1987 Developmental Stages In Human Embryos - Stage 23
| Embryology - 26 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
O'Rahilly R. and Müller F. Developmental Stages in Human Embryos. Contrib. Embryol., Carnegie Inst. Wash. 637 (1987).
| Online Editor Note |
|---|
| O'Rahilly R. and Müller F. Developmental Stages in Human Embryos. Contrib. Embryol., Carnegie Inst. Wash. 637 (1987).
The original 1987 publication text, figures and tables have been altered in formatting, addition of internal online links, and links to PubMed. Original Document - Copyright © 1987 Carnegie Institution of Washington.
|
- 1987 Stages: Introduction | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | References | Appendix 1 | Appendix 2 | Historic Papers | Embryonic Development
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
Stage 23
| Carnegie Collection - Stage 23 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Serial No. | Size (mm) | Grade | Fixative | Embedding Medium | Plane | Thinness (µm) | Stain | Point Score | Sex | Year | Notes |
| 45 | E,28 Ch, 40x35x20 | Poor | ? | P | Coronal/Transverse | 50 | Al. coch. | 51.5 | Female | 1895 | |
| 75 | E,30 | Good | Alc. | P | Sagittal | 50 | Coch. | 57 | Male | 1897 | |
| 86 | E,30 | Good | ? | ? | Coronal | 50 | Coch. | Male | 1897 | May be an early fetus | |
| 100 | E,27 | Poor | ? | P | Sagittal | 50 | Al. coch. | 57.5 | ? | 1897 | |
| 108 | E, 28 (est.) | Poor | Piurosulph. acid | P | Sagittal | 45 | Borax carm. | 52.5 | Male | 1897 | |
| 227 | E30 Ch, 60x40x20 | Poor | Formalin | P | Sagittal | 50, 100 | Al. coch. | 54 | Female | 1903 | |
| 417 | E,32 Ch., 0x60x40 | Good | Formalin | P | Transverse | 100 | Al. coch. | 58.5 | Female | 1907 | |
| 756A | E, 27 Ch. 60x45x35 | Good | Formalin | P | Coronal | 50 | Al. coch. | 56 | Male | 1913 | |
| 882 | E , 28 Ch, 80x80x40 | Good | Formalin | P | Transverse | 40 | Multiple | 53 | 8 | 1913 | |
| 950 | E, 29 | Good | Formol | P | Transverse | 50 | Al. coch. | 54 | Male | 1914 | |
| 1199 | E.,26 Ch,, 60x40x30 | Good | Formalin | C | Coronal | 40 | (Stain - Haematoxylin Eosin) aur , or. G. | 54.5 | Male | 1915 | |
| 1535 | E , 28 Ch. 50x45 x15 | Poor | Formalin | P | Transverse | 40 | (Stain - Haematoxylin Eosin) | 495 | Female | 1916 | |
| 1945 | E., 27.3 Ch., 83x53x22.5 | Good | Formalin | C-P | Transverse | 50 | (Stain - Haematoxylin Eosin) aur., or. G. | 48 | Male | 1917 | |
| 2561 | E., 27 .5 | Good | Formalin | C-P | Transverse | 25 | (Stain - Haematoxylin Eosin) aur., or. G. | 48 .5 | Male | 1919 | |
| 4205 | E., 29.5 | Good | Bouin | P | Transverse | 50 | A1. coch. | 55.5 | Female | 1923 | |
| 4289 | E., 32.2 Ch., 52x35x25 | Good | Formalin | P | Transverse | 15, 20 | A1. coch., Mallory | 59 | Female | 1923 | |
| 4525 | E., 30 | Good | Formalin | P | Sagittal | 20 | (Stain - Haematoxylin Eosin) | 57 | Male | 1924 | |
| 4570 | E, 30.7 Ch., 52X50X28 | Exc. | Bouin | P | Transverse. | 15 | (Stain - Haematoxylin Eosin) , phlox. | 55 | Male | 1924 | |
| 5154 | E.,32 | Good | Bouin | P | Transverse | 20 | (Stain - Haematoxylin Eosin) | 59.5 | Male | 1926 | |
| 5422 | E., 27 | Good | Formalin | P | Sagittal | 40 | (Stain - Haematoxylin Eosin) | 52.5 | Female | 1927 | |
| 5621A | E., 27.5 | Good | Formalin | P | Transverse | 20 | (Stain - Haematoxylin Eosin) | 52.5 | Male | 1927 | Other twin has spina bifida and fused kidneys |
| 5725 | E, 23 | Good | Formalin | P | Coronal | 25 | (Stain - Haematoxylin Eosin) aur., or. G. | 50.5 | Female | 1928 | |
