Book - Manual of Human Embryology 18-6
| Embryology - 26 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Keibel F. and Mall FP. Manual of Human Embryology II. (1912) J. B. Lippincott Company, Philadelphia.
- XVIII. Development of Blood, Vascular System and Spleen: Introduction | Origin of the Angioblast and Development of the Blood | Development of the Heart | The Development of the Vascular System | General | Special Development of the Blood-vessels | Origin of the Blood-vascular System | Blood-vascular System in Series of Human Embryos | Arteries | Veins | Development of the Lymphatic System | Development of the Spleen
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
C. Arteries
Evans HM. The development of the vascular system. In Keibel F. and Mall FP. Manual of Human Embryology II. (1912) J. B. Lippincott Company, Philadelphia. pp570-708.
By Herbert M. Evans. Johns Hopkins University, Baltimore.
Development and Fate of the Aortic Arches
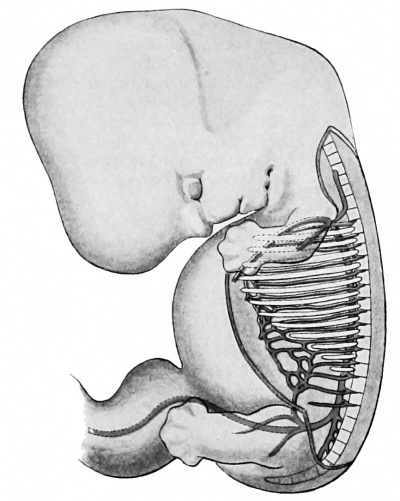
Since the human embryo, like that of all other vertebrates, possesses a row of definite gill bars or visceral arches, separated distinctly, externally by clefts, internally by entodermal pockets or pouches, so also its primitive vascular system is in conformity with this fundamental plan, and strong branches connecting the dorsal and ventral aortae — the aortic arches — each course in a visceral arch (Fig. 421). It has been known for a long time that in all vertebrates above the fishes, — i.e., in the amphibia, the sauropsida, and the mammalia — the number of these arches is five.
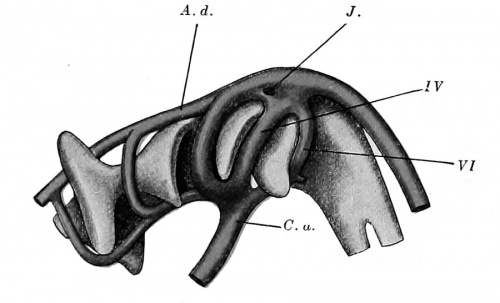
Fig. 421. Model of the pharynx and aortic arches in a human embryo 5 mm. long. (After Tandler, Morph. Jahrb., xxx, 1902, Taf. v, Fig. 17.) A. d., aorta dorsalia; C. a., conus arteriosus; J., island; IV, fourth aortic arch; VI, sixth aortic arch.
Within the last three decades, however, it has gradually been shown that in reality six arches exist in these classes, the fifth aortic arch being everywhere an exceedingly transitory vessel.[1]
Ziimnemiann (1889) was the first to indicate that there was any tendency to the formation of a fifth arch in man, reporting the separation of the fourth arch into two distinct vessels in a seven millimetre human embryo.
