated from the adjacent connective tissue. The superficial epithelium is sharply defined from the tunica albuginea and prevents thickening where connective-tissue strands ascend vertically towards it. In the stroma there are still remains of "medullary cords" and masses of genital cells.
The form of the fully developed ovary is very variable, since it must adapt itself to the space left free by the coils of the intestine. Even more than in the adult the position of the portions of the intestine with reference to each other and to the abdominal wall is very variable in older embryos, and this variability will produce a variability in the space available for the ovaries. The usual form of the ovary may best be compared with a three-sided prism, whose principal surfaces are pointed. The ovaries are sometimes short and thick, sometimes long and slender, sometimes straight, sometimes angled, sometimes twisted spirally. When the ovary has a sagittal direction one can distinguish a dorsal, a ventral, and a medial edge; when it is rotated into a horizontal position the dorsal edge becomes caudal, very rarely cranial, the ventral one cranial, very rarely caudal, the medial one dorsal, very rarely ventral. The three edges are generally sinuous and especially in the case of the medial, later the dorsal ones do not always extend throughout the whole length of the organ. Corresponding to the position of the edges one can recognize a lateral (later dorsal), a dorsal (later caudal) and a ventral (later cranial) surface. In the middle of the lateral surface the mesovarium is attached. Since the dorsal and ventral edges overhang slightly, the lateral surface is sometimes strongly, sometimes weakly concave (Figs. 619 b, 620 b, 621 &). The three edges are sometimes smooth, at other times they are sparingly or frequently notched. The notches are for the most part quite superficial, rarely deep, in which case they may extend for a considerable distance on the surfaces. They are either arranged parallel to one another or may radiate somewhat towards the hilus, but they are never so arranged as to permit of the derivation from them of that form of ovary which, on account of its likeness to the surface of the cerebral hemisphere, is termed an ovarium gyratum. This is not a persistent embryonic form, but one sui generis.
The position of the ovary in older embryos is, like its shape, very variable. The two organs may be quite symmetrical, but they may both be crowded to the same side and assume various positions. In the displacement of both ovaries to the same side portions of the intestine (rectum and sigmoid flexure), excessively filled with meconium, play an important part. The rotation of the ovaries into the horizontal position occurs at very different periods, frequently one still finds the sagittal position in new-born children, frequently the rotation is completed in the fourth fetal month. It is quite possible that each ovary again becomes upright after the rotation and is later again brought into the horizontal position, and these changes may be repeated several times. Until the first year of life both organs lie for the most part in the false pelvis or above the entrance into the true pelvis; usually they are symmetrically placed, the most striking asymmetry being produced by one ovary having descended into the true pelvis, with its long axis placed sagittally; it is usually the left ovary that undergoes this displacement. According to the position of the coils of the small intestine one finds the ovaries at one extreme pressed against the posterior abdominal wall and at the other against the anterior wall. Between the two extremes all intermediate positions occur; usually they lie in the dorsal portion of the false pelvis, as is to be expected from their development.
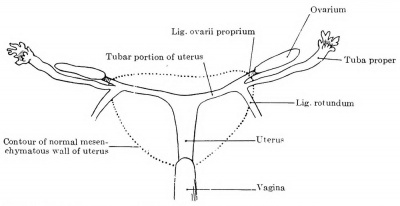
The relative position of the ovary and tube also varies. If both are still sagittal, the ovary usually lies medial to the tube. As a result of the degeneration of the mesonephros, especially in its cranial portion, the tube, however, acquires a very broad mesentery, and may be pushed by other viscera over the ventral surface of the ovary to its medial side, in which case the ovary lies in a bay of the plica mesonephridica, completely surrounded by the mesosalpinx. If the rotation to the horizontal position is completed, the tube usually lies ventral and somewhat caudal to the ovary. If the tube and ovary are forced strongly ventrally, the ovary may be pushed over the upper edge of the ligamentum latum and come to lie in the excavatio vesico-uterina, instead of in the excavatio rectouterina; the tube then lies dorsal to the ovary.
The following table shows data concerning the growth of the ovary. One may see from it that a slow and continuous growth is maintained throughout the entire embryonic period. After birth the rate of increase seems to be somewhat accelerated, but it diminishes again, to increase a second time at puberty. A difference in growth between the left and right ovaries can hardly be perceived.
Table showing Ovary Growth
| Table showing the Growth of the Ovary | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Vertex-breech length |
Greatest diam. of head |
Right Ovary | Left Ovary | Comparison between | |||||
| Breadth | Length | Breadth | Length | Length | Breadth | ||||
| R. | L. | R. | L. | ||||||
| 50.0 | 42.6 | 0.9 | 1.9 | 0.9 | 2.5 | .. | + | — | — |
| 125.0 | 123.0 | 1.2 | 5.9 | 1.5 | 4.1 | + | .. | .. | + |
| 138.0 | 115.0 | 1.6 | 5.0 | 2.0 | 5.0 | — | — | .. | + |
| 156.0 | 131.0 | 1.9 | 7.2 | 2.0 | 7.1 | + | .. | .. | + |
| 173.0 | 163.5 | 3.0 | 9.0 | ... | ... | .. | .. | .. | .. |
| 190.0 | 175.0 | 2.9 | 7.7 | 2.1 | 7.8 | .. | + | + | .. |
| 223.0 | 162.0 | 2.9 | 10.5 | 3.0 | 9.1 | + | .. | .. | + |
| 235.0 | 190.0 | 4.2 | 10.0 | 3.8 | 12.0 | .. | + | + | .. |
| 260.0 | 213.0 | 3.6 | 11.1 | 4.0 | 11.4 | .. | + | .. | + |
| 272.0 | 213.0 | 3.0 | 10.0 | 3.5 | 9.2 | + | .. | .. | + |
| 305.0 | 238.0 | 3.0 | 9.9 | 3.9 | 10.9 | .. | + | .. | + |
| 347.0 | ... | 3.5 | 10.8 | 4.9 | 8.5 | + | .. | .. | + |
| 355.0 | 273.0 | 4.0 | 14.0 | 5.2 | 9.9 | + | .. | .. | + |
| 386.0 | 324.0 | 5.1 | 11.5 | 3.0 | 9.9 | + | .. | + | .. |
| 402.0 | 301.0 | 5.05 | 10.5 | 3.0 | 12.0 | .. | + | + | .. |
| 3 weeks | ... | 5.0 | 17.0 | 5.0 | 14.0 | + | .. | — | — |
| 6 weeks | ... | 7.5 | 15.0 | 7.0 | 14.7 | + | .. | + | .. |
| 6 weeks | ... | 7.0 | 18.0 | 8.0 | 17.0 | + | .. | .. | + |
| 10 weeks | ... | ... | 14.0 | ... | 16.0 | + | .. | — | — |
| 2 months | ... | 6.0 | 14.5 | 4.0 | 13.0 | + | .. | + | .. |
| 3 months | ... | 6.0 | 15.5 | 5.0 | 14.7 | + | .. | + | .. |
| 7 months | ... | 5.9 | 15.5 | 4.5 | 18.1 | .. | + | + | .. |
| 15 months | ... | 9.0 | 18.0 | 9.0 | 19.5 | + | .. | — | — |
| I.75 years | ... | 7.0 | 20.0 | 8.5 | 15.0 | + | .. | .. | + |
| 4 years | ... | 10.0 | 27.0 | 12.7 | 23.2 | + | .. | .. | + |
| 5.5 years | ... | 11.1 | 29.0 | 9.1 | 26.1 | + | .. | + | .. |
| 14 years | ... | 11.9 | 26.5 | 12.0 | 29.5 | .. | + | .. | + |
| The measurements are all given in millimeters, breech length is measured along the nape and the back. | |||||||||
| Links: 1912 Ovary Growth | Table Ovary Growth Table | Collapsible Table | Ovary Development | |||||||||
Malformations of the Ovary
A complete absence of both ovaries is very rare, and, according to Nagel (1897) and.Gebhard (1899), occurs only in monsters incapable of maintaining life. Menge reports a case during life in which it was readily seen that in an otherwise normal person both ovaries, the uterus and the vagina were wanting. Such cases must, however^ be accepted only with caution, since observations made upon the living body can never give assurance that the case is not one of secondary atrophy. Even an autopsy is not always sufficient to demonstrate this process; I may recall the case recorded by Braun (1896) in which several years after ovarial atrophy not even the slightest trace of scar tissue was to be found at the point of constriction (Menge, 1910).
The absence of one of the ovaries is also very rare, yet it has certainly been observed, usually, but not always, with a concomitant absence of the Mullerian duct of the same side (Kossmann, 1899). Hypoplasias are more frequent and are associated with a hypoplastic condition of other portions of the genital apparatus (Menge, 1910). Supernumerary ovaries occur; von Winckel has described a third ovary in the vesico-uterine fold, together with a third tube.
Divided ovaries — ovaria partita, usually bipartita — are usually secondary formations; they may be derived from the lobed condition.