| 6573 | E,31.5 | Good | Bouin | C | Transverse | 20 | (Stain - Haematoxylin Eosin) | 58.5 | Female | 1932 | |
| 7425 | E, 27 | Exc. | Bouin | C-P | Coronal | 20 | (Stain - Haematoxylin Eosin) | 47 | Female | 1937 | Ag added |
| 9226 | E, 31 | Exc. | Formalin | C—P | Transverse | 12 | Azan | ? | Female | 1954 | |
| D.122 | E, 27 | Exc. | ? | ? | Transverse | 19 | Ag | ? | ? | 1976 | Yntema and Truex |
Abbreviations
| |||||||||||
|
|
|
|
|
|
|
|
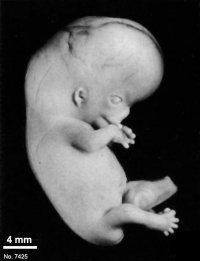
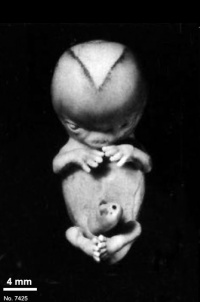
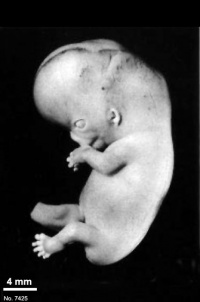
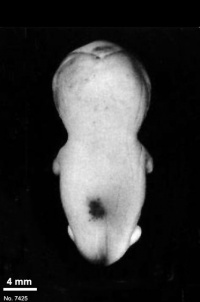
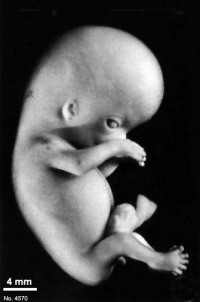
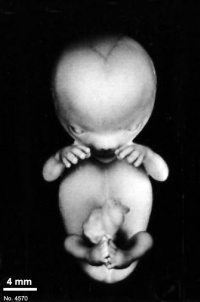
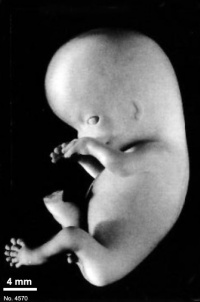
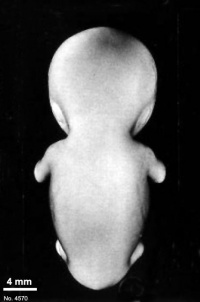
Fig. 23-1. Photographs of two embryos belonging to stage 23. The superficial vascular plexus of the head has spread nearly to the vertex. A considerable growth in length of the limbs has occurred during the past 2 days. The forearm is sometimes raised to a level above that of the shoulder (H), and the hands extend far out in front of the trunk. “Praying feet” are visible in B and F. Upper row, No 7425. Lower row, No. 4570. All views are at the same magnification.
Size And Age
The middle group of embryos of stage 23 measures 28–30 mm in length but the full range extends from 23 to 32 mm.
The age is believed to be approximately 56–57 postovulatory days (Olivier and Pineau, 1962), and not 47 days, as thought formerly on the basis of comparisons with the rhesus monkey. It should be emphasized that the ages of human embryos cannot be obtained “by matching the specimens against macaque embryos of known ovulation age having a similar degree of development” (Streeter, 1942). In point of fact, the rhesus monkey (Macaca mulatta) develops more rapidly during the embryonic period, such that stage 23 is reached at or before 7 weeks (Hendrickx and Sawyer, 1975; Gribnau and Geijsberts, 1981) compared to 8 weeks in the human. That a 30-mm human embryo may be expected to have an age of 8 postovulatory weeks has been confirmed ultrasonically in vivo (Drumm and O’Rahilly, 1977).
External Form
The head has made rapid progress in its bending toward the erect position. The head is distinctly rounded out, and the cervical region and trunk are of a more mature shape. The eyelids may show some fusion laterally and medially, but the eyes may be largely open (fig. 23-1E). Streeter (1942) had originally intended to extend his staging system “up to fetuses between 32 and 38 mm long, the stage at which the eyelids have come together.”
The limbs have increased markedly in length and show more-advanced differentiation of their subdivisions. The forearm ascends to or above the level of the shoulder.
The superficial vascular plexus is rapidly approaching the vertex of the head, leaving only a small nonvascular area that will soon become bridged by anastomosing branches.
The external genitalia are well developed but do not suffice for the detection of sex. In particular, some males tend to be diagnosed as females (Wilson, 1926b). Only in fetuses of about 50 mm is it safe to make an assessment.
Features for Point Scores
- Cornea. The cornea now comprises the anterior epithelium and its basement membrane, the substantia propria, and the posterior epithelium (Streeter, 1951, fig. 18, and O’Rahilly, 1966, figs. 51 and 59).