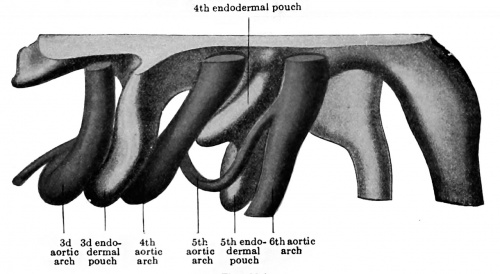
In his article on the development of the head arteries in mammals, Tandler (1902) described two very clear cases of a human fifth aortic arch, neither of which, it may be noted, corresponded to Zimmerrnann 's description, for in both cases the fifth arch took origin from the aorta ventralis and joined the dorsal portion of the pulmonary arch. A diverticulum of the fourth endodermal pouch (postbranchial body) separated the fifth and sixth arches, whereas the fourth pouch lay between the fifth vessel and the fourth arch. Since then other observers (Elze, 1907) have reported the partial presence of this vessel in the same situation. The question of the existence of a true fifth aortic arch was soon seen to involve the identification of the postbranchial body as the fifth branchial pouch. Hammar (1904), now, had described an embryo of 5 mm. (N.T. 20), in which five pouches were present, the fifth {using with the ectoderm of a fifth branchial cleft in the manner typical for these structures. Elze (1907), aware of Hammar 's report, and finding a fifth ectodermal cleft opposite the post-branchial body in an embryo of 7 mm. (N.T. 28), felt no hesitancy in identifying the postbranchial or ultimobranchial body as the fifth branchial pouch. Finally Tandler (1909) has examined a considerable number of embryos bearing on this point and brought together all that has been ascertained about the fifth arch. His conclusions seem to put the question at rest and tc show that in man, very transitorily, in embryos from five to ten millimetres in length, a true fifth arch exists (Figs. 422 and 423, A and B), springing from the truncus aorticus just before the fourth arteries are given off, and coursing dorsally in what is sometimes a distinct fifth gill bar to open into the sixth arch close to its upper end. In relation with it is a special transitory branch of the vagus nerve (ramus posttrematicus, Elze),[2] in front of it is the fourth entodermal pouch, and behind it the postbranchial body (fifth pouch). The latter is indeed in early stages apparently only a caudal ventral division of the fourth pouch. It is later incorporated in the thyroid gland (Tandler, 1909, Grosser, 1910), although apparently not contributing true thyroid tissue (Grosser).
Fig. 422. Model of the pharynx and aortic arches of a human embryo 7 mm. long. (After Tandler, 1909.) ( Embryo H8 of the I anat. Lehrkanzel, Vienna.)
Fig. 423. A and B. — Model of the pharynx and aortic arches of a human embryo 9 mm. long (NT. 37). (After Tandler, 1909.)
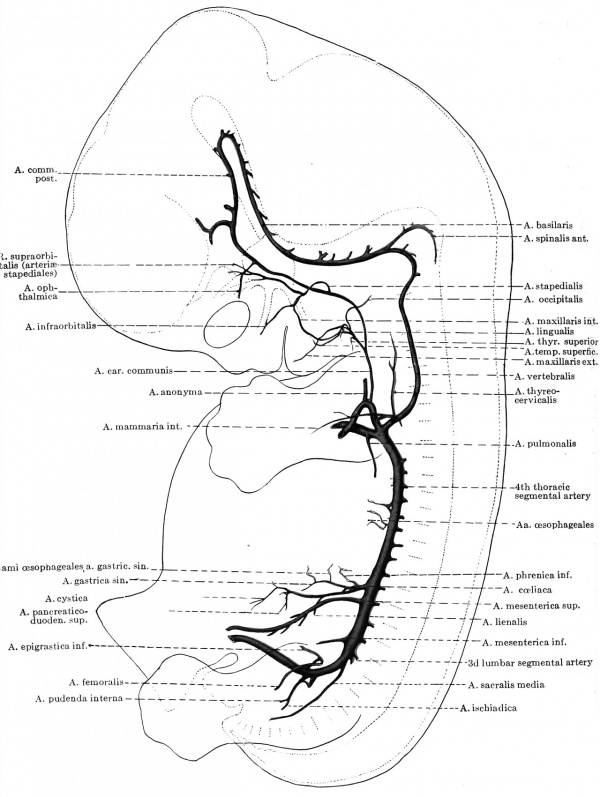
The only certain facts which have been established in the metamorphosis of the human arches into the trunks of the permanent vascular system have been incorporated in the diagram of Fig. 424. As far as their actual arch portions are concerned, the first two aortic arches are commonly lost, but the third and left fourth arches are retained, becoming the root portion of the internal carotid and the arcus aortae respectively. On the other hand, both the ventral and dorsal aortae beyond the position of the third arches are preserved, the former to furnish the stem of the external carotid, the latter the second part of the internal carotid
Fig. 424. Diagram of the aortic arches and their fate in man.