The Development of the Blood Vessels of Both Reproductive Glands
The aorta develops on either side about 30 mesonephric arteries, and of these 9-11 persist on each side (see p. 820). The majority of these persisting arteries form the rete arteriosuin urogenitale in the angle between the metanephros, mesonephros and reproductive gland, and this rete unites with vascular lumina which are formed independently inside the three organs. The first vascular lumina in the reproductive gland occur in an embryo of 18 mm. greatest length. Of the 9-11 persisting mesonephric arteries one, and that the lowest, is destined to become the a. spermatica interna, the rest become obliterated or else one or two persist as aa. spermaticae accessorise. Up to this point the development is exactly alike in the two sexes (see p. 822), but now it begins to differ. In the male (embryo of 60 mm. head-foot length) the artery passes from its origin from the aorta downwards in the retroperitoneum, meets the mesonephric fold a little below the cranial pole of the testis, traverses it obliquely cranio-dorsally to caudo-ventrally, and, finally, reaches the surface of the testis at the level of its lower pole. Here it lies in the tunica albuginea, runs as a rule on the dorsal surface around the caudal pole, reaches the lateral surface and again ascends cranially, lying always in the albuginea. Thus the artery forms an actual loop, between the limbs of which the testis is situated. A medial descending and a lateral ascending limb may be recognized ; with the exception of a small lateral twig to the caudal extremity of the testis, the descending limb is without branches, and it is important to note that it also passes the hilus without branching. The ascending limb divides into several branches, in the present case into two ventral and one dorsal, which ascend respectively on the ventral and dorsal surfaces of the testis. From these three branches the actual nutritive vessels of the testis arise, penetrating between the testis tubules and branching among them as far as the mediastinum. In this they are collected into venous stems, pass out as veins from the hilus into the mesorchium and there open into the v. spermatica interna. The branch given off by the descending limb at the lower pole of the testis behaves differently on the two sides, but it eventually ascends to the middle of the testis and participates in the supply of the testis cords. The three or four ascending arteries run upwards in the tunica albuginea, and mark out a tunica vasculosa (Fig. 614).
The a. spermatica of female embryos has at first the same path as in males, but very early it meets the mesovarium and there gives off branches to the hilus of the ovary until it is exhausted. It never reaches the caudal pole of the ovary.
The very different relations of the aa. spermaticse in the two sexes furnish a means for determining at once the sexual character of a reproductive gland. If the artery consists of merely a descending limb and this runs only along the hilus of the gland, this is an ovary; if the artery consists of a descending and an ascending limb and the latter lies on the surface of the gland, this is a testis.
The development of the vv. spermatica? is so intimately associated with the development of the veins in general that it may better be considered in that chapter.
Comparison of Testis and Ovary
The testis and ovary agree in that the superficial epithelium, the so-called germinal epithelium, either plays no part or, as in the ovary, only a small, diminishing one. The epithelial nucleus has the principal role in the development of both glands. But while in the male the nucleus is developed quickly and at a single stroke and then enlarges by the growth of all its parts, in the female its formation is slow and by steps; special germinal zones may be recognized in it and their growth is independent. The testis forms from the epithelial nucleus active testis cords, which only secondarily become surrounded by a connective tissue investment; no cords are formed in the ovary, but its epithelium is passively divided by inwandering connective tissue into a plump network of masses and cords of cells, whose individual constituents show very varied forms. The division goes so far that eventually each genital cell, or the young ovum developing from it, forms a unit by itself. In the testis the epithelium, in the ovary the connective tissue determines the form of the epithelial constituents. The testis tubules are formed mainly from indifferent cells, the form of the cord and later of the tubule is determined by these alone, the genital cells or spermatogonia are intrusions which have no influence on the form. The primary follicles of the ovary have their form, determined by the ova, the indifferent cells play, as follicle epithelium, a role which is unimportant so far as the form of the follicle is concerned. In Graafian follicles, it is true, the follicle epithelium has a dominant influence, but we cannot take these structures for comparison, since they are secondary formations, without homologues in the testis. We arrive, then, at this conclusion: testis cord and primary follicle, the foundation stones of the two sexes, arise from the same parent tissue, but follow very different developmental paths. The testis cords have no homologues in the female reproductive gland.
The tunicae albugineae may be regarded as homologous, in spite of the difference in their mode of formation, both are formed ultimately from the outermost layer of the epithelial nucleus. The rete testis and the rete ovarii are completely homologous, both as regards their anlage and their further development.
The Development of the Female Ducts.
THE EARLY DEVELOPMENT OF THE MULLERIAN DUCT.
As an efferent path for the products of the female reproductive gland a round duct, the Müllerian duct, is formed in both sexes on either side of the body ; it attains complete development only in the female and undergoes degeneration in males while they are still in the fetal period. According to its development the duct is divisible into a very short cranial portion (ostium abdominale tubae) and a very long caudal portion (the tube proper). The ostium abdominale is formed by an invagination of the ccelomic epithelium into the summit of the urogenital fold, the tube proper by an independent outgrowth of the blind end of this invagination. From the beginning the Müllerian duct lies in the secondary summit of the urogenital fold (p. 784), lateral to the primary excretory duct. This summit portion of the fold prepares itself for the reception of the Müllerian duct by beginning to separate from the rest of the fold as the tubar portion, and its epithelial covering becomes higher, at least in the region of the thoracic segments. The first anlage of the ostium abdominale is noticeable in embryos of 10 mm. greatest length as a circumscribed thickening of the epithelium at the summit of the urogenital fold on a level with the third thoracic segment; this place is termed the funnel area; in an embryo of 11 mm. greatest length the area has extended into the region of the fourth thoracic segment. The funnel area is situated immediately below the dorsal limb of the pleuro-peritoneal membrane at the opening of the pleuro-peritoneal duct into the abdominal cavity. In an embryo of 11 mm. greatest length a deep groove (Fig. 622) appears in the region of the posterior part of the third and in the whole of the fourth thoracic segment, in the middle of the transverse section of the funnel area, projecting into the subjacent mesenchyme of the urogenital fold; the groove presents, therefore, a dorsal and a ventral lip. The deeper posterior part of the groove closes to form a tube by the ventral lip growing toward and fusing with the dorsal lip. The tube thus formed separates from the epithelium of the urogenital fold; and the anlage of the cranial portion of the Mullerian duct is thus completed. When fully formed it is cornet-shaped, the opening of the cornet corresponding to the cranial portion of the groove which remains open, while its tip corresponds to the closed portion, separated from the coelomic epithelium; the opening of the cornet into the abdominal cavitv is termed the ostium abdominale tubse. The separated apex of the cornet lies in the immediate neighborhood of the primary excretory duct, between it and the coelomic epithelium ; it has, however, no connections with either.
Fig. 622. Transverse section of the urogenital fold of a human embryo of 11 mm greatest length and 9 mm head-foot length. (Embryo P. 1; slide 13, row 3, section 5; from the collection of Professor Hochstetter, Vienna.) The section passes through the middle of the third thoracic foramen intervertebrale. The mesonephros is undergoing degeneration in its cranial portion, and the urogenital fold has therefore diminished in size and appears to be triangular; its covering of coelomic epithelium is greatly split on the ventral surface. At the apex of the triangle the coelomic epithelium of the urogenital fold is greatly thickened (funnel area) and invaginated. This funnel-like invagination is the anlage of the ostium abdominale of the Mullerian duct. The funnel is placed frontally, and one can therefore distinguish a dorsal and a ventral lip. The latter has grown out and is curved into a hook to close the opening of the funnel. Into the primary excretory duct the degenerating tubulus collectivus of the second mesonephric tubule opens, beside it and medially is the tubulus secretorius of the third tubule, then the third Malpighian corpuscle, and, finally, the second Malpighian corpuscle.
In addition to the principal funnel, whose development has just been described, two to four accessory funnels are also formed as solid growths of the funnel area in the neighborhood of the principal funnel. These growths also separate partly from the parent tissue and their ends, which have thus become free, unite with the principal funnel; when this union is completed they become hollow. The accessory funnels are situated both on the dorsal and on the ventral side of the principal funnel; the scattered dentations of the margin form the anlage of the fimbriae. The first distinct fimbriae occur in embryos of from 28 to 30 mm.; in those of 60 mm. head-foot length the fimbria ovarica appears as a grooved projection directly caudally towards the cranial pole of the ovary. These accessory funnels I have so far found only in female embryos, but in these they occur regularly; in the males they are wanting and the male tubes develop no fimbriae. From accessory funnels the accessory tubes are to be distinguished; these occur in female embryos, but not regularly. They are usually situated somewhat more caudally than the accessory funnels and — what is characteristic — they never unite during development with the principal funnel or principal tube; they end blindly after a short course. The portion of the urogenital fold that contains such an accessory tube may be grooved out from the rest of the fold so that it remains in connection with it only by a narrow stalk (Fig. 618) ; in this way the appendix vesdculosus, the hydatid of Morgagni, is formed. The number of accessory tubes in the best example was four; they also appear to occur only in female embryos. All accessory tubes, with the exception of that one which forms the appendix, appear to vanish in the course of development. Concerning the origin of a double tube and concerning the tubar appendages such as have been described in the adult (Kossmann. 1895) I have no personal knowledge.
At the very time when the posterior end of the groove is separating from the epithelium it begins to grow out caudally, and in this process we come to the development of the distal portion of the Müllerian duct; it is formed by the gradual outgrowth of the tip of the cornet. The path that it will follow is already laid out ; it must pass, caudally between the ccelomic epithelium laterally and the primary excretory duct medially, as if between two bars. Its growth results entirely from its own forces, the outgrowing tip being always free and connected neither with the ccelomic epithelium nor .the primary excretory duct ; only poorly preserved or poorly fixed preparations could deceive one on this point. The growth depends on the increase of cells partly along the entire extent of the duct, as is shown by the mitoses, partly at the outgrowing end, which is frequently swollen and presents all the peculiarities of a so-called cone of growth. The reader will find the various stages of growth in the following table .