- Optic nerve. The sheath is quite distinct, at least in the more advanced specimens (O’Rahilly, 1966, fig. 55). A vascular canal is present.
- Cochlear duct. The tip of the duct, having proceeded “horizontally” again, now points “downward” for the second time (fig. 19-6). The duct is coiled to nearly its final extent of 2½ turns.
- Adenohypophysis. Scant trace of the stalk remains (fig. 19-7). Lobules of epithelium project into the mesodermal component of the gland, and oriented epithelial follicles are present (Streeter, 1951, plate 2). Abundant angioblasts and capillaries are found.
- Vomeronasal organ. A narrow canal is seen in the long, tapering duct. The sac is beginning to shrink and retrogress (fig. 19-9).
- Submandibular gland. Lumina are found in many terminal branches of the duct (fig. 19-10). Orientation of the epithelial tree is beginning, and angiogenesis is commencing around the epithelium. A mesodermal sheath is beginning to form around the gland.
- Metanephros. The secretory tubules are changing from short to long, and becoming more convoluted. The epithelium in some tubules is high. Renal tubules of fourth and fifth orders are present. Large glomeruli are numerous (fig. 19-12).
- Humerus. All five cartilaginous phases are now present (Streeter, 1949, figs. 3 and 18).
Stage 23 marks the end of the embryonic period proper, as defined by Streeter. The arbitrary but useful criterion that he used was the replacement of the cartilage of the humerus by bone marrow. “If the onset can be recognized in a given specimen, that specimen is straightway classed as a fetus.” A drawing of a photomicrograph showing this process in a 34-mm fetus was provided by Streeter (1949, fig. 6).
Additional Features
- Blood vascular system. The left superior vena cava is obliterated during stages 21-23 (McBride, Moore, and Hutchins, 1981).[1]
- Heart. The heart has been described in some detail by Licata (1954)[2], and the nerve supply and conducting system were investigated by Gardner and O’Rahilly (1976b).
- Palate. The palate at stages 18–23 has been described and classified by Waterman and Meller (1974)[3] and the palate at stages 19–23 has been studied by Diewert (1981)[4]. According to the latter, in half of embryos of stage 23 the palatal shelves have made contact and “epithelial adhesion in the midpalatal region” is present.
- Intestine. Although nerves reach the extremity of the intestinal loop, fibers are not yet anchored to the visceral musculature (Bossy, 1981).
- Larynx. The larynx has been reconstructed and described by Müller, O’Rahilly, and Tucker (1981, 1985).
- Diaphragm. The diaphragm and adjacent abdominal organs have been illustrated by Wells (1954, fig. 33).
- Mesonephros. A detailed study of, and comparison between, the mesonephros and the metanephros in staged embryos is unfortunately lacking.
- Metanephros. The kidneys have ascended from a sacral level at stages 13–15 to a lumbar level at stages 17–23. At stage 23 they are generally at the level of lumbar vertebrae 1–3 (Müller and O’Rahilly, 1986a).
- Suprarenal gland. The development of the suprarenal gland at stages 13–23 was studied in detail by Crowder (1957).
- Testis. Testicular tubules are identifiable. The rete testis makes contact, but no actual union with the mesonephric elements has occurred yet (Wilson, 1926a). Clusters of cells have started their differentiation into interstitial cells.
- Ovary. The rete ovarii is closely related to but not united with the mesonephric elements (Wilson, 1926a).
- Paramesonephric ducts. The paramesonephric ducts meet the urogenital sinus and fuse with each other in the median plane (Koff, 1933; Pillet, 1968). The sinusal tubercle has appeared.
- Skull. The chondrocranium has been reconstructed and described in detail by Müller and O’Rahilly (1980b). In the more advanced specimens, ossification may commence in several skeletal elements of the skull, and also in the scapula and distal phalanges of the hand (O’Rahilly and Gardner, 1972).
- Vertebrae. The vertebral column has been studied by O’Rahilly, Müller, and Meyer (1980, 1983). Either 33 or 34 cartilaginous vertebrae are present. Spinous processes have not yet developed, so that a general appearance of total spina bifida occulta is given.
- Joints. Stages 22 and 23 were included in Moffett's (1957) study of the temporomandibular joint. In the more advanced embryos, cavitation may have begun in the shoulder, elbow, wrist, hip, knee, and ankle joints (O’Rahilly and Gardner, 1975).
- Brain. A general view of the organ was given by Gilbert (1957, fig. 22), and the cranial nerves were included by Müller and O’Rahilly (1980b, fig. 10). The rhombencephalon by now shows a very advanced organization, and it presents striking resemblances to that of the newborn. In the cerebellum the external granular layer begins to develop on the rostral surface. Some of the cerebellar commissures have appeared. In the diencephalon the thin roof represents tela choroidea of the third ventricle. The interventricular foramen is reduced to a dorsoventral slit. The cerebral hemispheres cover almost the whole lateral surface of the diencephalon. The brain is now surrounded by loose tissue that is the forerunner of the subarachnoid space (O’Rahilly and Müller, 1986b). Most of the cisternae of the adult are already present.