artery; whereas the ventral aorta between the third and fourth arches becomes the stem of the a. carotis communis. The corresponding part of the dorsal aorta disappears, so that now all of the internal carotid blood courses by way of the ventral stem. The sixth arch is lost on either side beyond the origin of the corresponding pulmonary artery, but on the right its proximal portion, between the truncus and the a. pul. dextra, persists and is the root portion of the adult right pulmonary artery. On the left side, however, this proximal portion of the pulmonic arch is apparently incorporated as part of the truncus pulmonis, and the adult a. pul. sinistra consequently is merely the exact analogue of the embryonic vessel (Bremer).[3]
This, then, is the general outcome of the arches, although we are now in the possession of some facts concerning the fate of the first two arches about which nothing hitherto has been known.
Before proceeding to consider details of the changes undergone by the various arches, mention may be made now of several kinds of shifting or growth displacements which affect these vessels and which make it easier to understand the relations which characterize the chief trunks derived from them in the adult. In the first place, as His clearly showed, the place of insertion of the aortic truncus into the anterior pharyngeal wall, whence it is split up into the arches, moves gradually lower down, so that, while at first the arches go off horizontally and even more caudally placed from the truncus, they soon course in an ascending direction from the caudally placed root stem. These changes have been described as a " moving down " of the insertion place of the truncus, and are doubtless due to the same phenomena of unequal growth which cause the apparent rapid descent of the heart from its earliest position at the end of the fore-gut. This change takes place in a regular and characteristic way, as Figs. 425, 426, 427 clearly indicate. Originally, when only two arches exist, the truncus may be described as splitting to send on either side of the gut an ascending and descending limb — the first and second arches respectively. Soon the full complement of arches is present, and the downward progression of the aortic truncus with respect to the gill bars now gives a different arrangement of its arches from the parent stem. Both of the first two arches arise together from an ascending stem, while the third arch courses back practically horizontally from the truncus and the last two come off together from a descending stem. Next an exaggeration of the length and importance of the common ascending stem for the first two arches (the stem which will later constitute the external carotid trunk) occurs and a truly ascending course for the third arch, although the latter has not yet been incorporated in the larger ascending trunk (Fig. 426). In the next changes which take place, the third arch has been carried up in the general ascending trunk (now the common carotid trunk), whereas the fourth tends to course more nearly horizontally. Eventually even the fourth and sixth arches come to have an ascending course.
|
|
Figs. 425 and 426. Reconstruction of the aortic arches in two human embryos, measuring 2.15 mm. and 3.2 mm. respectively. (After W. His, Anat. mensch. Embry., iii, Leipzig, 1885, p. 1S6, Fig. 119, and Atlas iii, Taf. ix, Fig, 12 and 15.)
Fig. 427. Reconstruction of the aortic arches in a human embryo 4.2 mm. long. (See Figs. 425 and 426.) whereas the fourth tends to course more nearly horizontally, Eventually even the fourth and sixth arches come to have an ascending course.
At the same time that these changes in the arrangement of the arches have been taking place, another of a more general nature has transpired, for not only the heart but the whole system of arches also has moved down toward the thorax. A reliable criterion of this general dislocation is furnished by the relation of the arches to the dorsal segmental arteries, for the latter have a fixed relation to the somites of the dorsal body wall. Before the stage of five millimetres, all the series of dorsal segmental arteries, including the hypogiossus artery, are considerably below the junction place of the sixth arch with the dorsal aorta. By the stage of seven millimetres this place corresponds to the first cervical dorsal segmental, by the stage of nine millimetres to the second vessel, and by the time the embryo has reached eleven and a half millimetres to the sixth or even the seventh cervical segmental, from which trunk the subclavian and vertebral arteries arise (Tandler). This relation is at last almost that of the adult, where the subclavian comes off the transverse portion of the aortic arch.