The lumen extends continuously from the funnel into the caudal portion of the duct, and follows the growth of the duct so closely that all that is ever solid is a small terminal portion. In this way the larger proximal portion of the duct becomes hollow; in its distal portion the lumen arises in loco, and is, accordingly, frequently discontinuous.
When one considers that the duct rapidly grows from the third or fourth thoracic segment to the sacral region it is clear that one has to deal with a decided increase in length; from the third row of the table one sees in fact the rapid increase. In an embryo of 12.5 mm. greatest length the length of the duct is 330 p, in one of 17 mm. it is already 1440 or 1220 In this latter embryo the duct has just reached the level of the second lumbar vertebra. Notwithstanding that the duct has still to grow over four or five vertebrae and these the highest in the entire vertebral column, there is from this time onwards a remarkably slight increase in length. This fact finds an explanation when one compares in the two first columns of the table the position of the ostium in successive stages. It lies at the beginning of its development in the third and fourth thoracic segment and at the close of development it is in the fourth lumbar segment, having wandered downwards through twelve segments. Since the ostium is always the same one, no traces of a new structure being shown, this wandering must be regarded as a true descensus. This is the more peculiar as the similar wandering seen in the cranial part of the mesonephros and of the reproductive gland is, in both these organs, due to the degeneration of the cranial portion. The wandering of the ostium abdominale is, of course, a passive process, and is probably due to three causes: First, the upper portion of the Mullerian duct no longer increases in length, it therefore lags behind in the total growth. Secondly, the ostium is attached to the eras of the diaphragm; since this descends, the ostium must follow it. Thirdly, the cranial part of the urogenital fold degenerates when its principal contents, the mesonephros and the reproductive organ, vanish; the upper portion of the Mullerian duct then hangs by a very loose fold and may therefore curve and bend and thus bring the ostium into a lower position. The end of the tube never quite reaches the cranial pole of the ovary, it always projects beyond it even in the adult; in the new-born child the cranial or lateral pole of the ovary still lies frequently at about the middle of the tube.
Table showing the Growth of the Mullerian Duct
| Table showing the Growth of the Müllerian Duct | ||||||
|---|---|---|---|---|---|---|
| Length of the embryo (mm) |
Right | Left | Right, absolute length in mikra. |
Left, absolute length in mikra. | ||
| Beginning | End | Beginning | End | |||
| 12.5 | 7 Th. | 8 Th. | ... | ... | 330 | ... |
| 13 | 9 Th. | 11/12 Th. | 8/9 Th. | 11 Th. | ... | ... |
| 13.5 | 9 Th. | 11/12 Th. | 11 Th. | 1 L. | 990 | 1035 |
| 14.75 | ... | ... | 10 Th. | 1 L. | ... | ... |
| 17 | 10/11 Th. | 2 L. | 10/11 Th. | 2 L. | 1440 | 1220 |
| 18 | 10 Th. | 2 L. | 10/11 Th. | 2 L. | 1420 | 1270 |
| 19.4 | 11/12 Th. | 3 L. | ... | ... | 1785 | ... |
| 21 | 1 L. | 1 Sa. | 1 L. | 1 Sa. | 1560 | 1740 |
| 22 | 12 Th. | ... | ... | ... | ... | ... |
| 28.5 | 2 L. | Müller's tubercle | ... | ... | ... | ... |
| 30 | 2/3 L. | Müller's tubercle | 3 L. | Müller's tubercle | ... | ... |
| 35 | 2/3 L. | Müller's tubercle | 4/5 L. | Müller's tubercle | ... | ... |
| 50 | 4 L. | Müller's tubercle | 5 L. | Müller's tubercle | ... | ... |
| 60 | 4 L. | Müller's tubercle | 5 L. | Müller's tubercle | ... | ... |
| 70 | 1 Sa. | Müller's tubercle | ... | ... | ... | ... |
| Th. = thoracic, L. = lumbar, Sa. = sacral. | ||||||
| Links: 1912 Mullerian Duct Growth | Mullerian Duct Growth Table | Collapsible Table | Renal Development | Male Development | ||||||
The Müllerian duct grows along the primary excretory duct. This makes a double bend in its course (Fig. 552) ; we may distinguish (1) a cranial vertical portion, (2) a horizontal one, and (3) a lower vertical one. The bends of the excretory duct appear before the Müllerian duct in its caudal growth has reached the places where the bends occur; Fig. 558, for example, shows the tip of the tube not yet arrived at the first bend of the excretory duct. As the Müllerian duct now grows onwards it lies ventral to the horizontal portion of the excretory duct, sometimes more cranially, sometimes slightly caudally. As it grows along the horizontal portion of the excretory duct the union of the two urogenital folds to form the genital cord occurs, and the Müllerian ducts now transverse this, situated between the two primary excretory ducts, lying now medial to these and close together. The space between the two primary excretory ducts is, as is shown in Fig. 552, exceedingly small. The two Müllerian ducts, accordingly, grow downwards close together until they reach the Müllerian tubercle. When they have arrived at this level they suddenly bend almost at right angles and run horizontally on the wall of the urogenital sinus. This horizontal portion is usually thickened, and without a lumen ; very frequently it is filled from the beginning with a vesicular epithelium. Müller's tubercle is at first merely the entire dorsal wall of the vesico-urethral anlage at the level of the orifice, and projects into the lumen of this. The vesico-urethral anlage then grows greatly in breadth by taking up into itself the two cloacal limbs (see p. 878), and thus Müller's tubercle becomes marked off as a special projection within the dorsal wall of the vesico-urethral anlage.
The left Müllerian duct reaches Müller's tubercle at the earliest in embryos of 21 mm. greatest length, the right in embryos of 28.5 mm. greatest length. The union of the duct with the sinus epithelium does not occur for some time; in embryos of 45 mm. the blind ends of the ducts first bore into the stratified epithelium of the sinus ; they do not reach its surface, however, but end blindly under the superficial layer of cylindrical epithelium. The actual breaking through takes place first in embryos of 70 mm. head-foot length.
Formation of the Uterovaginal Canal
In both sexes there is a union of the right and left Müllerian ducts within the genital cord, and the unpaired canal so formed is termed the utero-vaginal canal. The union occurs at the earliest in embryos of 22 mm. and at the latest in those of 28.5 mm. ; the sex seems to have no influence on the earlv or late union. The union takes place first in the second fourth of the later uterovaginal canal and advances thence in both the cranial and caudal direction; frequently, especially in the caudal portion, the union takes place discontinuously. It extends upwards nearly to the upper end of the genital cord, i.e., to the level of the second bend of the urogenital fold (Fig. 552), between the horizontal and lower vertical portions. This bend later on lies almost at the same level as the first bend between the horizontal and upper vertical portions (Fig. 552) at which the inguinal fold arises; but on this account one cannot give this first bend as the cranial limit of the union of the ducts. While the union in male embryos proceeds at once in the horizontal terminal portions of the Müllerian ducts as far as their blind ends, in females these portions remain separate for a long time ; their union first occurs in embryos of 50 mm. headfoot length.
The length of the uterovaginal canal is 600 M in embryos of 26 mm.; at 28 mm. it is 860 /*, at 30 mm. lOOO^to 1550 ^, at 50 mm. 2000 /*, at 60 mm. 2040 m.
When the union of the two Müllerian ducts is completed above we term the portions that remain separate the tubar anlagen. The primitive tube consists of two portions, a sagittal and a horizontal, the sagittal one lying in the upper vertical and the horizontal one in the horizontal portion of the urogenital fold (compare Figs. 552 and 623).
The union of the two ducts is at first only an external one. They are placed together so that they form a single external contour, but in the interior each has still its own medial wall. Later the two medial walls fuse to form a septum, and then this becomes resorbed in the caudo-cranial direction. Since frequently the two Müllerian ducts do not lie in the same frontal plane of the embryo, the utero-vaginal canal is frequently placed obliquely, and since, furthermore, the ducts change their position during their course, sometimes the right one and sometimes the left lying more ventrally, a spiral twisting of the canal may be brought about.
Formation of the Wall of the Uterovaginal Canal
GENERAL STATEMENT AND EXTERNAL FORM.
After the completion of the utero-vaginal canal the following relations obtain in the genital cord : In the mesonephric fold the primitive tubes run vertically downwards (vertical portion), reach the lateral edge of the genital cord, bend at right angles and run almost horizontally towards the middle line (horizontal portion) and there unite to form the utero-vaginal canal (Fig. 623 a). At the transition of the vertical into the horizontal portion of the tube the plica inguinalis (shown in Fig. 623 as the lig. rotundum) arises from the mesonephric fold.
The first anlage of the supportive tissue portion of the uterine or vaginal wall consists of a thickening of the surrounding mesenchyme ; from the beginning a rather sharp and smooth line of demarcation exists between the connective tissue investment of the uterus or vagina and the loose mesenchyme of the broad ligament, so that the external contour of the uterus is clearly defined (Fig. 623 b). The mesenchymatous wall of the uterus appears in both sexes, but in quite different form. In the female embn'o it surrounds the utero-vaginal canal and the horizontal portion of both tubes (Fig. 623 b) ; in the male embryo, where it develops after the degeneration of the horizontal portion of the tubes, it surrounds only the utero-vaginal canal (Fig. 624).
Fig. 623 a, b, c, and d. Four diagrams of the development of the external form of the female uterus. The mesonephric folds and the genital cord are shaded obliquely, the mesenchymatous uterine wall vertically. The various parts of the primitive uterine wall and of the utero-vaginal canal are lettered. S vertical portion; h, horizontal portion of the wall of the primitive tube; F, fundus uteri; C, cervix uteri; V, vagina.
