Assisted Reproductive Technology
| Embryology - 2 Mar 2026 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
| Educational Use Only - Embryology is an educational resource for learning concepts in embryological development, no clinical information is provided and content should not be used for any other purpose. |
Introduction
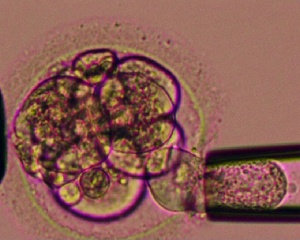
In vitro fertilization is one of now many different reproductive options know collectively as Assisted Reproductive Technology (ART). These techniques continue to grow worldwide with development of new medical technologies. In vitro fertilization covers the aided fertilization process, in contrast with in vivo fertilization which is the normal uterine occuring fertilization process.
The earliest experiments 1945-48, involved fertilising oocytes that had been collected from women with spermatozoa in a petri dish.[2] The first successful IVF was carried out in the UK in 1978 by Edwards RG, et al.[3], receiver of the 2010 Nobel Prize in Medicine.
The Latin, In vitro = "in glass" meaning in essence a test tube as apposed to in vivo (in life or a living body), fertilisation (Aus spelling) and fertilisation (US spelling). Even in vivo fertilization can also now be considered "assisted" through some fertility drug treatments. Both processes have the same biological outcome, fusion of male and female gametes to form a diploid zygote.
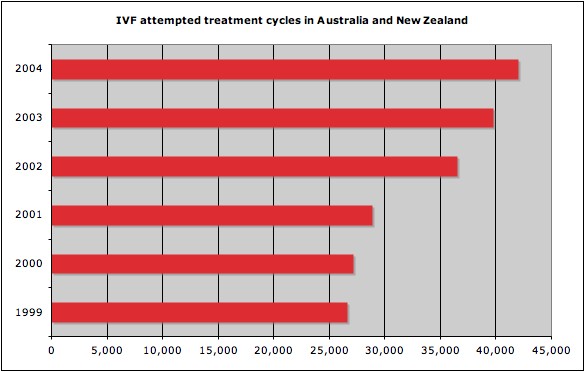
In Australia, the first successful IVF occurred in 1980.[4] and during 2005 1,596 IVF babies were born. In the same year in Australia and New Zealand 51,017 treatment cycles were reported, an increase of 13.7% of ART treatment cycles from 2004. In all countries using Assisted Reproductive Technologies (ART), pregnancy rates vary for the different methods of treatment and also between individual IVF or GIFT units. In Australia best clinical pregnancy rate (per 100 oocyte retrieval cycles) by most successful 25% of all clinics increased from 24.9% (1998) to 34.4% (2001) (NPSU data - ART 2002 report)

Robert Edwards |
The Nobel Prize in Physiology or Medicine 2010 - Awarded to Robert G. Edwards "for the development of in vitro fertilization" who battled societal and establishment resistance to his development of the in vitro fertilization procedure, which has so far led to the birth of around 4 million people. (More? Assisted Reproductive Technology | Nobel Prize 2010)
|
Some Recent Findings
|
| More recent papers |
|---|
|
This table allows an automated computer search of the external PubMed database using the listed "Search term" text link.
More? References | Discussion Page | Journal Searches | 2019 References | 2020 References Search term: Assisted Reproductive Technology |
| Older papers |
|---|
| These papers originally appeared in the Some Recent Findings table, but as that list grew in length have now been shuffled down to this collapsible table.
See also the Discussion Page for other references listed by year and References on this current page.
|
Trends in ART procedures
- In the last 5 years there has been a shift from day 2-3 embryo (cleavage stage) transfers to day 5-6 embryo (blastocyst) transfers.
- The proportion of blastocyst transfers has increased from 27.1% in 2006 to 52.1% in 2010.
- Increase in the transfer of vitrified (ultra-rapid frozen) embryos. Compared with 2009, the proportion has more than doubled from 18.3% to 38.2%.
- reduction in the rate of multiple birth deliveries, with a decrease from 12% in 2006 to 7.9% in 2010.
- shifting to single embryo transfer, the proportion of which increased from 56.9% in 2006 to almost 70% in 2009 and 2010.
- decrease in the multiple delivery rate was achieved while clinical pregnancy rates remained stable at about 23% per cycle.
Data: Assisted reproductive technology in Australia and New Zealand 2010[22]
18 Ways to Make a Baby
- Natural sex
- Artificial insemination - of mother with father's sperm
- Artificial insemination - of mother with donor sperm
- Artificial insemination - with egg and sperm donors, using surrogate mother
- In vitro fertilization (IVF) - using egg and sperm of parents
- IVF - with Intra-Cytoplasmic Sperm Injection (ICSI)
- IVF - with frozen embryos
- IVF - with Preimplantation Genetic Diagnosis (PGD)
- IVF - with egg donor
- IVF - with sperm donor
- IVF - with egg and sperm donor
- IVF - with surrogate using parents' egg and sperm
- IVF - with surrogate and egg donor
- IVF - with surrogate and sperm donor
- IVF - with surrogate using her egg, sperm from baby's father
- IVF - with surrogate using egg and sperm donors*
- Cytoplasmic transfer**
- Nuclear transfer and cloning
In Vitro Fertilization
First IVF Baby
Louise Brown was born at 1147 BST on 25 July, 1978, in Oldham, United Kingdom.
- Links: BBC Profile Louise Brown
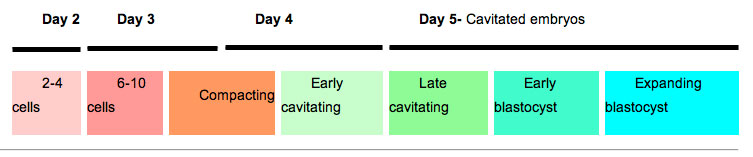
Blastocyst Formation (in vitro)
The table below shows human blastocyst in vitro changes during week 1 development.[23]
|
|
|
- Links: Week 1 | Blastocyst
Embryo Culture Milestones
- 1944-48 human oocytes fertilised by spermatozoa in vitro[2][24]
- 1949 8 cell mouse embryo -> blastocyst (in saline and egg yolk)
- 1956 8 cell mouse embryo -> blastocyst (first embryo culture medium)
- 1957 2 cell mouse embryo -> blastocyst
- 1958 8 cell mouse embryo -> blastocyst, then transferred to pregnant recipient
- 1960's development of culture requirements for mouse mebryos
- 1965 2 cell mouse embryo -> blastocyst, then transferred into pseudopregnant recipient
- 1968 zygotes from mouse -> blastocysts
- 1968,70 2 & 4 cell rabbit embryos -> blastocyst in serum supplemented medium
- 1970,71 1 & 2 cell rabbit embryos -> blastocyst in defined medium
- 1970,81 Culture of in vitro fertilized human embryo -> 16 cells -> blastula
- 1998 Cloning of adult sheep "dolly"
- 2004 Cloning of human blastocysts
Data modified from[25]
Oldest IVF Mother
There is still risk, ethical and genetic debate about very old women becoming pregnant by IVF.