At the stage of seven millimetres, a splitting of the truncus begins, proceeding from above downward and separating the fourth arches, with the system lying above them, from the sixth ones. The latter then come to have an independent common trunk, — the truncus pulmonalis, — and this, as is well known, is exclusively connected with the right heart, whereas the truncus aorticus is similarly in relation with the left.
Still another growth change in the arrangement of these vessels is to be mentioned. We left the last three arches in a markedly ascending course. Such a course obtains for the pulmonic arches so long as they persist, but after the division of the truncus the systemic truncus elongates much, pushing, as it were, the proximal portions of the third and fourth arches again upward and giving them a horizontal or even slightly descending course (Tandler).
The dorsal part of the right fourth arch now atrophies beyond the origin of the subclavian stem, and this whole segment now constitutes but a branch of the persisting a. anonyma.
A. Carotis Interna and its Branches
It has already been emphasized that the earliest branch of any of the arches consists in that given off by the dorsal part of the first arch toward the embryonic mid-brain. This persists and is of increasing importance, and when the atrophy of the connecting portion of the dorsal aorta between the third and fourth arches results, it constitutes, together with this part of the dorsal aorta and third arch, the internal carotid artery. The internal carotid, then, consists of three morphologically different portions, — a proximal or root portion derived from the third arch, an intermediate portion consisting of the original aorta dorsalis from here to the first arch, and an end portion which is the earliest branch of the first arches and is the chief supply of the brain.[4]
It is to be noted that, besides the larger internal carotid which is given off from the end of the first arch, the aorta dorsalis also sends several smaller branches toward the hind-brain before the region of the primitive segments is reached, and, when, at length, the latter territory is reached, the dorsal segmental vessels. Those dorsal branches which are in front of the segmental area are very transitory, and attract onr interest chiefly because they represent the first vascular sprouts sent out by the dorsal aorta into the tissues of the embryo in this region and, directed toward the sides of the medullary tube, are directly responsible for the formation of the v. capitis medialis.[5]
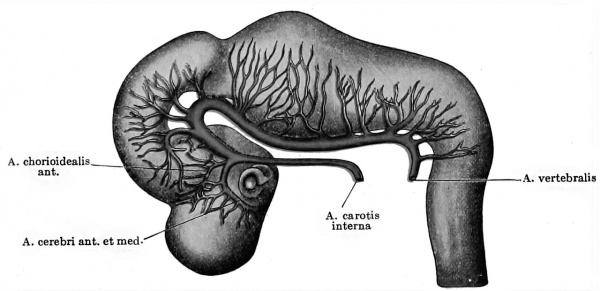
As soon as the region of the somites is reached the dorsal aortic branches are strictly segmentally arranged, — i.e., they course between successive somites. The pair between the first and second somites, however, early atrophy, and the pair situated between the second and third somites and which are in relation with the hypoglossus nerve remain somewhat longer and, as the so-called hypoglossus arteries, constitute the first of the series. In embryos of five mm. length (Tandler 1902, Ingalls 1907) the hypoglossus can be seen giving off a long longitudinal cranial-coursing branch, which headward anastomoses with the a. carotis interna on each side, thus making two long arterial arches. This branch of the hypoglossus artery is the a. vertebralis cerebralis. Later, as has been mentioned, the a. vertebralis cerebralis is taken over by the first cervical segmental artery, and the hypoglossal artery atrophies, and still later, as was first shown by Hochstetter (1890), an anastomosis between the first seven cervical segmentals (aa. vertebrates cervicales) enables the seventh of these vessels to act as the origin for the vertebral artery. De Vriese has pointed out that in all early embryos the carotid, after giving off the ophthalmic artery, may be considered as dividing into two terminal