Diagram a shows the position of the primitive tubes and of the utero-vaginal canal after it is completed. Diagram b shows the relation of the mesenchymatous uterine wall to the primitive tubes and to the utero-vaginal canal. It encloses the whole of the horizontal portion of both tubes in the uterine region and as a result the lig. rotundum is brought into relation with the uterine wall. The fundus uteri is bent in at an angle (uterus introrsum arcuatus). Diagram c shows the broadening of the horizontal portion of both tubes to form the fundus uteri, the broadening taking place in such a way as to straighten out the inward bend {uterus plani/undus) . Diagram d: The broadening of the fundus has increased and it is curved outward (uterus foras arcuatus). lig. rotund, (chorda gubernaculi)
Development of the External Form of the Female Uterus
In consequence of the horizontal portion of both tubes being taken up into the uterine wall, the primitive tubes may be divided into the definitive tubes and the tubar portion of the uterus. Since the two tubar portions of the uterus give the lumen of the uterus a triangular form from the beginning and produce in the fundus an angular bend towards the lumen (uterus introrsum arcuatus, Fig. 623 b), we may distinguish between the broader corpus (uterine portions of the tubes) and the narrow cervix portion (uterovaginal canal). Then the corpus portion begins to broaden, its angled inward bend being thus straightened out (uterus planifundus, Fig. 623 c) and then curved out cranially (uterus foras arcuatus, Fig. 623 d). How much of the later uterine mucous membrane is formed from the uterine portions of the tubes and how much from the utero-vaginal canal can hardly be determined with certainty; probably the point of entrance of the primary excretory duct into the wall of the uterus forms the limit between the two. This much is certain, that the fundus is formed from the tubar portion, the cervix from the utero-vaginal canal ; the boundary between the two must lie in the isthmus and may coincide with the boundary between the corpus epithelium and the cervix epithelium of the adult. By the extension of the mesenchymatous uterine wall outwards upon the tubes the inguinal fold, or the round ligament derived from it, first comes into relation with the uterine wall (Fig. 623 b-d) ; the ligament has nothing to do with the utero-vaginal canal.
Development of the External Form of the Male Uterus
On account of the non-participation of the horizontal portions of the tubes in its formation the fundus, and perhaps also the entire corpus, must be wanting in the male uterus ; it represents, accordingly, from the beginning only the cervix and perhaps a part of the isthmus (Fig. 624). We shall learn later that in it more than the half of the utero-vaginal canal is degenerated in the cranio-caudal direction, so that in the male there is no persistence of the uterine portions but only of the vaginal one ; the term uterus masculinus is therefore certainlv incorrect.
Fig. 624. Diagram of the development of the male uterus. Only the utero-vaginal canal becomes enclosed by the mesenchymatous uterine wall, the horizontal portions of both primitive tubes had previously degenerated. Consequently the plica inguinalis, or the chorda gubernaculi which is derived from it, does not come into relation with the uterus.
Formation of the Definite Wall of the Tubes.
In embryos of 50 mm. head-foot length the loose connective tissue of the tubar fold begins to arrange itself in concentric rings around the epithelium of the Müllerian duct, and so forms the anlage of the supportive tissue of the tube (Fig. 618). In fetuses of 80 mm. trunk length the mesenchyme of the tube wall separates into two layers, an inner one composed of round cells, no longer arranged regularly, and an outer one, three times as broad as the inner, formed of curved, spindle-shaped cells in regular layers ; thus the mucosa and muscularis are separated. In embryos of 180 mm. trunk length the first muscle fibres appear, the circular musculature being the first to form and then an outer and an inner longitudinal layer.
The epithelium of the tube is from the beginning singlelayered, but is in several rows. According to the degree of extension it is sometimes high cylindrical, sometimes cubical. In newborn children one finds between stretches with cylindrical epithelium groups of cubical cells which have the appearance of single alveolar glands. As to the time at which the ciliation begins I possess only negative results; according to Popofr" (1893) the epithelium bears cilia in the ninth fetal month.
An embryo of 50 mm. head-foot length already shows folds projecting into the lumen, two ventral and two dorsal (Fig. 617), giving a cross section of the lumen the form of a four-rayed star. The formation of the folds begins in the ostium abdominale and proceeds slowly towards the uterus. At first the folds are produced almost altogether by the difference in the height of the epithelium, but later the connective tissue of the mucosa grows into them and forces the epithelial covering apart. In embryos between 80 and 250 mm. trunk length secondary folds form on the primary ones and in the eighth month in addition to folds (directed towards the lumen), depressions (directed towards the mucosa) occur. The tube of the new-born child already shows almost completely the form of that of the adult. As soon as the folds have reached a certain size the connective tissue in them forms a special scaffolding of parallel bundles of connective tissue fibrils, which are surrounded by looser connective tissue.
Transformation of the Tubar Portion of the Uterus and the Uterovaginal Canal into the Uterus and Vagina.
The utero-vaginal canal is a long tube (Fig. 625). It runs vertically downwards parallel to the posterior surface of the bladder and urethra, bends almost at right angles at the level of Müller's tubercle, and accordingly at the boundary between the urethra and the urogenital sinus, forming a horizontal portion, and opens into the sinus. The vertical portion is long, the horizontal quite short (Fig. 625). In transverse section the utero-vaginal canal forms a flattened oval, whose longest axis lies in the frontal plane. The epithelium is in general arranged so that it is high on the ventral and dorsal surfaces and low at the angles ; at first it is everywhere one-layered and high cylindrical. The nuclei are in several rows. To this rule only the horizontal portion forms an exception from the beginning, it being completely filled with a vesicular polygonal epithelium. In embryos of 38 mm. greatest length differences between the epithelium of the tubar portion of the uterus and that of the uterovaginal canal appear; in the tubar portion a cylindrical onelayered epithelium, varying in height, occurs; in the uterovaginal canal it is several layered and the superficial layer is sometimes cylindrical, sometimes cubical. This many-layered epithelium appears first in the cranial part and gradually extends into the caudal part of the uterovaginal canal, meeting (in embryos of 70 mm. greatest length) the vesicular epithelium that gradually ascends from the horizontal portion; wherever the vesicular epithelium extends it completely fills the lumen. Towards the middle of the fourth fetal month three regions may be distinguished in the uterine and vaginal canals by means of differences in their epithelium: first, about an upper fourth, hollow and lined with a simple cylindrical epithelium, the future corpus uteri (probably formed from the tubar portion of the uterus) ; second, a second fourth, hollow and lined by a manylayered, cylindrical epithelium, the future cervix uteri ; and, finally, a distal half, filled with a vesicular, polymorphous epithelium, the future vagina ; the cervix uteri and vagina are derived from the utero-vaginal canal. The boundary between the corpus and cervix epithelium corresponds almost with the internal os uteri, and it is also marked externally by a bend or at least a greater distinctness of the ventrally concave curvature. I shall consider the topography of the boundary between the cervix and vagina later on.
Fig. 625. Primary excretory duct and uterovaginal canal seen from behind and somewhat from the left. Human embryo of 29 mm. nape length. (After Keibel 1896, from Felix-Biihler, Entwicklung der Geschlechtsorgane in Hertwig's Handbuch der Entwicklungsgeschichte, Vol. 3.) The two Müllerian ducts have united to form the utero-vaginal canal. The latter shows a long vertical and a short horizontal portion. Also the two primary excretory ducts are bent and accordingly also possess a horizontal portion at their orifices.
Development of the Vagina
In the third to the sixth fetal month (the statements of authors vary, apparently on account of a variability in the appearance of the structure) the portio vaginalis is formed by the following processes: first, the wall of the utero-vaginal canal thickens in all its dimensions — even in the third month, and, second, the epithelium forms at the boundary between the cervix and vagina, but always in the vaginal territory, a ventral and a dorsal solid projection (Fig. 626), and these grow out into the mesenchyme in a shovelshaped form; they are the solid anlagen of the fornix anterior and posterior vaginas and therefore bound the anterior and posterior lips of the external os uteri. The projection that represents the anterior fornix is always considerably lower than that representing the posterior fornix and in consequence the anterior lip of the os is lower than the posterior, a relation that may be retained even in the adult. With the anlage of the portio vaginalis or of the two fornices the upper limits of the vagina become sharply determined.
Fig. 626. Median longitudinal section of the utero-vaginal canal at the level'of the portio vaginalis uteri of a human embryo of 260 mm. (After Werth and Grusdew, from Felix-Buhler, Entwicklung der Geschlechtsorgane in Hertwig's Handbuch der Entwicklungsgeschichte, Vol. 3.) The portio vaginalis is beginning to be defined and the supravaginal circular muscle is developed in it. Its lumen extends to its lower end, which is closed by an epithelial plug. The vagina is still altogether solid, but its future lumen is indicated by the solid epithelial cord that traverses it. From this cord there grow into the surrounding mesenchyme, forward and backward at different levels, two solid projections of epithelium, the anlagen of the fornix anterior and posterior.
Only after the formation of the fornices does the vagina become hollow. The lumen first appears in embryos of 150 to 200 mm. trunk length (Nagel 1891) in the distal portion of the vagina. It is formed by the breaking down of the central cells and the arrangement of the peripheral ones into a stratified cubical and later a stratified pavement epithelium; starting in the distal portion the breaking down of the central cells proceeds cranially. In this manner the vagina, together with the two fornices, becomes hollow and the protuberant portio vaginalis is grooved out from its surroundings. Since the utero-vaginal canal is bent or strongly curved at the point where the portio vaginalis projects, the angle between the uterus and the vagina is present from the beginning.