- 2003 India - A 65-year old Indian woman was the oldest in the world to give birth by IVF.
- 2006 United Kingdom - A 62-year old woman has become the UK's oldest woman to give birth to a child.
- 2008 Australia - A 54-year old woman was Australia's oldest woman pregnant by IVF (most Australian IVF clinics do not treat women over 50)
- 2010 Australia - A 57-year old woman is now the oldest mother to give birth in Australia, has delivered IVF twins in Western Australia.
IVF Sex Ratios
A recent paper looked at Australian assisted reproductive technology (ART) data (2002-2006) studied the effect on human sex ratio at birth by different procedures. PMID:20875033
- "More males were born following in vitro fertilisation single embryo transfer (IVF SET) (53.0%) than intracytoplasmic sperm insemination (ICSI) SET (50.0%), and following blastocyst SET (54.1%) than cleavage-stage SET (49.9%). For a specific ART regimen, IVF blastocyst SET produced more males (56.1%) and ICSI cleavage-stage SET produced fewer males (48.7%). The change in the sex ratio at birth of SET babies is associated with the ART regimen. The mechanism of these effects remains unclear. Fertility clinics and patients should be aware of the bias in the sex ratio at birth when using ART procedures."
Oocyte and Embryo Quality
There have been many attempts to establish morphological, biochemical or molecular markers of oocyte quality with variable results by different groups. Recently studies have also looked at the molecular quality of cumulus and granulosa cells.[26] Earlier studies of this support cell population have looked at markers of mitosis and apoptosis, with positive and negative correlation respectively. Following removal of these support cells, oocyte features such as; nuclear maturation status, meiotic spindle presence, cytoplasm morphology, zona pellucida structure, and polar body presence and structure have all been investigated.[27]
Several different microscopic techniques have also been used to visually analyse oocyte appearance:
- normal light microscopy
- phase contrast microscopy
- differential interference microscopy
- polarized light microscopy - A study has used polarization microscope to identify the spindle in rescue ICSI of unfertilized oocytes appears to result in better normal fertilization rate and less 3PN rate compared to the control group.[28]
- confocal microscopy (not for clinical use)
A 2010 meeting of the Alpha Executive, and ESHRE Special Interest Group of Embryology, held in Istanbul, led to the "Istanbul consensus"[29][30] established a consensus criteria and terminology for grading oocytes, zygotes and embryos that would be amenable to routine application in any IVF laboratory. The following sub-heading are brief summary points of the complete consensus.
Oocyte Scoring
Optimal oocyte morphology is that of a spherical structure enclosed by a uniform zona pellucida, with a uniform translucent cytoplasm free of inclusions and a size-appropriate polar body. Oocytes undergo both nuclear and cytoplasmic maturation, and that these processes are neither the same nor necessarily even synchronous.
- Cumulus-oocyte complex (COC) scoring - provides a tool for troubleshooting not a correlation with embryo developmental competence. This should be a binary score (0 or 1), with a ‘good' COC (score of 1) defined as having expanded cumulus and a radiating corona.
- Zona pellucida scoring- no specific benefit to measuring zona thickness, as evidence for any effect on outcome. However, it was noted that there could be patient-specific effects, and so a note should be made of exceptional observations regarding the colour or thickness of the zona pellucida.
- Perivitelline space - presence of inclusions in the perivitelline space is anomalous. However, there was insufficient evidence to support any specific prognosis associated with this observation. Therefore, the observation of inclusions should be noted, there is no requirement to count or measure them, only noted if it is exceptionally large.
- Polar body scoring - presence or absence of the first polar body should be noted in the uninseminated oocyte, where possible. Exceptionally large polar body should be noted and should not be inseminated, due to the risk of oocyte aneuploidy.
- Cytoplasm scoring - homogeneous cytoplasm is expected, and that non-homogeneous cytoplasm is of unknown biological significance.
- ‘Granularity' of the cytoplasm is ill-defined, and distinctly different from clustering of organelles.
- Organelle clustering is associated with lower implantation potential.
- smooth endoplasmic reticulum (sER) disks are associated with the risk of a serious, significantly abnormal outcome.
- Vacuolization - observation of large vacuoles in the oocyte should be noted.
- small vacuoles (5–10 µm in diameter) few small fluid filled and transparent are unlikely to be of biological consequence.
- large vacuoles (>14 µm in diameter) are associated with fertilization failure.
Zygote Scoring
Pronuclear scoring - three categories can provide additional information to the fertilization check, and that both should be performed at the same time.
- symmetrical
- non-symmetrical
- abnormal - pronuclei with no NPBs (‘ghost pronuclei’), single nucleolar precursor body (‘bulls-eye pronuclei”).
Cleavage-stage Scoring
- Assessment of cell number - 4 cells on Day 2 and 8 cells on Day 3 and related to embryo cell cleavage rates.
- more slowly than the expected rate have a reduced implantation potential
- faster than the expected rate are likely to be abnormal and have a reduced implantation potential.
- Fragmentation - an extracellular membrane-bound cytoplasmic structure that is <45 µm diameter in a Day-2 embryo and <40 µm diameter in a Day-3 embryo. Relative degrees of fragmentation were defined as:
- mild (<10%)
- moderate (10–25%)
- severe (>25%).
- Multi nucleation - the presence of more than one nucleus in a blastomere, and includes micronuclei, and is associated with a decreased implantation potential, increased level of chromosome abnormality and increased risk of spontaneous abortion.
- Cell size - embryos at the 2-, 4- and 8-cell stages, blastomeres should be even sized. For all other cell stages, one would expect a size difference in the cells, as the cleavage phase has not been completed.
Day 4 Assessment (Morula stage)
- optimal embryo at this stage (92 ± 2 h) would be compacted or compacting, and have entered into a fourth round of cleavage. T the consensus system uses three grades, the reason for a fair or poor grade should also be included.
Day 5 Assessment (Blastocyst stage)
- optimal embryo at this stage (116 ± 2 h) would be a fully expanded through to hatched blastocyst with an ICM that is prominent, easily discernible and consisting of many cells, with the cells compacted and tightly adhered together, and with a TE that comprises many cells forming a cohesive epithelium. It was agreed that while the ICM has a high prognostic value for implantation and fetal development, a functional TE is also essential. The consensus for a blastocyst scoring system was that there should be a combination of stage and score. It was agreed that ‘hatching' is defined as the obvious emergence of the TE with enclosed blastocoel through a thinning zona pellucida. It was also agreed that hatching cannot be reliably assessed in embryos with an artificially breached zona pellucida (with the exception of the breach made during ICSI). The ICM and TE should be graded relative to the Gardner A–C scale, but that a grade of 1–3 (rather than A–C) should be given—with Grade 1 equivalent to Gardner A.