branches, anterior and posterior, the latter of which turns round to anastomose with the a. vertebralis cerebralis and is by far the more important of the two. When the cerebral vertebrals fuse to a basilar artery beneath the hind-brain, the two posterior terminal branches of the carotids consequently join each other in this trunk. This is the condition of the arteries in the head in embryos measuring nine millimetres (Fig. 428). Here the ophthalmic artery is not illustrated, but the carotid is seen splitting into its two terminal trunks, a small anterior and a strong posterior, the latter continued into the basilar. The anterior terminal trunk immediately gives off the anterior chorioidal artery and proceeds as a prominent vessel on the side of the fore-brain, encircling the optic cup from above and meeting its fellow of the opposite side just behind the olfactory pit. This vessel is the a. cerebri anterior, and gives off many rami to the cerebral vesicle, which are later represented by a single trunk, the middle cerebral. The posterior terminal branch of the carotid gives off many branches to the sides of the mid-brain, and these later are also represented by a single trunk, the posterior cerebral. In the next succeeding stages we see an increase in the of the opinion that we must consider the last-mentioned artery as being represented originally by all the small branches which come off from the carotid between the third and fourth nerves behind and the middle cerebral in front. In older embryos (48 mm. long) these many branches are represented by a large mesencephalic artery and a small true posterior cerebral (Mall) ; in older fetuses the latter branch absorbs the former.
Fig. 428.Graphic reconstruction of the arterial system in the brain of a human embryo 9 mm long. (After Mall, Amer. Jour. Anat., vol. iv, Plate I, Fig. 4.) (Mall No. 163)
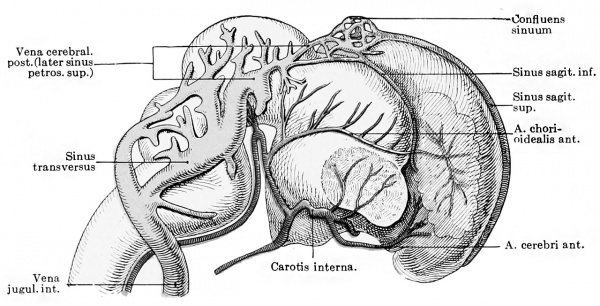
importance of the anterior chorioidal artery (Fig. 429), but it is remarkable that single large stems representing either the middle or posterior cerebral artery are very late in appearing. Mall is
Fig. 429. Graphic reconstruction of the vessels of the brain in a human embryo 38 mm. long. (From Kollmann, after Mall.) (Mall No. 145)
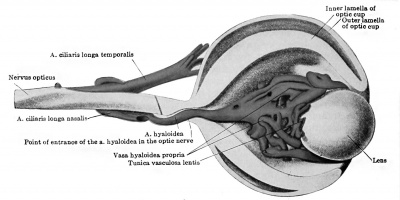
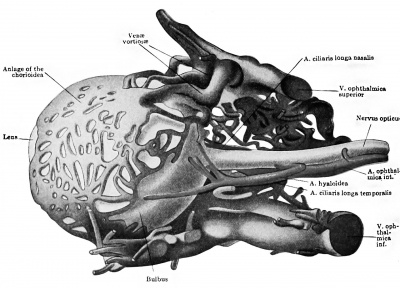
The ophthalmic artery is the first branch of the internal carotid to develop. In embryos measuring seven millimetres it can be seen to course toward the eye, dividing in its mid course into the a. ciliaris longa temporalis and a common trunk, afterwards splitting into the a. ciliaris longa nasalis and the a.hyaloidea. The latter artery pierces the optic cup, courses through the vitreous body, and reaches the posterior surface of the lens in capillaries. The arrangement and size of these branches of the ophthalmic are such that the a. ciliaris longa temporalis appears as the continuation of the main stem, and this is true up to the stage of 20 millimetres at least. The ciliary arteries supply a capillary plexus representing the chorioidea. Dedekind (1908) has reconstructed this simple vascular scheme in an embryo measuring 19 millimetres (Fig. 430 and 431). The hyaloid artery is noted by Dedekind as turning into an arterial plexus before being resolved into the capillaries constituting the tunica vasculosa lentis. Here, then, is another instance of several paths being used by the arterial blood before the reduction to a single path. The hyaloid artery serves as the later a. centralis retina, but no retinal vessels are present till late. The researches of 0. Schulze (1892) had indicated the same fact in other mammals. Versari (1903) has stated, indeed, that the human embryo reaches 120 millimetres in length before the retinal vessels are formed. In an embryo of 33.4 millimetres Dedekind has recorded the a. lachrymalis, aa. ethmoidales, and a. nasofrontalis.