In older embryos, new-born children and young girls until puberty, the outer surface of the portio vaginalis of the uterus is marked in the most delicate manner by fine grooves, which appear quite symmetrically. They occur always on the borders of both lips, almost always on the outer surface of the anterior lip and more rarely on the outer surface of the posterior lip. They appear as principal grooves that usually run radially towards the os and are connected by transverse grooves; more rarely transverse grooves are the principal ones and in this case they are connected by very few radial accessory grooves.
The epithelium of the vagina is henceforward a many-layered pavement epithelium, that of the cervix is a stratified cylindrical epithelium; the question arises where the boundary between the two occurs. According to R. Meyer (1910) it always lies at first in the cervical canal. Then "the cervical epithelium differentiating in the mucous epithelium destroys the pavement epithelium as far as the external os uteri and in about one-third of the cases even further." "There is thus formed Fischel's congenital histological ectropion." "Some islands of the basal cell rows of the pavement epithelium are spared and these always regenerate the pavement epithelium of the outer surface of the portio vaginalis uteri, raising up and compressing the mucous epithelium. " " The definitive abolition of the congenital histological ectropion occurs, however, only in childhood." We see then that the cervical and vaginal epithelium are engaged in a mutual struggle. At its close the boundary between the cervical and vaginal epithelium coincides in general with the level of the external os. With a small portio vaginalis, a narrow cervical canal and a narrow external os the boundary lies above the os; with a large portio vaginalis and a broad os externum it lies below the os, that is on the vaginal surface of the portio vaginalis (R. Meyer, 1898 c). Since the boundary struggle does not progress equally over the whole periphery, the boundary line comes to be wavy and, indeed, islands of one kind of epithelium are included within the other ; islands of cervical epithelium on the vaginal surface of the portio vaginalis have been termed physiological erosion.
With the formation of the portio vaginalis uteri and the anlage of the two fornices the cranial limits of the vagina are determined once and for all. The length relations between the vagina and uterus remain the same during the late fetal period and the first year of extra-uterine life, that is to say, the vagina is as long as or longer than the uterus.
The colunmae rugarum are formed by the ingrowth of numerous solid epithelial projections into the subjacent mesenchyme at a time when the lumen of the vagina is not quite filled by epithelium.
Fig. 627 a and b. External genitalia of a female embryo of 40.2 cm. vertex-breech length (measured over nape and back) and 30.1 cm. greatest head circumference. x1.5 The labia minora surround a long slit-like opening, the urogenital orifice, and present two distinct portions, an oral high portion (6 mm.) and an anal low one (1-1.5 mm.). Between the oral portions is the shallow urethral groove, between the anal portions the urogenital sinus. The hymen appears between the anal portions of the labia minora.
The lower boundary of the vagina is formed from the hymen, which is formed from Müller's tubercle. It has been shown above that the distal end of the utero-vaginal canal fuses with the epithelium of the urogenital sinus, and that this point of fusion persists as a solid mass of epithelium even when the rest of the vagina becomes hollow. Immediately above Müller's tubercle the vagina then forms an ampulla-like enlargement, by which Müller's tubercle is compressed to a disk. This is lined on one surface by vaginal epithelium and on the other by epithelium from the urogenital sinus ; the two layers of epithelium and the intervening mesenchyme form the hymen. In its centre the hymen has for a long time a cavity closed by a solid knob of epithelium, the remains of the point of penetration of the utero-vaginal canal.
Later the disk-like hymen becomes funnel-shaped; the funnel is compressed from right to left so that the ostium vaginaa no longer is a circle, but a sagittal slit. In embryos of 272 mm. vertexbreech length (measured over nape and back) and 213 mm. in the greatest circumference of the head, there regularly develops between the hymen cleft and the anal periphery of the hymen, usually somewhat to the left of tlie middle line, a sagittal fold, the hymen fold (Figs. 627, 645 and 646). At first small and inconspicuous it may increase in height up to birth to such an extent that it projects out like a partition between the labia minora. Rarely the fold is double, that is to say, a right one may be present in addition to a left one, the two folds then uniting at their anal ends and thus enclosing a groove that extends orally as far as the ostium vaginae (Fig. 646).
Recently it has been suggested that the vagina has a double origin, a pars Mülleriana and a pars adjuneta being distinguished. The pars Mülleriana is supposed to be derived from the utero-vaginal canal and the pars adjuneta from a frontal division of the urogenital sinus (Bolk, 1907). If this were true the hymen could not correspond with Müller's tubercle, but would be a new formation inside the urogenital sinus. Bolk's suggestion is, however, incorrect. In the first place direct embryological observation shows that the hymen arises from the tubercle, and, in the second place, cases in which the distal end of the primary excretory duet persists show this running to the hymen ; the opening of the primary excretory duct is always at Müller's tubercle.
Development of the Uterine Wall
The transformation of the tubar portions of the uterus and of the proximal portion of the utero-vaginal canal into the corpus and cervix uteri will first be considered together. In this connection I would refer to the work of R. Meyer (1898) . The epithelium of both forms in transverse section an oval, whose longest axis is placed in the frontal plane (Fig. 628). In embryos of the fourth month the oval becomes a wavy slit, the number of waves depending on the width of the uterus and of the slit. In the lower part of the uterus one finds, therefore, only one entire wave, in the upper part 1% or 2. The anterior and posterior uterine walls are so close together that the summit of a wave on one wall fits into the depression between two waves of the other and vice versa.
This wavy form of the uterine slit persists as the fundamental form until birth and is often found in children and, in rare cases, even in the adult. In embryos of about 150 mm. secondary folds arise from the depressions between the waves and, indeed, from their deepest parts, these folds appearing as longitudinal folds of the mucous membrane; in the upper part of the uterus, where there are two waves, there are two folds on each of the walls, in the lower part only one. From these folds other smaller accessory folds arise and produce throughout the entire cavity of the uterus a very complicated pattern. But as the accessory folds develop the entire lumen of the corpus uteri elongates and, consequently, the folds again become flattened out, until, finally, the surface of the mucous membrane in the adult corpus uteri appears quite smooth. The folds are retained most distinctlv in the cervix.
Fig. 628. Transverse section of the utero-vaginal canal at the level of the upper anlage of the prostatic glands of a female embryo of 80 mm. head-foot length. (Embryo R. Meyer 266; slide 69, row 1, section 1, from the collection of R. Meyer, Berlin.) The epithelial cylinder of the utero-vaginal canal is surrounded by an extensive layer of thickened mesenchyme, the primitive utero-vaginal wall. In this a middle zone is beginning to be defined by a closer arrangement of the nuclei; this is the future muscularis. Inwards from this is the future mucosa and outwards the future subserosa and serosa. The urogenital sinus develops on its dorsal surface two solid epithelial projections, the anlagen of the prostatic glands.
Long transverse folds are added to the longitudinal ones in embryos of 125 mm. at the earliest, and these form broad swellings projecting into the lumen. When these folds become so long that there is no longer space for them in the cervix, they ascend obliquely and the uppermost may then separate the corpus from the cervix like a valve. In such cases, if the mucous membrane of the corpus secretes mucus there will necessarily be a retention of the secretion in the uterine cavity. The transverse folds — forerunners of the plicae palmata? — appear in the cervix in such a manner that in embryos of 160 mm., for example, an upper third and also a small marginal zone above the ostium externum remain free from them. Consequently the upper third of the cervix — frequently even more (see the following table showing the growth in length of the uterus) — belongs macroscopically to the cervix but microscopically to the corpus uteri. On this account this portion of the cervix has been termed the isthmus and as a result two different internal ostia have been recognized, the ostium internum anatomicum, between the corpus and isthmus, and the ostium internum histologicum, between the isthmus and cervix.
The mucous glands of the cervix develop in embryos of from 110 mm. (Bosger 1894) to 175 mm. (Tourneux and Legay 1887), and always at the base of a fold. The cylindrical glands of the corpus uteri appear at very different times; one may find them abundant and well developed even in new-born children and fail to find them in young girls of 12 or 14 years. (Wyder 1878). Their appearance and growth in length is, therefore, until puberty, altogether independent of age. The cilia of the uterine epithelium first appear with the approach of puberty (Wyder 1878).
Development of the Musculature of the Vagina and Uterus
Around the epithelial cylinder of the utero-vaginal canal the mesenchyme thickens to form a closed investment. This primitive utero-vaginal wall extends forward and backward to the surface of the genital cord and, accordingly, fills the cord completely in the sagittal direction, but in the frontal direction a strip occurs right and left along the lateral walls of the primitive true pelvis, which still contains the original loose mesenchyme. In this way the genital cord becomes divided into three parts ; the uterus and the right and left ligamentum latum (Fig. 629). The boundary between the uterus and the ligamenta lata almost corresponds with the course of the primary excretory duct, which is enclosed in the uterine wall so far as this corresponds to the utero-vaginal canal. In the primitive uterine and vaginal walls strands of spindleshaped cells arise in embryos of 60 mm. head-foot length, and are arranged concentrically around the lumen in such a manner that they mark off three zones; first and most internallv, a zone of v 7 v 7 round cells, the future mucosa, then the layer of spindle-shaped cells, the future muscularis, and, finally and outermost, a second layer of round cells, the foundation for the future subserosa and serosa. The same arrangement of layers is also found in the wall of the tube and the tubar layers pass over praeter propter into those of the uterus. The blood-vessels, already present in considerable numbers, still lie in the subserosa. and thereby define this from the serosa. In embryos of 80 mm. head-foot length the primitive muscularis becomes stronger and splits into a series of concentric rings, but it is only in embryos of from 120 to 140 mm. trunk-length that the first muscle cells are distinct.