Non-viable Embryo
- a non-viable embryo is an embryo in which development has been arrested for at least 24 h, or in which all the cells have degenerated or lysed.
In Vitro Maturation
In Vitro Maturation (IVM) is a term used to describe a range of techniques for developing ovarian follicles and oocytes outside of the body. These oocytes are generally retrieved from the antral follicles of either unstimulated or minimally stimulated ovaries. The technique has been suggested for polycystic ovary syndrome and other ovarian pathologies for fertility preservation. This is a relatively new approach to human fertility.
Assisted Reproductive Technology (Australia and New Zealand)
- Assisted Reproductive Technology in Australia and New Zealand
- There were 88,929 ART treatment cycles reported from Australian and New Zealand fertility clinics in 2019 (81,049 and 7,880 respectively), representing an increase of 6.2% in Australia and 2% in New Zealand from 2018. This equates to 15.6 cycles per 1,000 women of reproductive age (15–44 years) in Australia, compared with 7.9 cycles per 1,000 women of reproductive age in New Zealand. Women used their own oocytes or embryos (autologous cycles) in 95% of treatments. Embryos and oocytes that had been frozen and thawed were used in 36.7% of autologous cycles.
- Overall, live birth rates per embryo transfer have risen from 25.3% in 2015 to 28% in 2019.
| Australia Birthweight Data 2002-2010 |
|---|
|
Birthweight percentiles by gestational age for births following assisted reproductive technology in Australia and New Zealand, 2002-2010[31]
|
| Australia 2010 Data | |||||
|---|---|---|---|---|---|

|

| ||||
ART in Australia and New Zealand 2010[22]
|
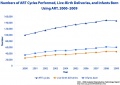
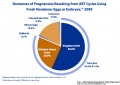
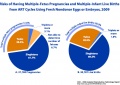
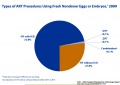
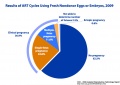
ART in Australia and New Zealand 2009[32] 9 Nov 2011
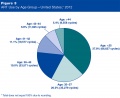
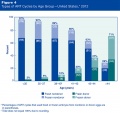
Average age of women was 35.5 years (35.2 years in 2002). Women aged older than 40 years has increased from 14.3% in 2002 to 15.3% in 2005. Since ANZARD was established in 2002 there has been a significant increase in the number of embryos transfer cycles where women received single-embryo transfers (SET). SET cycles accounted for 48.3% of embryos transfer cycles in 2005, compared to 28.4% in 2002. The increase of SET cycles resulted more singleton deliveries. The proportion of singleton deliveries was 85.9% in 2005, the highest proportion ever reported. Babies born to women who had a single-embryo transfer had better outcomes compared to babies born to women who had a double-embryo transfer (DET). In 2005, there were 3,681 SET babies and 5,589 DET babies. In SET babies, 96.1% were singletons, compared to 61.6% singletons in DET babies. SET babies had a lower proportion of preterm babies (11.7%), compared to 30.6% in DET babies. Similarly, 8.0% of SET liveborn babies were low birthweight, compared to 25.0% in DET liveborn babies. Perinatal mortality rate is a measure of perinatal outcomes. In 2005, for all babies born following ART treatment, the perinatal mortality rate was 14.7 deaths per 1,000 births, a 23.8% decrease from 19.3 deaths per 1,000 births in 2004. The perinatal mortality rate was the lowest among singletons born following SET (7.3 deaths per 1,000 births) in 2005.[33] | ||||
ART 2004
In Vitro Fertilization - ABC News Baby born from frozen embryo "In what's thought to be a world first, a baby has been born in Melbourne using a woman's frozen egg and a donor's frozen sperm which created an embryo that was also frozen, then thawed and implanted into the mother" "JOHN MCBAIN: Oh egg freezing is very difficult. Embryo freezing itself is very well established. We would probably have about 55 per cent of all the babies born from our program, and that's about 1,400 a year, come from frozen embryos. So, that's very well established technology. But even with these embryos, only 70 per cent of the embryos survive the freezing and thawing. With eggs, it's closer to 40 to 50 per cent, and then you have to have the number which don't fertilise following that, and then you have to have those which end up being frozen, possibly not surviving the embryo freezing stage too, and that's a reason we don't promote it." |
Regulation of Assisted Reproductive Technology
Assisted Reproductive Technology (ART) , including IVF (or in vitro fertilisation) is regulated in Australia through both legislative and voluntary compliance frameworks. Legislation in three states is underpinned by a national system of accreditation by the Reproductive Technology Accreditation Committee (RTAC) of the Fertility Society of Australia, which is in turn is underpinned by guidelines produced by the NHMRC. This is outlined in more detail below.
Current legislation
- ART is regulated by specific legislation in three States, the Victorian Infertility Treatment Act (1995) , the South Australian Reproductive Technology Act (1988) and the Western Australian Human Reproductive Technology Act (1991) . Each of these pieces of legislation establishes a State regulatory body which issues Licences to clinics that provide ART services.
- There is no Commonwealth legislation covering the regulation of ART clinical practice.
- The Research Involving Human Embryos Act 2002 includes a requirement that use of non-excess ART embryos occurs in an RTAC-accredited ART clinic.
Reproductive Technology Accreditation Committee (RTAC). RTAC, under the Fertility Society of Australia, administers a national Code of Practice and a system for the accreditation of ART clinics in Australia. Through its Code of Practice, RTAC sets professional and laboratory standards for ART clinical practice.
(Information from the NHMRC)
- Links: NHMRC information | RTAC
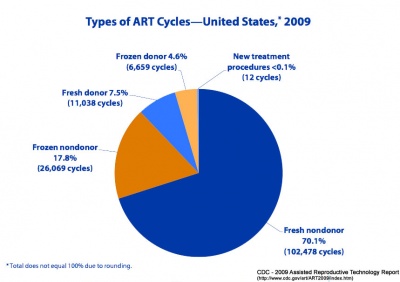
Assisted Reproductive Technology (USA)
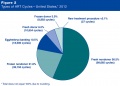
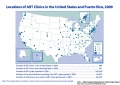
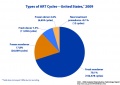
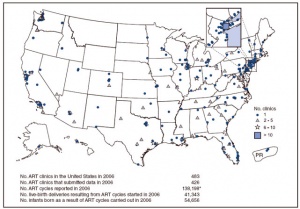
USA 2016 Data
Based on CDC’s 2016 Fertility Clinic Success Rates Report, there were 263,577* ART cycles performed at 463 reporting clinics in the United States during 2016, resulting in 65,996 live births (deliveries of one or more living infants) and 76,930 live born infants. Of the 263,577 ART cycles performed in 2016, 65,840 were banking cycles in which the intent of the ART cycle was to freeze all resulting eggs or embryos for future ART cycles and for which we would not expect a resulting pregnancy or birth. Although the use of ART is still relatively rare as compared to the potential demand, its use has doubled over the past decade. Today, approximately 1.7% of all infants born in the United States every year are conceived using ART.