We have as yet only an incomplete record of the development of the eye vessels in man, but Versari has furnished important observations on older stages (beginning with 22 mm.). In the splendid paper by Schultze the older stages in many mammals were beautifully portrayed, and some of the eye vessels in human fetuses of the sixth and eighth months shown. However, only Fuchs's careful study in the rabbit can lay any claim to completeness.
Fate of the Second Aortic Arches
As a rule, no trace of the first arch is seen in embryos of 7 millimetres and only the dorsal and ventral ends of the second arch are evident. Tandler (1902) has recently declared that in man and other mammalian embryos the dorsal parts of the second arches become the root portions of the stapedial artery on each side.[6]
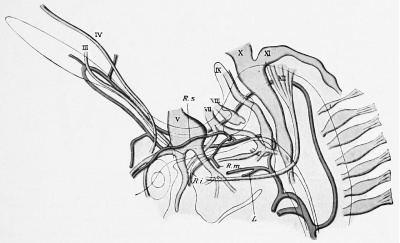
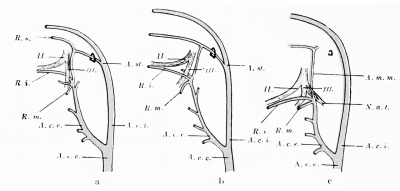
The a. stapedialis persists throughout life in some mammals, — e.g., the rat, — but normally atrophies in man. At the height of its development it possesses, after piercing the anlage of the stapes, three branches, which follow the three divisions of the fifth nerve ; these are the supra-orbital, the infra-orbital, and the mandibular rami, respectively. The first of these (ramus supra-orbitalis) leaves the main stem, shortly after the stapes is passed, so that the infraorbital and lower-jaw rami have a common stem (Fig. 432). The infra-orbital division of this stem passes behind the third division of the fifth nerve to gain the second division, which it follows. Later (in embryos of 15 to 17 mm.) the external carotid artery anastomoses with the common trunk for the infra-orbital and mandibular rami, just at the point where these vessels are given off. The infra-orbital ramus gains the outer side of the third branch of the fifth nerve by the development of an arterial loop around the nerve and the atrophy of the medial limb of the loop. Soon the original common trunk of the infra-orbital and mandibular rami (which lies above the point of the anastomosis witli the external carotid) becomes surrounded by the auriculo-temporal nerve and we can recognize in it the future a. menmgea media. Now the stapedial atrophies from its origin to its division place into the three rami, and consequently these branches are then all supplied by the a. carotis externa, the stem of supply for the supra-orbital branch being the old common stem for the two lower branches, in which the flow is now reversed ; this is, as has been said, the middle meningeal artery, whereas the ramus infra-orbitalis is the a. infraorbitalis of the internal maxillary, and the ramus mandibularis, the a. alveolaris inferior. This is clear from the diagrams in Fig. 433.
Fig. 430. Left eye of a human embryo 19 mm. long, opened through a horizontal section. X 66. (After Dedekind, 1908.)
Fig. 431. Left eye of the same embryo seen from the temporal side. X 66. (After Dedekind, 1908.)