The musculature of the uterus and vagina may be traced back to three primitive layers: inner and outer longitudinal and middle circular musculature. In the uterus the circular musculature appears first, in the vagina the longitudinal. Since the anlage of the cervix musculature is entirely under the influence of the vaginal musculature, we must distinguish embryologically between the corpus musculature on the one hand and the cervix-vaginal musculature on the other.
In embryos of from 240 to 300 mm. (according to Werth and Grusdew 1898) two superposed muscle rings appear in the muscularis of the uterus, the one, strong and compact in the corpus, extends almost to the cervix, the other, weak and scattered, occurs in the neighborhood of the portio vaginalis. The vagina at the same time forms a longitudinal layer at the boundary between the subserosa and the primitive muscular wall, and this layer extends upwards over the vagina and into the cervix. In embryos of from 340 to 355 mm. the circular muscle layers of the corpus and those of the cervix fuse, and, in addition, the circular musculature of the corpus fuses with that of the tube. The two tubes meet the body of the uterus at an angle, and that produces a certain amount of confusion in the previously simple arrangement of the musculature. The inner strands of the musculature of the tubes pass directly over into the circular laver of the uterus, but the outer strands stream out as longitudinal bundles both on the ventral and on the dorsal surface of the uterus, and thus form an outer longitudinal muscle layer on the body of the uterus. The circular musculature of the cervix, which has hitherto remained weak, now becomes very strong and forms the supravaginal muscle ring. This develops in the region of the longitudinal musculature streaming up from the vagina, and becomes intermingled with it. In the meantime a circular layer has appeared in the vagina itself, in the region of the longitudinal layer which has now become considerably thickened ; it intermingles with the longitudinal layer and a pure longitudinal layer is left only on the outer and inner surface. With this the anlage of the vaginal musculature is completed; there is an outer and an inner longitudinal musculature and an intermediate circular one.
Fig. 629. Transverse section of a human embryo of 32 mm. vertex-breech length at the level of the utero-vaginal canal. (Embryo 426, I, IS in the collection of the Anatomical Institute, Zurich, IX. 1, 2.) X 15. The section shows the genital cord stretched transversely through the cavity of the true pelvis, and in it are the two primary excretory ducts and the utero-vaginal canal. The mesenchymatous uterine wall, which encloses the two primary excretory ducts, is so situated that it divides the genital cord into three portions, the uterus and the two lig. lata.
The inner longitudinal musculature of the uterus has a double origin; on the one hand longitudinal bundles stream from the orifices of the tubes on the inner surface of the circular musculature, and, secondly, fibres separate from the inner ring of the supravaginal circular muscle, and these also lie on the inner side of the circular musculature of the uterus, passing upwards towards the muscle bundles streaming down from the tubes and fusing with these to form the stratum longitudinale submucosum.
From the seventh fetal month to the end of pregnancy there is at first only an enlargement of the layers already present. In the body of the uterus the circular musculature always constitutes the principal mass. It grows in a three-fold manner : by increase in the thickness of its parts, by the development of new layers at the periphery and between the bundles of the circular muscle. These last, the interstitial bundles, are sometimes oblique and sometimes longitudinal, and produce the felted mass of the adult uterus, whose constituent parts can scarcely be made out. The outer longitudinal bundles that stream down from the tubes become included in this confused mass.
The vessels situated in the subserosa still lie on the outer surface of the musculature, but later they become enclosed by it, bundles of circular muscle fibres forming a coarse-meshed network, the stratum vasculare, around them. External to this network an outermost layer, the stratum supravasculare, is also formed, consisting principally of longitudinal bundles, which are in connection with the muscle bundles in the ligamentum rotundum, the ligamentum ovarii proprium and the plicae recto-uterinse.
Growth of the Uterus in the Postfetal Period
The following table, compiled from data given by Hegar (1908), will furnish information as to the growth of the uterus. It gives the length of the corpus uteri, isthmus, and cervix and the entire length.
| Growth of the Uterus in the Postfetal Period | ||||
|---|---|---|---|---|
| Age | Length of corpus (mm) | Length of isthmus (mm) | Length of cervix (mm) | Total length (mm) |
| Fetus of 7 months | 22 | |||
| Child of 5 weeks | 27 | |||
| 1 year | 10 | 23 | ||
| 14 months | 10 | 5 | 12 | 27 |
| 2.5 years | 8 | 6 | 12 | 26 |
| 3 years | 9-10 | 5-6 | 10 | 25 |
| 3.5 years | 6 | 5 | 16 | 27 |
| 9 years | 9 | 4.5 | 13 | 27 |
| 11 years | 12 | 6 | 19 | 37 |
| 13 years | 27 | 56 | ||
| 15 years | 59 | |||
| 16 years | 41 | 12 | 25 | 78 |
| 17 years | 27 | 6 | 22 | 55 |
| 17 years | 20 | 4 | 16 | 40 |
| 18 years | 36 | 5 | 31 | 72 |
| 19 years | 27 | 5 | 28 | 60 |
| 19 years | 28 | 6 | 27 | 61 |
| 19 years | 24 | 8 | 21 | 53 |
| 20 years | 30 | 6 | 16 | 52 |
| 20 years | 30 | 7 | 21 | 58 |
| 22 years | 35 | 5 | 29 | 69 |
| 28 years | 40 | 10 | 28 | 78 |
| 29 years (nulliparous wife) | 34 | 10 | 34 | 78 |
| 30 years (virgin) | 38 | 7 | 29 | 74 |
| Data compiled from: Hegar K. Anatomical investigations on the nullipara uterus with special consideration of isthmus (Anatomische Untersuchungen am nullipara Uterus mit besonderer Berücksichtigung der Isthmus). (1908) Beitraae zur Geburtsh. u. Gynak., vol. 13, 1908. reference list | Uterus Growth Table | Collapsible Table | Uterus Development | ||||
The table shows that the entire uterus passes through a resting stage in the early years of life, the uterus of a child of five weeks having the same length as that of a child of nine years. From this time onward an actual increase in length takes place, at first slowly, but more rapidly with the beginning of puberty. The increase is not, however, equally distributed among the various portions of the uterus. The body and neck elongate, but the isthmus practically not at all. Furthermore the body grows decidedly more than the neck.
Degeneration of the Tubes and of the Uterovaginal Canal in Male Embryos
We have seen that the Müllerian ducts are formed in both sexes and that in both sexes they unite with the lower portion of the utero-vaginal canal; it has also been stated that this union occurs in embryos between 22 mm. and 28.5 mm. greatest length. Almost immediately after the union, that is to say in embryos of 30 mm., the degeneration of the ducts and of the utero-vaginal canal begins in male embryos. It makes its appearance in the middle of the anlage, in the portion of the tube that lies between the lower pole of the testis and the utero-vaginal canal. Both tubes lose their lumina, their epithelial cells show signs of degeneration at the centre and of proliferation at the periphery. In embryos of 60 mm. the tubes have degenerated as far as the portion between the testes and epididymides, and the utero-vaginal canal as far as the lower horizontal portion: in the intervening space only scattered remains occur, as a rule in the neighborhood of the point of crossing of the ureter and the primary excretory duct; these remains regularly show proliferation phenomena. In embryos of 90 mm. remains of the tubes always occur along the upper two-thirds of the testis and the ostia abdominalia are still open; data are wanting as to the time of their closure. Finally, of each entire tube only a very small portion remains, situated between the testis and the head of the epididymis ; it is known in systematic anatomy as the hydatid of Morgagni. It contains a simple, rarely stalked, epithelial canal, frequently without pouchings. Of the utero-vaginal canal only the epithelium degenerates at first, the dense mesenchyme enclosing it persisting for a long time as a sort of primitive uterine wall. A collapse of the epithelial canal regularly precedes its degeneration, so that one frequently sees a small, solid, sagittal portion resting upon the persisting, broad horizontal terminal piece; evidences of nuclear degeneration are, however, distinctly visible, so that one has to do not with a new formation, but with a degeneration. Only the broad horizontal portion persists throughout life, forming the vagina masculina. The portion of its wall surrounding the slit-like opening will represent a male hymen. The vagina masculina frequently shows glandlike growths in older fetuses, but very rarely true open gland sacks (E. Meyer 1909). Müller's tubercle persists as the colliculus seminalis, the upper and lower cristas urethrales are defined quite early in the embryo.
Inhibitions of the Development of the Uterus and Vagina
It is almost self-evident that an organ with so complicated a development as that shown by the uterus and vagina would show disturbances of the development. The many forms under which these disturbances present themselves may be arranged in groups, each of which may be brought into relation with a definite stage of development. In the following schema I follow, with slight modifications, the subdivisions proposed by von YVinekel (1S99) ; more important departures for it will be considered later.
Normal Development. Inhibitions of Development
- The mesonephric fold is completely developed, but as yet shows no erian ducts, together with complete abtrace of an anlage of the Mullerian sence of the tubes, uterus and vagina duct. (probably never occurs [von Winckel, 1899] or only in association with extensive bodily malformations [Nagel, 1S97]).