- Spinal cord. The general appearance of the spinal cord and spinal ganglia as a whole has been illustrated by O’Rahilly, Müller, and Meyer (1980, figs. 3-5).
- Cutaneous innervation of the limbs. The sequence follows the proximodistal development of the external form but with a delay of 1–2 stages. At stage 23, the exploratory buds have reached the tips of the fingers and almost those of the toes (Bossy, 1982).[5]
- Eye. The retina comprises the pigmented layer, external limiting membrane, proliferative zone, external neuroblastic layer, transient fiber layer, internal neuroblastic layer, nerve fiber layer, and internal limiting membrane (O’Rahilly, 1966, fig. 53). The secondary vitrous body and secondary lens fibers are forming.
- Ear. A detailed account of the middle and internal ear in embryos at the later stages is unfortunately lacking. The otic capsule at stage 23 has been reconstructed by Müller and O’Rahilly (1980b, fig. 5).
Embryonic vs. Fetal Period
The distinction between the embryonic and the fetal periods at 8 postovulatory weeks has proved valuable. It is based primarily on the probability that more than 90 percent of the more than 4,500 named structures of the adult body have appeared by that time (O’Rahilly, 1979[6]). Nevertheless, it is not to be denied that this useful distinction is arbitrary and that alternative divisions of prenatal life are feasible. For example, based largely on mathematical and hormonal criteria (teratogenetic data are totally unconvincing in this regard), it has been argued that “‘la période embryonnaire paraît bien s’étendre de la fécondation á la fin du 3ème mois et qu'on peut y démarquer un premier sous-stade de 45j allant jusqu’à la lère ébauche typiquement humaine (18 ou 19C); et un second sous-stade de finition histogénétique, de réglage des proportions, lui-même exigeant aussi 45 jours” (Guyot, 1985). The total, some 90 days, would result in a length of approximately 90 mm.
It has been considered preferable to retain Streeter’s system here because:
- there is no really convincing reason to change it at this time,
- to do so would cause endless confusion (as would adopting a different staging system, as certain authors have done),
- external changes would probably be too slight to be useful in adding further stages,
- in practical terms, adequately detailed information is not yet available beyond 8 postovulatory weeks.
References
| Online Editor Note |
|---|
| O'Rahilly R. and Müller F. Developmental Stages in Human Embryos. Contrib. Embryol., Carnegie Inst. Wash. 637 (1987).
The original 1987 publication text, figures and tables have been altered in formatting, addition of internal online links, and links to PubMed. Original Document - Copyright © 1987 Carnegie Institution of Washington.
|
- ↑ Mcbride RE, Moore GW & Hutchins GM. (1981). Development of the outflow tract and closure of the interventricular septum in the normal human heart. Am. J. Anat. , 160, 309-31. PMID: 7223678 DOI.
- ↑ LICATA RH. (1954). The human embryonic heart in the ninth week. Am. J. Anat. , 94, 73-125. PMID: 13124266 DOI.
- ↑ Waterman RE & Meller SM. (1974). Alterations in the epithelial surface of human palatal shelves prior to and during fusion: a scanning electron microscopic study. Anat. Rec. , 180, 111-35. PMID: 4411922 DOI.
- ↑ Diewert VM. (1982). Contributions of differential growth of cartilages to changes in craniofacial morphology. Prog. Clin. Biol. Res. , 101, 229-42. PMID: 7156139
- ↑ Bossy J. (1982). [Sequence of development of cutaneous innervation of the limbs in the human embryo]. Bull Assoc Anat (Nancy) , 66, 57-61. PMID: 7139140
- ↑ O'Rahilly R. 1979. Early human development and the chief sources of information on staged human embryos. Europ. J. Obstet. Gynec. Reprod. Biol., 9, 273-280. PMID 400868
See also Müller F & O'Rahilly R. (1990). The human brain at stages 21-23, with particular reference to the cerebral cortical plate and to the development of the cerebellum. Anat. Embryol. , 182, 375-400. PMID: 2252222
Müller F & O'Rahilly R. (1990). The human rhombencephalon at the end of the embryonic period proper. Am. J. Anat. , 189, 127-45. PMID: 2244584 DOI.
Search Pubmed
- 1987 Stages: Introduction | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | References | Appendix 1 | Appendix 2 | Historic Papers | Embryonic Development
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
Cite this page: Hill, M.A. (2024, April 26) Embryology 1987 Developmental Stages In Human Embryos - Stage 23. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/1987_Developmental_Stages_In_Human_Embryos_-_Stage_23
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G