Fig. 432. Profile reconstruction of the head vessels and nerves in a human embryo 12.5 mm. long. (After Tandler, Morph. Jahrb., xxx, Taf. v, Fig. 21.) R. s., R. i., R. to., ramus supra-orbitalis, infraorbitalis, and mandibulars of the a. stapedia; L., a. lingualis of the a. car. ext.
The place of origin of the stapedial artery and its relation to the stapes identify it accurately with the second visceral arch, but its territory of supply, when its three typical rami are developed, is entirely in the province of the first arch. This becomes intelligible when we know that these rami are later acquisitions of the stapedial, that primarily they arose from the first arch, and were later added to the a. stapedialis. Such, at any rate, is the case in the rat, as Tandler was able to show that the blood supply of the jaws (upper and lower) came originally from the dorsal part, of the first arch. To the stem supplying the jaws, a supra-orbital vessel was added, and then from the stapedial vessel an anastomosis with this common stem developed, whereby the three branches went to the a. stapedialis. This early history of the three stapedial branches has not as yet been secured in man, but the facts at present known make it none the less certain that the stapedial artery here has gained the territory of the first arch only secondarily. In man the three branches of the stapedial, instead of being derived from the dorsal end of the first arch, are probably derivatives of the ventral portion of that arch and the aorta ventralis.[7]
Fig. 433. Schemata showing the fate of the a. stapedialis in the human embryo. (After Tandler, 1902.) a represents the conditions present in a human embryo 17 mm. long, b those in one 19 mm. long, and c those in one 23 mm. long. II., second branch of the trigeminus; III. , third branch of the trigeminus; A.m.m., a. meningea media; A.c.c, a. carotis communis; A.c.e., a. carotis externa; A.c.i., a. carotis interna; N.a. t., nervus auriculotemporal; R.i,, ramus infra-orbitalis; R.m., ramus mandibularis; R.s,, ramus supra-orbitalis.
A. Carotis Externa
The trunk of this vessel may be considered the aorta ventralis from the origin of the third arches cranialward. His indicated that the lingual artery was among the first of its important branches to develop, and at 17 millimetres (N.T. 65) Tandler identified the superior thyroid, lingual, and external maxillary arteries. These vessels are, in fact, present at 14 millimetres, when the internal maxillary is also being evolved from the anastomosis of its trunk of origin with the stapedial (Fig. 434). At this stage one also sees a prominent branch of the carotis externa coursing dorsalward. This is the a. occipitalis, having the position and typical relations of this vessel to the muscle masses. Its proportionately 7 great development in these early stages is probably to be explained by its importance as a meningeal vessel.
 Fig. 434. Graphic reconstruction of the face vessels in a human embryo measuring 14 mm. (No. 144, Mall collection.)
Fig. 434. Graphic reconstruction of the face vessels in a human embryo measuring 14 mm. (No. 144, Mall collection.)
At 15.5 millimetres, the chief superficial branches of the carotis externa are evident, the a. auricularis posterior and a. temporalis super ficialis.
Nothing is known of the development of the coronary arteries. Tandler has noted their beginnings in a 17 mm. embryo (N.T. 65).
The only observations known to me (1904) on this subject are the fragmentary ones of Martin (1894) and those of F. T. Lewis (1904). Lewis has called attention to the fact that the heart of early embryos is nourished by diverticula of the ventricular lumen which course between the muscular trabecular — sinusoids of Minot, the chief method of nourishment of the myocardium in the lower vertebrates. Later the coronary system supervened and there was a great regression of the extensive sinusoidal system characteristic for the preceding stages. Lewis records the coronary arteries being first recognizable hi rabbits of 14 days and 18 hours.
Variations
The variations in the great vessels arising from the aortic arch have been known for a long time and could be explained satisfactorily on an embryological basis ever since the work of Rathke. They have been classified by Krause, for instance, and by so many, following him, that it will not be necessary to consider them here. De Vriese's work has shown the morphological character of the posterior communicating artery, — i.e., this vessel represents the original caudal continuation of the posterior terminal branch of the carotid. Consequently, cases in which the posterior cerebral arteries appear to be supplied by strong posterior communicating vessels, represent merely a retention of normal embryonic conditions, whereas the complete atrophy of the posterior communicating is an exaggeration of normal development. Islands in the course of the basilar are readily intelligible from the original paired nature of this vessel.