- Complete absence of both Mullerian ducts
- Complete absence of one Mullerian duct occurs only in association with a total absence of the urogenital fold, its organs, and the kidney of the same side (uterus unicornus verus [semiuterus]).
- Formation of the funnel of the tube in the mesonephrie fold, outgrowth of both blind ends as Mullerian ducts into the urogenital fold along the primary excretory duct.
- The Mullerian ducts unite, at first in the middle of the later uterovaginal canal, extending thence cranially and caudally. The union is at first only an adhesion, later the septum between the two ducts is resorbed. Around the epithelial utero-vaginal canal the mesenchymatous wall of the uterus is formed like a sheath, and in such a manner that portions of the walls of the primitive tubes enter into the formation of the uterus (see Fig. 623, p. 894), and for this reason the fundus of the uterus appears slightly depressed.
- The flat fundus uteri gradually becomes convex outwards (uterus foras arcuatus). The form of the uterus fetalis is thus acquired.
- The uterus infantilis is formed by a strong growth of the cervix and a weak growth of the corpus.
- The uterus virgineus develops from the uterus infantilis by a stronger growth of the corpus.
- The sexually mature uterus develops from the uterus virgineus by equal growth.
2a. Complete failure of the fusion of the two Mullerian ducts (uterus didelphys [= uterus duplex separatus], vagina duplex separata.
2b. Combined with a failure of one vagina to communicate with the exterior.
2c. Combined with a failure to develop lumina (uterus didelphys solidus, vagina duplex solida).
2d. Combined with the formation of only a partial lumen in the uterovaginal canal (uterus didelphys partim excavatus, vagina duplex partim excavata).
2e. Combined with a varying amount of inhibition of one-half (uterus didelphys asymmetrieus, uterus unicornis spurius [semiuterus spurius] cum rudimento uteri alterius).
3a. The union of the Mullerian ducts fails completely or partially (uterus bicornis saeptus, subsseptus, simplex; vagina sagpta, subsaepta, simplex).
3b. Uterus introrsum arcuatus saeptus, subsaeptus, simplex; vagina saepta, subsaepta, simplex.
3c. Combined with a failure of one side to open to the exterior.
3d. Combined with incomplete development of lumen.
4a. The fundus of the uterus remains flat (uterus planifundus).
4b. Combined with various degrees of inhibition of resorption of the septum.
4c. Combined with a partial failure of the lumen.
4d. Uterus foras arcuatus.
4e. Combined with various degrees of inhibition of the resorption of the septum, and with partial failure of the lumen.
5. The growth of the uterus does not take place; it remains a uterus fetalis.
6. The stronger development of the body fails, the uterus infantilis persists.
7. The growth takes place unequally or insufficiently and there is formed a uterus virgineus inaequalis or hypoplasia of the uterus.
Various theories have been proposed to account for the origin of malformations of the uterus and vagina. To-day they all have the fault that they are based on a plan of development that needs correction in important points. We have at present — basing our opinion on the facts described above (pp. 916-920) — to distinguish between 1. Disturbances of development in the formation of the mesenchymatous wall, and 2. Disturbances of development in the anlage and in the further development of the epithelial canal.
The epithelial canal is formed in the first place from the utero-vaginal canal, and, secondly, from the uterine portions of the two tubes. Since the tubar portion of the uterus practically forms the corpus uteri, and the utero-vaginal canal the cervix and the vagina, the development of these two portions of the uterus takes place under quite different ' conditions. The difference in their manner of development leads one to expect differences in the causes of their disturbances of development, and one may therefore subdivide the disturbances in the development of the epithelial canal into 2a. Disturbances in the development of the utero-vaginal canal; they affect the cervix uteri and the vagina.
2b. Disturbances in the further development of the tubar portions of the uterus; they affect the corpus uteri.
1. Disturbances of Development in the Formation of the Mesenchymatous Wall of the Uterus.
It has been pointed out above that the mesenchymatous wall of the uterus is developed in such a manner that it encloses the utero-vaginal canal as well as the two uterine portions of the tubes (Fig. 623 b). By this inclusion of the horizontal portion of the tubes in the mesenchymatous wall the plica inguinalis, or the ligamentum rotundum that is developed in it, is brought into connection with the uterus. If now one finds in autopsies uteri in which the round ligament is attached to the wall of the tube, but not to the uterine wall, it must either be the result of an inhibition of the development of the mesenchymatous wall of the uterus or a hermaphroditic character. In the fundamental work of Kussmaul (1859) there are two figures, Figs. 17 and 18 (of which Fig. 17 is here reproduced as Fig. 630), which show conditions that are identical so far as our present purpose is concerned. The uterus is T-shaped, the transverse bar of the T being twice as long as the vertical one. and having attached to its ends the two round ligaments and the ligamenta ovarii propria. The point of insertion of both ligaments marks the boundary between the tube proper and the uterine portion.
Fig. 630. — Copy of Fig. 17 by Kussmaul (1859). The normal extent of the mesenchymatous wall of the uterus is indicated by a dotted line on the figure of the uterus rudimentarius solidus.
The normal extent of the mesenehymatons wall of the uterus is shown in Fig. 630 by the dotted line. A comparison of the normal and inhibited uterus shows the remarkable lack of development of the latter. This anomaly was termed a uterus rudimentarius by Kussmaul (1859), but we now describe it more in detail as a uterus with its mesenchymatous wall developed. It can naturally, and it usually does, occur in association with inhibitions in the development of the epithelial canal, complete or partial degeneration of this, obliteration of the lumen, a failure of the union of the two ducts, etc.
In considering a uterus with imperfect mesenchymatous wall one should not fail to notice its great similarity with the male type. We have seen (p. 916) that the formation of the mesenchymatous wall differs in the two sexes. In the male the mesenchyme surrounds only the utero-vaginal canal, the uterine portions of the tubes remaining free from it; furthermore, in the male the epithelial canal of the utero-vaginal wall within the mesenchymatous investment degenerates, though the investment itself persists for a longer time. In all cases of uterus with imperfect development of the mesenchymatous wall, accordingly, one must take into account the possibility of its being a case of hermaphroditism, and eventually consider whether there may not have been an error in the determination of the sex.
2A. Developmental Disturbances In The Region Of The Epithelial Portion Of The Utero-Vaginal Canal
(Cervix Uteri And Vagina).
Here belong all cases of double vagina, double cervix, vagina septa and cervix septa. They arise by the non-union or incomplete union of the two Mullerian ducts or by the non-resorption or incomplete resorption of the septum after the union. What can hinder the union of the two Mullerian ducts to form the uterovaginal canal? I have collected the following suggested causes from the literature :
- Hydronephrosis.
- Abnormal distention of the bladder and rectum.
- Anomalies in the formation of the abdominal wall (hernia umbilicalis, cleft pelvis, abdominal clefts, abnormally short yolk stalk).
- Fetal peritonitis.
- Ligamentum recto-vesieale.
- Shrinkage of the lig. rotunda.
- Too great breadth of pelvis.
- Long persistence and too great separation of the mesonephroi or the primary excretory ducts.
- Congenital tumors.
The first four of these causes can hardly be effective. In the first place, the utero-vaginal canal is formed before the metanephroi become functional, and consequently hydronephrosis and an abnormal distention of the bladder are excluded. Secondly, the formation of meconium begins a considerable time after the completion of the utero-vaginal canal. Meconium was first found in an embryo of 223 mm. vertex-breech length (measured over the back) and 162 mm. greatest head circumference; filled with meconium, but not dilated, was the rectum of an embryo of 235 mm. vertex-breech length (measured over the back) and 190 mm. greatest head circumference; a strongly dilated rectum was first found in an embryo of 347 nun. vertex-breech length (measured over the back). An abnormally dilated rectum cannot, therefore, prevent the union, which has already been completed for a very long time (embryos of 22-28.5 mm.). Thirdly, the normal hernia umbilicalis occurs long after the completion of the uterovaginal canal. If it could prevent the union, non-union would be the rule, union the exception. I shall discuss the disturbance produced by an abdominal cleft later on; what influence a too short yolk-stalk can have upon the union I cannot understand. Finally, in the fourth place, a fetal peritonitis at the time of the development of the utero-vaginal' canal is practically excluded and has never been observed. The ligamentum vesico-rectale — a short falciform fold of peritoneum that extends sagittally from the posterior wall of the bladder to the anterior wall of the rectum, through the true pelvis (Krieger, 1858) — is rather the result of a non-union of the two Mullerian ducts than its cause; it also is frequently lacking in cases of divided uterus (Kehrer, 1899).
According to Thiersch (1852) the non-union of the Mullerian ducts is to be attributed to a strong development and long persistence of the two mesonephroi; Frankl (1902) and Holzbach (1909) have endeavored to establish this theory on a broader basis. The ontogenetic foundations upon which it is built, however, I cannot — at least for man — admit as correct. Firstly, the mesonephros never descends into the region of the utero-vaginal canal and never lies in the true pelvis. Secondly, the union of the two Mullerian ducts is accomplished even although the caudal end of the mesonephros increases considerably in man at the time. If persistence and stronger growth of the mesonephros could prevent the formation of the utero-vaginal canal, its non-formation would be the rule, and its formation the exception. Thirdly, the caudal portion of the mesonephros is actually not degenerated but persists as the epigenitalis and paragenitalis. The results of autopsies also argue against this theory. If an excessively developed mesonephros prevented the union of the Mullerian duct it should, as Kussmaul pointed out, leave behind it a paragenitalis more strongly developed than usual, but this is not the case.