Assisted Reproductive Technology (ART)
- "Fertility clinics in the U.S. report and verify data on the assisted reproductive technology (ART) cycles started and carried out in their clinics, and the outcomes of these cycles, during each calendar year. ART includes all fertility treatments in which either eggs or embryos are handled. The main type of ART is in vitro fertilization (IVF). IVF involves extracting a woman’s eggs, fertilizing the eggs in the laboratory, and then transferring the resulting embryos into the woman’s uterus through the cervix."
USA 2013 Data
- 6.9 million (11%) of the 61 million women aged 15–44 years had received infertility services at some time in their lives.
- 93,787 fresh nondonor ART cycles were started in 2013.
- 33,425 (36%) led to a pregnancy, but only 27,406 (29%) resulted in a live birth.
- 6,019 (almost 1 in 5) of ART pregnancies did not result in a live birth.
USA 2012 Data
| ART USA 2012 Data |
|---|
|
2012 Assisted Reproductive Technology National Summary Report[34]
|
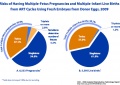
Multiple births resulting from assisted reproductive technology (2012)[35]
- 134,381 ART transfer cycles performed in 2012
- 51,262 resulted in live births
- 13,563 (26.5%) were multiple births
- 3,123 twin and 440 triplet and higher order births.
- 46.1%) of these multiple births resulted from 4 cycle types:
- two fresh blastocyst transfers among favorable prognosis patients less than 35 years (1,931 multiple births)
- two fresh blastocyst transfers among average prognosis patients less than 35 years (1,341 multiple births)
- two fresh blastocyst transfers among donor-oocyte recipients (1,532 multiple births)
- two frozen/thawed ETs among patients less than 35 years (1,452 multiple births).
- More than half of triplet or higher order births resulted from the transfer of two embryos
- 52.5% of births among fresh autologous transfers
- 67.2% of births among donor-oocyte recipient transfers
- 42.9% among frozen/thawed autologous transfers
ART USA Archive
| ART USA 2011 - 2009Data |
|---|
| ART 20011
Births Resulting From Assisted Reproductive Technology: Comparing Birth Certificate and National ART Surveillance System Data, 2011[17]
|
| ART USA 2010 Data
2010 Assisted Reproductive Technology National Summary Report | 2010 Assisted Reproductive Technology Report
|
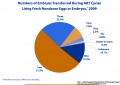
| ART USA 2009 Data
|
| ART USA 2006 Data
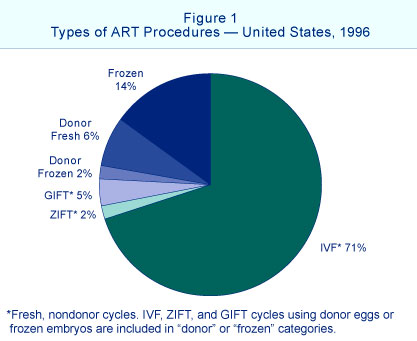
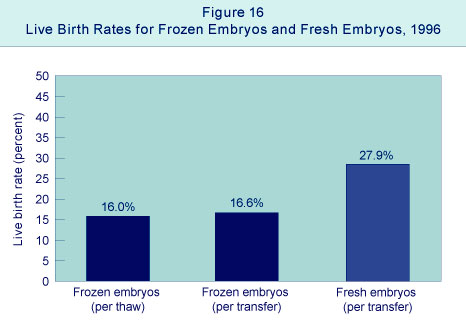
1996 Assisted Reproductive Technology Success Rates National Summary and Fertility Clinic Reports
1995 Assisted Reproductive Technology Success Rates National Summary and Fertility Clinic Report.]
|
European Society of Human Reproduction and Embryology

|
Citation: ESHRE Atlas of Human Embryology: from Oocytes to Preimplantation Embryo (2012)
This online textbook edition is designed mainly for ART clinicians covering only the developmental period between oocytes to the preimplantation embryo.
|
- Assisted reproductive technology in Europe, 2007[18] "This 11th European IVF-monitoring report presents the results of assisted reproductive technology (ART) treatments initiated in Europe during 2007.
Highlights from the 2007 Report
- From 33 countries, 1029 clinics reported 493 184 treatment cycles
- IVF (120 761), ICSI (256 642), frozen embryo replacement (91 145), egg donation (15 731)
- preimplantation genetic diagnosis/preimplantation genetic screening (4638)
- in vitro maturation (660) and frozen oocytes replacements (3607)
- overall a 7.6% increase since 2006.
Reports annually (in the journal Human Reproduction) on the European results of assisted reproductive techniques. Listed below are some statistical information gathered from reporting clinics for the current 2001 report. ESHRE Report 2001
Highlights from the 2001 Report
- From 23 countries, 579 clinics reported 289,690 cycles
- IVF 120,946, ICSI 114,378, frozen embryo transfer (FER) 47,195 and egg donation (ED) 7,171 (4% increase since the year 2000)
- European data on intra-uterine inseminations (IUIs) were reported from 15 countries. A total of 67 124 cycles [IUI husband'sperm (IUI-H) 52 949 and IUI donor sperm (IUI-D) 14 185] were included.
- In 12 countries where all clinics reported to the register, a total of 108 910 cycles were performed in a population of 131.4 million (829 cycles/million inhabitants).
- IVF- clinical pregnancy rate per aspiration and per transfer was 25.1 and 29.0%, respectively.
- ICSI- clinical pregnancy rate per aspiration and per transfer was 26.2 and 28.3% (similar to the results from 2000).
- IUI-H- clinical pregnancy rate was 12.8% in women less than 40 and 9.7% in women 40 years of age.
- After IVF and ICSI, the distribution of transfer of one, two, three and 4 embryos was 12.0, 51.7, 30.8 and 5.5%, respectively.
- Distribution of singleton, twin and triplet deliveries for IVF and ICSI combined was 74.5, 24.0 and 1.5%, respectively.
- Range of triplet deliveries after IVF and ICSI differed from 0.0 to 8.2% between countries.
- After IUI-H in women less than 40 years of age, 10.2% were twin and 1.1% were triplet gestations.
Human Fertilisation and Embryology Authority UK (HFEA)
The UK Human Fertilisation and Embryology Authority (HFEA) was established in August 1991 following the passing of the Human Fertilisation and Embryology Act 1990 (HFE Act).