According to R. Meyer (189S) the cause of a non-union of the ducts is frequently an abnormal shortness and strength of the ligamentum rotundum, eventually strengthened by a disproportion between the breadth of the lig. latum and the transverse diameter of the true pelvis. I hold, on the contrary, the shortness of the round ligament to be a result of the non-union of the ducts, but I agree as far as the influence of an excessive breadth of the pelvis, associated with a short lig. latum, is concerned.
Pick (1896, 1S98) endeavored to explain the non-union by intervening detached germinal tissue and by the tumors formed from them. Since he observed a simultaneous occurrence of tumors and deformed or double uteri in 30 cases, his theory cannot be dismissed off-hand. It fails, however, to explain cases of double formation unaccompanied by tumors — and these are the most frequent.
Pick's idea is strengthened by the observation of von Recklinghausen (1896), who, in his search for adenocysts in uterine tumors, structures which he showed to be pathologically altered remains of the mesonephros or paragenitalis, found his best material in deformed uteri of the smooth broad forms and in uteri bicornes.
Von Winckel (1899), who accepts the theories of both  and Pick, has called attention to the relation between the Mullerian and the primary excretory ducts. The Mullerian ducts lie at first lateral to the excretory ducts, then they cross over their ventral surfaces and come to lie medial to them, so that a separation of the excretory ducts laterally must produce a lateral pull on the Mullerian ducts. The displacement laterally of the primary excretory ducts may be caused by the mesonephros and the metanephros. Whoever is familiar with the relations which the primary excretory duct holds to the lateral wall of the pelvis at the time when the utero-vaginal canal is formed, will admit that a lateral displacement of the primary excretory duct is possible only with an enlargement of the true pelvis; von Winckel's theory, therefore, agrees closely with that of R. Meyer.
and Pick, has called attention to the relation between the Mullerian and the primary excretory ducts. The Mullerian ducts lie at first lateral to the excretory ducts, then they cross over their ventral surfaces and come to lie medial to them, so that a separation of the excretory ducts laterally must produce a lateral pull on the Mullerian ducts. The displacement laterally of the primary excretory ducts may be caused by the mesonephros and the metanephros. Whoever is familiar with the relations which the primary excretory duct holds to the lateral wall of the pelvis at the time when the utero-vaginal canal is formed, will admit that a lateral displacement of the primary excretory duct is possible only with an enlargement of the true pelvis; von Winckel's theory, therefore, agrees closely with that of R. Meyer.
An account of how the mesonephric fold is united to the lateral abdominal wall by the plica inguinalis has been given in the section treating of the urogenital fold (p. 793). At the same place it is furthermore pointed out that on the formation of the ventral abdominal wall all its parts are pushed eranially and medially. With the abdominal wall, the point where the urogenital fold is attached to it and, finally, also, the urogenital fold itself, must alter their positions in the directions named. The best evidence of the nature and extent of the displacement is the varying position of the m. rectus abdominis during the formation of the anterior abdominal wall; it lies at first at the middle of the lateral wall and later immediately beside the ventral median line. This passive displacement of the two urogenital folds towards the median line, however, favors their union. Perhaps it is also the cause of the spiral twisting of the mesonephric folds and thus of the spiral course of the Mullerian ducts around the primary excretory ducts. If the displacement of the anterior abdominal wall takes place early, that is to say, before the enlargement of the transverse diameter of the true pelvis — as actually happens — then a genital cord of normal extent will be formed and the mesonephrie fold will be twisted spirally. If the displacement is retarded, that is, until after the enlargement of the true pelvis, then the formation of the genital cord at least becomes difficult, and the difficulty will increase in proj)ortion as the amount of retardation increases, until finally the union is impossible.
If, however, the fusion of the two mesonephrie folds fails partially or wholly, the pull falls on the plica inguinalis or on the round ligament developed in it, and thereby the ligament becomes shortened. This shortening of the round ligament is in this ease, in my opinion, the result and not the cause of the nonunion of the Mullerian ducts. In this connection I would call attention to an observation by Rudolph (1909), in which the ligamentum rotundum was stated to be completely wanting in a case of uterus didelphys.
I have already called attention to the influence of extensive displacements of various portions of the intestine on the position of the mesonephrie fold. On the left side the influence is so strong that we must accept a right-angled bend of the mesonephrie fold as a regular result of its action (p. 791). If we suppose that the pressure of the intestinal tract on the mesonephrie fold becomes greater by some disturbance in its development, then Ave would have the abnormal pressure demanded by all the authors cited above, but produced by quite a different organ; it would be a second cause of the non-union of the Mullerian duets.
Finally, I must mention the topographical relations of the ascending and descending colons on the one hand and the mesonephrie fold on the other. It must be noted, in the first place, that the ascending colon lies from the beginning lateral to the right fold, and, further, that the descending colon usually runs along the medial surface of the left fold; it is this latter condition, principally, that produces its bend. If we suppose that the descending colon does not come to lie on the lateral side of the mesonephrie fold at the end of the process of readjustment of position, as is normally the case, the outward pressure, already existing, would be active beyond the usual time and so offer an obstacle to the union of the folds.
The same result would occur if the ascending colon came to lie on the medial side of the mesonephrie fold. The colon would then force the fold laterally and so again prevent its union with the fold of the opposite side. That such a thing is possible is shown by a case observed by Descomp (1909), in which the right ovary and tube were covered by the caecum, that is to say. were lateral to it. The influence of abnormalities in the position of the large intestine forms a third cause for the non-union.
All these considerations lead to the conviction that the causes for the occurrence of double formations of the utero-vaginal canal are more manifold than had been supposed, and that in any case several causes may act.
2B. Disturbances In The Development Of The Epithelial Canal In The Region Of The Corpus Uteri
Here belong the uterus bicomis in all its different degrees of development, the uterus introrsum arcuatus and the uterus planifundus.
The corpus uteri — it must he continually borne in mind — is not formed by a union of the two Mullerian ducts, but the two uterine portions of the tubes, just as they are and just as they lie, combined with the cranial blind end of the uterovaginal canal, are transformed into the corpus uteri by simple enlargement (Fig. 623 a and b). The two uterine portions of the two tubes lie almost in the same line, and, consequently,— compare Fig. 623 b — there is sufficient room craraally for their former medial, now their cranial, walls to be raised up and so form the fundus uteri (Fig. 623 c and d). On the other hand, if the two portions meet at an acute angle, if they form with the utero-vaginal canal a Y instead of a T, room is lacking for the upgrowth of the fundus, that is to say, the developmental stage of the uterus planifundus cannot be formed and the epithelial canal of the corpus remains two-horned. The more acute the angle at which the tubar portions of the uterus meet, the more the two-homedness of the corpus uteri will be emphasized, and if this form of the epithelial canal persists until the musculature develops, it remains persistent; thus the uterus bicornis is formed. From this mode of development it follows that the forces that produce the uterus bicornis act in exactly the opposite direction from those that prevent the union of the Mullerian ducts to form the utero-vaginal canal. If the urogenital folds are drawn too far laterally, the genital cord is not formed and there is therefore no development of the utero-vaginal canal; if, on the other hand, the urogenital folds are pressed too far medially, although the Mullerian ducts will unite to form a utero-vaginal canal, the formation of a single corpus uteri will be prevented.
The urogenital fold is influenced most strongly and for a very long time by changes in the position of the developing intestine. In different embryos one finds the fold in very different positions: 1, completely rotated outwards, so that its medial surface rests upon the posterior and lateral abdominal walls; 2, placed sagittally so that it projects into the abdominal cavity as a ridge; 3, completely bent over medially, so that its medial surface rests on the posterior abdominal wall and its apex, which contains the Mullerian duct, reaches the root of the mesentery. This third position especially is interesting in connection with the formation of a uterus bicornis; if both Mullerian ducts run downward close to the root of the mesentery a horizontal tubar portion of the uterus cannot be formed (compare Fig. 552). Thus the condition is produced from which we started in the explanation of the uterus bicornis. The size of the angle between the two limbs of the Y will determine the degree of the two-hornedness — if the word is allowable ; the more acute the angle the longer the horns and vice versa.
If the formation of the utero-vaginal canal is prevented there can, of course, be no formation of a single corpus uteri, and a totally divided cervix uteri must always be associated with a divided corpus. On the contrary, even after • the completed union of the utero-vaginal canal, the formation of the corpus uteri may be prevented; the force which makes the formation of the fundus uteri impossible, must even increase the force that unites the utero-vaginal canal. Also in cases of uterus didelphys it is possible — at least in fetuses and young girls in the early years of life — to distinguish between the portion of the Mullerian duct intended for the formation of the utero-vaginal canal and the portion intended for the formation of the tubar portion of the uterus, and this by the relations of the primary excretory duct, which lies only within the mesenchymatous wall of the utero-vaginal canal or the portion homologous with it.
The formation of the utero-vaginal canal throughout its entire length is not necessary for the formation of a single corpus uteri, a union at the cranial end is sufficient and the rest of the canal need not be formed. That such a malformation is possible is shown by the occurrence of a uterus unicorporeus bicollis (that is, with doubled cervix).
The different septa that occur, in the female genital canal have, naturally, a different genesis. The septa in the region of the vagina and cervix are the persistent medial walls of the two Müllerian ducts, those of the corpus uteri, on the contrary, are the remains of an inward bending of the fundus uteri.
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2024, April 27) Embryology Book - Manual of Human Embryology 19-2. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Book_-_Manual_of_Human_Embryology_19-2
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G