The HFEA's principal tasks are to:
- License and monitor clinics that carry out in vitro fertilisation (IVF) and donor insemination
- License and monitor research centres undertaking human embryo research
- Regulate the storage of gametes and embryos
HFEA also provide a downloadable patient booklet: Your Guide to Infertility and website information on Patients' Guide to Donor Insemination (DI)
Canada
Society of Obstetricians and Gynaecologists of Canada summary statements (for January 2005 to December 2012) lists 15 key statements for pregnancy outcomes after assisted human reproduction[36]
- Links: Canada ART | [[Canada Statistics
Sweden
Sweden had its first child born after in vitro fertilisation 20 years ago. A recent paper in BMJ looks at the change in multiple birthrates since a change in the early 1990s, to reduce the number of embryos transferred in the clinic from three to two.[37]
"The rate of multiple births after in vitro fertilisation increased to a maximum of 29% in 1991 but fell to 18.5% by 2001, resulting in a 70% reduction of preterm births"
Controlled Ovarian Stimulation
A variety of drug based techniques are used to stimulate maternal oocyte development, called controlled ovarian stimulation (COS), for any in vitro fertilization procedure. The recommended for technique will vary for some procedures and also from clinic to clinic and between countries.
An example of ovarian stimulation (based on[38])
- Gonadotrophin releasing hormone agonist (GnRHa) triptorelin acetate (0.1 mg/day) treatment started on the 22nd day of the preceding menstrual cycle.
- Human menopausal gonadotrophin (HMG) and/or follicular stimulating hormone (FSH) was carried out daily 12 to 15 days later.
- Dosage may vary dependent upon patient response and can be monitored by hourmone levels (oestradiol) and transvaginal ultrasound (follicular size).
- The resulting ovulatory wave generates large follicles (greater than 18 mm in diameter).
- Human chorionic gonadotrophin (HCG) is then administered (36 to 38 h later)
- Clinical transvaginal puncture is used to collect from these follicles cumulus-oocyte complexes.
- Oocytes are then isolated from these cumulus-oocyte complexes.
- Links: Menstrual Cycle | Ovary Development | Oocyte Development | Pituitary
Gamete Banking
Both men and women undergoing clinical procedures of chemotherapy and/or radiotherapy (ionizing radiation) can have induced gametogenic failure.
Female
Women undergoing clinical procedures of chemotherapy and/or radiotherapy (ionizing radiation) can have induced premature ovarian failure. Therefore a growing reproductive option has been the collecting of oocytes or ovarian tissue before commencing these procedures and storing ("banking") by cryopreservation for later use. One major issue is coordination of the two procedures, as most cancer therapies commence immediately, and most reproductive procedures require substantial preparation time. Currently the cryopreservation techniques required for ovarian tissue preservation are also improving all the time. In a number of clinics women with breast cancer and of reproductive age are being counselled about their reproductive options.[39]
Chemotherapy, alkylating and alkylating-like agents attach to the guanine base of DNA, cross-linking the DNA, preventing replication and cell division. Some examples include: busulfan, carboplatin, chlorambucil, cisplatin, cyclophosphamide, dacarbazine, ifosfamide, thiotepa
A third potential option that may also in future be available is the transplanting (allografting) of ovarian cortex between individuals, this has recently been carried out between genetically non-identical sisters.[40]
Male
A recent paper[21] described the current practice of spermatozoa banking in the United Kingdom in relation to cancer patients. The UK Human Fertilization and Embryology Authority have now extended the period of storage permitted by their regulations to 55 years. They point to a lack of "national and international guidelines for the provision, organization, maintenance and management of the cryopreservation services."
- Links: Environmental | Drugs | Radiation
Ovarian Reserve
See ovarian reserve section on ovary page.
Ovarian reserve is a term referring to the evaluation of ovary oocyte (egg) number and quality. A negative finding has been described as Diminished Ovarian Reserve, or an ovarian insufficiency or premature ovarian failure and may be seen in adult childhood cancer survivors and adult patients undergoing a number of therapies.
The anti-Müllerian hormone (AMH) level is currently the most sensitive marker of ovarian reserve.[41]
Single-cell analysis of human ovarian cortex identifies distinct cell populations but no oogonial stem cells[42]
- "The human ovary orchestrates sex hormone production and undergoes monthly structural changes to release mature oocytes. The outer lining of the ovary (cortex) has a key role in defining fertility in women as it harbors the ovarian reserve. It has been postulated that putative oogonial stem cells exist in the ovarian cortex and that these can be captured by DDX4 antibody isolation. Here, we report single-cell transcriptomes and cell surface antigen profiles of over 24,000 cells from high quality ovarian cortex samples from 21 patients. Our data identify transcriptional profiles of six main cell types; oocytes, granulosa cells, immune cells, endothelial cells, perivascular cells, and stromal cells. Cells captured by DDX4 antibody are perivascular cells, not oogonial stem cells. Our data do not support the existence of germline stem cells in adult human ovaries, thereby reinforcing the dogma of a limited ovarian reserve."
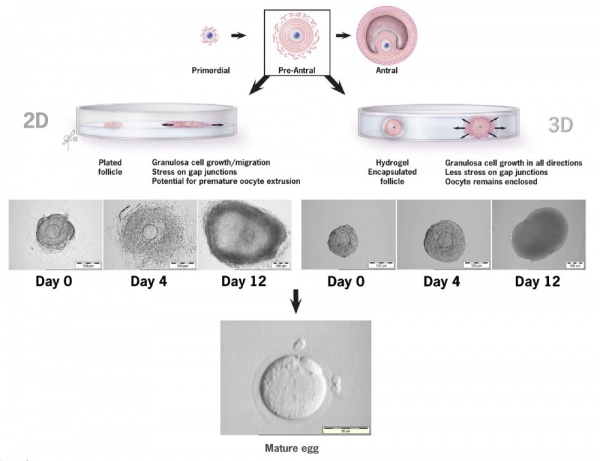
Ovarian Follicle Growth in vitro
2D and 3D methods of ovarian follicle growth in vitro.[43]
Germline Gene Editing
The European Society of Human Genetics and the European Society for Human Reproduction and Embryology have developed a consensus statement covering germline gene editing and the following ART-related topics.[44]
- "expanded carrier screening, direct-to-consumer genetic testing, voiding of the presumed anonymity of gamete donors by advanced genetic testing, advances in the research of genetic causes underlying male and female infertility, utilisation of massively parallel sequencing in preimplantation genetic testing and non-invasive prenatal screening, mitochondrial replacement in human oocytes, and additionally, issues related to cross-generational epigenetic inheritance following IVF"
Technology development in germline gene editing has allowed the development of many animal models of human congenital defects. More recently there has been debate around the ethics of using similar methods in "correcting" these abnormalities using similar techniques in humans. In 2018 the European Society of Human Reproduction and Embryology (ESHRE) and the European Society of Human Genetics (ESHG) together developed a background document and recommendations to inform and stimulate ongoing societal debates.[45][46]
Historic Embryology
| John Charles Rock (1890-1984) was a Boston gynaecologist and human fertility researcher. In 1938 he began a collaboration with pathologist Arthur Hertig and the researcher Miriam Menkin (1901 – 1992; née Miriam Friedman). Rock and Menkin were the earliest clinical researchers in the USA looking at human in vitro fertilisation techniques[2] for treatment of infertility. John Rock was also the main subject of a recent book on changes in reproduction.[47] |
References
- ↑ Milachich T. (2014). New advances of preimplantation and prenatal genetic screening and noninvasive testing as a potential predictor of health status of babies. Biomed Res Int , 2014, 306505. PMID: 24783200 DOI.
- ↑ 2.0 2.1 2.2 Rock J. and Menkin MF. In vitro fertilization and cleavage of human ovarian eggs. (1944) Science 100 (2588): 105-107. PMID 17788930
- ↑ Edwards RG, Steptoe PC & Purdy JM. (1980). Establishing full-term human pregnancies using cleaving embryos grown in vitro. Br J Obstet Gynaecol , 87, 737-56. PMID: 6775685
- ↑ Lopata A, Johnston IW, Hoult IJ & Speirs AI. (1980). Pregnancy following intrauterine implantation of an embryo obtained by in vitro fertilization of a preovulatory egg. Fertil. Steril. , 33, 117-20. PMID: 7353686
- ↑ McCulloh DH, Kutchukhidze N, Charkviani T, Zhorzholadze T, Barbakadze T, Munné S & Chkonia L. (2020). Follicle size indicates oocyte maturity and blastocyst formation but not blastocyst euploidy following controlled ovarian hyperstimulation of oocyte donors. Hum. Reprod. , , . PMID: 32142586 DOI.
- ↑ Wen SW, Miao Q, Taljaard M, Lougheed J, Gaudet L, Davies M, Lanes A, Leader A, Corsi DJ, Sprague AE & Walker M. (2020). Associations of Assisted Reproductive Technology and Twin Pregnancy With Risk of Congenital Heart Defects. JAMA Pediatr , , . PMID: 32091547 DOI.
- ↑ Bouet PE, Boueilh T, de la Barca JMC, Boucret L, Blanchard S, Ferré-L'Hotellier V, Jeannin P, Descamps P, Procaccio V, Reynier P & May-Panloup P. (2020). The cytokine profile of follicular fluid changes during ovarian ageing. J Gynecol Obstet Hum Reprod , , 101704. PMID: 32028036 DOI.
- ↑ Association of In Vitro Fertilization With Childhood Cancer in the United States Spector LG, Brown MB, Wantman E, et al. Association of In Vitro Fertilization With Childhood Cancer in the United States. JAMA Pediatr. Published online April 01, 2019. doi:10.1001/jamapediatrics.2019.0392
- ↑ Reigstad MM, Larsen IK, Myklebust TÅ, Robsahm TE, Oldereid NB, Brinton LA & Storeng R. (2016). Risk of Cancer in Children Conceived by Assisted Reproductive Technology. Pediatrics , 137, e20152061. PMID: 26908669 DOI.
- ↑ Tachibana M, Kuno T & Yaegashi N. (2018). Mitochondrial replacement therapy and assisted reproductive technology: A paradigm shift toward treatment of genetic diseases in gametes or in early embryos. Reprod. Med. Biol. , 17, 421-433. PMID: 30377395 DOI.
- ↑ Kushnir VA, Darmon SK, Barad DH & Gleicher N. (2018). New national outcome data on fresh versus cryopreserved donor oocytes. J Ovarian Res , 11, 2. PMID: 29304839 DOI.
- ↑ Patil AS, Nguyen C, Groff K, Wu J, Elliott J & Gunatilake RP. (2018). Severity of congenital heart defects associated with assisted reproductive technologies: Case series and review of the literature. Birth Defects Res , 110, 654-661. PMID: 29714054 DOI.
- ↑ Martínez-Granados L, Serrano M, González-Utor A, Ortíz N, Badajoz V, Olaya E, Prados N, Boada M & Castilla JA. (2017). Inter-laboratory agreement on embryo classification and clinical decision: Conventional morphological assessment vs. time lapse. PLoS ONE , 12, e0183328. PMID: 28841654 DOI.
- ↑ Bouillon C, Léandri R, Desch L, Ernst A, Bruno C, Cerf C, Chiron A, Souchay C, Burguet A, Jimenez C, Sagot P & Fauque P. (2016). Does Embryo Culture Medium Influence the Health and Development of Children Born after In Vitro Fertilization?. PLoS ONE , 11, e0150857. PMID: 27008092 DOI.
- ↑ Dunietz GL, Holzman C, McKane P, Li C, Boulet SL, Todem D, Kissin DM, Copeland G, Bernson D, Sappenfield WM & Diamond MP. (2015). Assisted reproductive technology and the risk of preterm birth among primiparas. Fertil. Steril. , 103, 974-979.e1. PMID: 25707336 DOI.
- ↑ Heisey AS, Bell EM, Herdt-Losavio ML & Druschel C. (2015). Surveillance of congenital malformations in infants conceived through assisted reproductive technology or other fertility treatments. Birth Defects Res. Part A Clin. Mol. Teratol. , 103, 119-26. PMID: 25684703 DOI.
- ↑ 17.0 17.1 Thoma ME, Boulet S, Martin JA & Kissin D. (2014). Births resulting from assisted reproductive technology: comparing birth certificate and National ART Surveillance System Data, 2011. Natl Vital Stat Rep , 63, 1-11. PMID: 25493705
- ↑ 18.0 18.1 de Mouzon J, Goossens V, Bhattacharya S, Castilla JA, Ferraretti AP, Korsak V, Kupka M, Nygren KG & Andersen AN. (2012). Assisted reproductive technology in Europe, 2007: results generated from European registers by ESHRE. Hum. Reprod. , 27, 954-66. PMID: 22343707 DOI.
- ↑ Müller A, Keller K, Wacker J, Dittrich R, Keck G, Montag M, Van der Ven H, Wachter D, Beckmann MW & Distler W. (2012). Retransplantation of cryopreserved ovarian tissue: the first live birth in Germany. Dtsch Arztebl Int , 109, 8-13. PMID: 22282711 DOI.
- ↑ Wang YA, Macaldowie A, Hayward I, Chambers GM, & Sullivan EA 2011. Assisted reproductive technology in Australia and New Zealand 2009. Assisted reproduction technology series no. 15. Cat. no. PER 51. Canberra: AIHW. Online Summary | PDF
- ↑ 21.0 21.1 Sharma V. (2011). Sperm storage for cancer patients in the UK: a review of current practice. Hum. Reprod. , 26, 2935-43. PMID: 21873609 DOI.
- ↑ 22.0 22.1 AIHW, Macaldowie A, Wang YA, Chambers GM & Sullivan EA 2012. Assisted reproductive technology in Australia and New Zealand 2010. Assisted reproduction technology series. Cat. no. PER 55. Canberra: AIHW. Online Summary | PDF | 26 Oct 2012
- ↑ Fong CY & Bongso A. (1999). Comparison of human blastulation rates and total cell number in sequential culture media with and without co-culture. Hum. Reprod. , 14, 774-81. PMID: 10221713
- ↑ MENKIN MF & ROCK J. (1948). In vitro fertilization and cleavage of human ovarian eggs. Am. J. Obstet. Gynecol. , 55, 440-52. PMID: 18903892
- ↑ Bavister BD. (1995). Culture of preimplantation embryos: facts and artifacts. Hum. Reprod. Update , 1, 91-148. PMID: 15726768
- ↑ Uyar A, Torrealday S & Seli E. (2013). Cumulus and granulosa cell markers of oocyte and embryo quality. Fertil. Steril. , 99, 979-97. PMID: 23498999 DOI.
- ↑ Rienzi L, Balaban B, Ebner T & Mandelbaum J. (2012). The oocyte. Hum. Reprod. , 27 Suppl 1, i2-21. PMID: 22811312 DOI.
- ↑ Moon JH, Son WY, Henderson S, Mahfoudh A, Dahan M & Holzer H. (2013). Spindle examination in unfertilized eggs using the polarization microscope can assist rescue ICSI. Reprod. Biomed. Online , 26, 280-5. PMID: 23352100 DOI.
- ↑ Alpha Scientists in Reproductive Medicine and ESHRE Special Interest Group of Embryology. (2011). The Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Hum. Reprod. , 26, 1270-83. PMID: 21502182 DOI.
- ↑ ESHRE Special Interest Group Embryology. (2011). Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Reprod. Biomed. Online , 22, 632-46. PMID: 21481639 DOI.
- ↑ Li Z, Wang YA, Ledger W & Sullivan EA. (2014). Birthweight percentiles by gestational age for births following assisted reproductive technology in Australia and New Zealand, 2002-2010. Hum. Reprod. , 29, 1787-800. PMID: 24908671 DOI.
- ↑ Wang YA, Macaldowie A, Hayward I, Chambers GM, & Sullivan EA 2011. Assisted reproductive technology in Australia and New Zealand 2009. Assisted reproduction technology series no. 15. Cat. no. PER 51. Canberra: AIHW. Online Summary | PDF
- ↑ 33.0 33.1 Wang YA, Dean JH & Sullivan EA. Assisted Reproduction Technology in Australia and New Zealand 2005 National Perinatal Statistics Unit (2007) AIHW Assisted reproduction technology series no. 11
- ↑ Centers for Disease Control and Prevention, American Society for Reproductive Medicine, Society for Assisted Reproductive Technology. 2012 Assisted Reproductive Technology National Summary Report. Atlanta (GA): US Dept of Health and Human Services; 2014. http://www.cdc.gov/art/ART2012
- ↑ Kissin DM, Kulkarni AD, Mneimneh A, Warner L, Boulet SL, Crawford S & Jamieson DJ. (2015). Embryo transfer practices and multiple births resulting from assisted reproductive technology: an opportunity for prevention. Fertil. Steril. , 103, 954-61. PMID: 25637480 DOI.
- ↑ Okun N & Sierra S. (2014). Pregnancy outcomes after assisted human reproduction. J Obstet Gynaecol Can , 36, 64-83. PMID: 24444289 DOI.
- ↑ Källén B, Finnström O, Nygren KG & Olausson PO. (2005). Temporal trends in multiple births after in vitro fertilisation in Sweden, 1982-2001: a register study. BMJ , 331, 382-3. PMID: 15894553 DOI.
- ↑ Assidi M, Montag M, Van der Ven K & Sirard MA. (2011). Biomarkers of human oocyte developmental competence expressed in cumulus cells before ICSI: a preliminary study. J. Assist. Reprod. Genet. , 28, 173-88. PMID: 20953827 DOI.
- ↑ Lawrenz B, Neunhoeffer E, Henes M, Lessmann-Bechle S, Krämer B & Fehm T. (2010). Management of fertility preservation in young breast cancer patients in a large breast cancer centre. Arch. Gynecol. Obstet. , 282, 547-51. PMID: 20499073 DOI.
- ↑ Donnez J, Squifflet J, Pirard C, Jadoul P & Dolmans MM. (2010). Restoration of ovarian function after allografting of ovarian cortex between genetically non-identical sisters. Hum. Reprod. , 25, 2489-95. PMID: 20663793 DOI.
- ↑ Lie Fong S, Laven JS, Hakvoort-Cammel FG, Schipper I, Visser JA, Themmen AP, de Jong FH & van den Heuvel-Eibrink MM. (2009). Assessment of ovarian reserve in adult childhood cancer survivors using anti-Müllerian hormone. Hum. Reprod. , 24, 982-90. PMID: 19153092 DOI.
- ↑ Wagner M, Yoshihara M, Douagi I, Damdimopoulos A, Panula S, Petropoulos S, Lu H, Pettersson K, Palm K, Katayama S, Hovatta O, Kere J, Lanner F & Damdimopoulou P. (2020). Single-cell analysis of human ovarian cortex identifies distinct cell populations but no oogonial stem cells. Nat Commun , 11, 1147. PMID: 32123174 DOI.
- ↑ Desai N, Alex A, AbdelHafez F, Calabro A, Goldfarb J, Fleischman A & Falcone T. (2010). Three-dimensional in vitro follicle growth: overview of culture models, biomaterials, design parameters and future directions. Reprod. Biol. Endocrinol. , 8, 119. PMID: 20946661 DOI.
- ↑ Harper JC, Aittomäki K, Borry P, Cornel MC, de Wert G, Dondorp W, Geraedts J, Gianaroli L, Ketterson K, Liebaers I, Lundin K, Mertes H, Morris M, Pennings G, Sermon K, Spits C, Soini S, van Montfoort APA, Veiga A, Vermeesch JR, Viville S & Macek M. (2018). Recent developments in genetics and medically assisted reproduction: from research to clinical applications. Eur. J. Hum. Genet. , 26, 12-33. PMID: 29199274 DOI.
- ↑ de Wert G, Pennings G, Clarke A, Eichenlaub-Ritter U, van El CG, Forzano F, Goddijn M, Heindryckx B, Howard HC, Radojkovic D, Rial-Sebbag E, Tarlatzis BC & Cornel MC. (2018). Human germline gene editing: Recommendations of ESHG and ESHRE. Eur. J. Hum. Genet. , 26, 445-449. PMID: 29326428 DOI.
- ↑ De Wert G, Heindryckx B, Pennings G, Clarke A, Eichenlaub-Ritter U, van El CG, Forzano F, Goddijn M, Howard HC, Radojkovic D, Rial-Sebbag E, Dondorp W, Tarlatzis BC & Cornel MC. (2018). Responsible innovation in human germline gene editing: Background document to the recommendations of ESHG and ESHRE. Eur. J. Hum. Genet. , , . PMID: 29326429 DOI.
- ↑ Marsh M. and Ronner W. The Fertility Doctor: John Rock and the Reproductive Revolution. (2008) Baltimore: Johns Hopkins University Press. Pp. x, 374. ISBN 978-0-801-89001-7.
Reviews
Articles
Smitz J, Dolmans MM, Donnez J, Fortune JE, Hovatta O, Jewgenow K, Picton HM, Plancha C, Shea LD, Stouffer RL, Telfer EE, Woodruff TK & Zelinski MB. (2010). Current achievements and future research directions in ovarian tissue culture, in vitro follicle development and transplantation: implications for fertility preservation. Hum. Reprod. Update , 16, 395-414. PMID: 20124287 DOI.
Meintjes M, Chantilis SJ, Ward DC, Douglas JD, Rodriguez AJ, Guerami AR, Bookout DM, Barnett BD & Madden JD. (2009). A randomized controlled study of human serum albumin and serum substitute supplement as protein supplements for IVF culture and the effect on live birth rates. Hum. Reprod. , 24, 782-9. PMID: 19147504 DOI.
Search Pubmed
July 2010 "Assisted Reproductive Technology" All (45041) Review (5016) Free Full Text (8551)
"in vitro fertilization" All (29785) Review (3172) Free Full Text (6189)
Search Pubmed Now: in vitro fertilization | assisted reproduction technology
External Links
External Links Notice - The dynamic nature of the internet may mean that some of these listed links may no longer function. If the link no longer works search the web with the link text or name. Links to any external commercial sites are provided for information purposes only and should never be considered an endorsement. UNSW Embryology is provided as an educational resource with no clinical information or commercial affiliation.
- Australia Assisted reproductive technology in Australia and New Zealand 2002 Report - Highlights Has links to the full report online. | (Australian) National Perinatal Statistics Unit | (Australian) National Perinatal Statistics Unit Assisted Reproduction Technology Reports | The Australian Infertility Support Group
- Victorian Assisted Reproductive Treatment Authority (VARTA) This group provides public education and resources for professionals and the community on fertility and issues related to assisted reproductive treatment, including IVF, surrogacy and donor-conception.
- Fertility Society of Australia http://www.fertilitysociety.com.au
- USA CDC - Assisted Reproductive Technology
- 2012 Assisted Reproductive Technology National Summary Report
- Society for Assisted Reproductive Technology The Society for Assisted Reproductive Technology (SART) promotes and advances the standards for the practice of assisted reproductive technology to the benefit of patients, members and society at large.
- The American Society for Reproductive Medicine (ASRM) is a multidisciplinary organization for the advancement of information, education, advocacy and standards in the field of reproductive medicine.
- American Fertility Association (AFA) is a national consumer organization that offers support for men and women dealing with infertility. Their purpose is to educate the public about reproductive disease and support families during struggles with infertility and adoption.
- RESOLVE: The National Infertility Association is a national consumer organization that offers support for men and women dealing with infertility. Their purpose is to provide timely, compassionate support and information to people who are experiencing infertility and to increase awareness of infertility issues through public education and advocacy.
- UK | Human Fertilisation and Embryology Authority (UK) | Your Guide to Infertility | Patients' Guide to Donor Insemination (DI) | Patients FAQs | Patients Guide to IVF Clinics (UK)
- IVF Directory
- The Merck Manual | The Merck Manual- Infertility | The Merck Manual- Pregnancy | Search The Merck Manual "Infertility" | Search The Merck Manual "Pregnancy"
- Sydney Commercial IVF Sites Sydney IVF | Citywest IVF | IVF South | North Shore Fertility Pty Ltd
- National Conference of State Legislatures (USA) Embryo and Gamete Disposition Laws Updated July 2007
Terms
For a full list of terms see ART Glossary
- empty follicle syndrome - (EFS) Term used to describe a condition in which no oocytes are recovered/obtained after an apparently successful ovarian stimulation.
- follicle stimulating hormone - (FSH, gonadotropin) A glycoprotein hormone secreted by anterior pituitary (adenohypophysis gonadotrophs, a subgroup of basophilic cells) and acts on gametogenesis and other systems in both males and females. In females, FSH acts on the ovary to stimulate follicle development. Negative feedback by inhibin from the developing follicle decreases FSH secretion. In males, acts on the testis Sertoli cells to increase androgen-binding protein (ABP) that binds androgens and has a role in spermatogenesis. FSH-defficiency in females results in infertile (block in folliculogenesis prior to antral follicle formation) and in males does not affect fertility (have small testes but are fertile). FSH protein has a molecular weight 30 kDa and a 3-4 hour half-life in circulation. Gonadotrophins have been used clinically in humans for the treatment of infertility.
- human chorionic gonadotropin - (hCG, human chorionic gonadotrophin) Placental hormone initially secreted by cells (syncitiotrophoblasts) from the implanting conceptus during week two, supporting the ovarian corpus luteum, which in turn supports the endometrial lining and therefore maintains pregnancy. Hormone can be detected in maternal blood and urine and is the basis of many pregnancy tests. Hormone also stimulates the onset of fetal gonadal steroidogenesis, high levels are teratogenic to fetal gonadal tissues.
- human menopausal gonadotropin - (HMG) A clinical hormone preparation used in assisted reproductive technologies (ART). This hormone is collected from the urine of menopausal women and has similar biological activity to that of follicle stimulating hormone (FSH). This is used in an injectable form along with human chorionic gonadotropin (hCG) to induce ovulation. Some commercial product names include Menogon or Organon.
- triptorelin acetate - A gonadotropin-releasing hormone (GnRH) agonist used clinically in an acetate or pamoate form inreproduction for assisted reproductive technologies (ART, in vitro fertilization, IVF). This decapeptide (pGlu-His-Trp-Ser-Tyr-D-Trp-Leu-Arg-Pro-Gly-NH2) agonist stimulates the pituitary to decrease secretion of gonadotropins luteinizing hormone (LH) and follicle stimulating hormone (FSH). Also used for other clinical conditions.
- zona pellucida birefringence - (ZPB) Optical property of the zona pellucida using polarization imaging when viewed microscopically. Used to qualitatively predict the developmental potential of a in vitro matured metaphase-II (MII) oocytes. High birefringence has been associated with oocytes contributing to conception cycles when compared with those of nonconception cycles and higher implantation, pregnancy, and live birth rates from transferred oocytes. (More? PMID18284880 | PMID20079896)
- Luteal-phase insufficiency - stimulated IVF cycles can disrupt the maternal pituitary gland pulsatile release of luteinizing hormone (LH) during the menstrual cycle luteal phase. Clinically, progesterone supplementation (support) is given during the luteal-phase of stimulated IVF cycles.
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2026, March 2) Embryology Assisted Reproductive Technology. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Assisted_Reproductive_Technology
- © Dr Mark Hill 2026, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G