Fig. 435. Reconstruction of the lung anlagen and their vessels in a human embryo 10.5 mm long. (After His, 1887.)
Comparative
In the fish, amphibia, birds, and reptiles the internal carotid arteries are the sole source of supply for the brain, or nearly so, since the vertebrals are unimportant. The carotid in these classes divides into its anterior and posterior terminal branches, and the latter are continuous down the spinal cord with the anterior spinal artery, baring formed the basilar in the region of the hind-brain. This is the simple scheme represented in early mammalian embryos.
The development of the main vessels in the early lung is known to us from the observations of His (1S87). His showed that the two pulmonary arteries are from the first asymmetrical, in that the right vessel passes in front of the so-called eparterial bronchus, whereas the remainder of its course, like the entire extent of the opposite artery (a. pulmonalis sinistra), is behind the bronchial tree (Fig. 435). The pulmonary veins, on the other hand, are placed ventral to the bronchial system, and this relation persists throughout life, giving us arteries separated everywhere from veins by the corresponding divisions of the bronchial tree. 30 Flint (1906) has followed the developing vessels in the lung of the pig, more completely than has been done in the case of any other mammal. The pulmonary veins are reported by most observers as growing out of the sinus venosus before the development of the pulmonary arteries (see also Federow, 1910). In this connection, Flint has suggested that the early appearance of a drainage channel ventral to the pulmonary anlage and the ventral projection of the anlage from the walls of the foregut combine to favor the mechanical establishment of arterial paths dorsal to the organ. These early relations are only repeated in growth, and hence may be regarded as fundamental in determining the architectural interrelations of bronchial and vascular trees in the adult organ. In relation with this is the fact that the eparterial bronchus receives a ventrally placed arterial supply, and that here, consequently, the veins and arteries are accompanying vessels. It seems hardly necessary to refute the error of Aeby (1880) and others who attempted to make the arrangement of the arteries responsible for the form of the bronchial tree. As Flint has emphasized, the arteries are mere passive followers of the bronchi in development, and arise secondarily from the capillary mesh which enveloped a newly formed diverticulum of the bronchus.[8]
The Branches of the Aorta
As has been seen from the preceding description, the history of the development of the arterial system in the human embryo shows that at first two long channels exist — the descending aortae — which course through the entire length of the embryonic body and emerge in the belly stalk without having sent off any branches into the tissues of the embryo. The aortae and their system of branches, then, do not develop like many other vessels of the body, but pursue an elongated unbranched course over an area into which later they are destined to send out a copious supply of arteries. When, as development proceeds, capillaries are finally sent into the embryonic tissues, these sprout from the aorta, dorsally at strictly inter- segmental points, often ventrally and laterally also at such points, but in the case of these vessels usually more irregularly.
The segmental position is strictly observed only in the case of the dorsal branches. These from the first course only in the planes between the primitive segments. The ventral branches, however, are often found arising at more frequent intervals from the aortic wall, while the lateral branches, except the earliest stages, depart furtherest from a segmental alignment. Both ventral and lateral branches, however, show a tendency to adhere to the segmental plan.[9] Recent investigations on mammals and birds indicate that the branches supplying the limb arise from the aorta at multiple irregular points as a typical capillary plexus (see beyond), but are later segmentally arranged, as is the case in the earliest stages yet seen in man.
The aortic branches fall into three groups or rows, a dorsal row, a lateral row, and a ventral row. At first the dorsal segmentals supply only the central nervous system (the spinal cord and its ganglia), the lateral row, only the Wolffian body, and the ventral row, only the primitive intestine.[10] But of these branches, those which are at first purely neural in their area of distribution come eventually to supply also the body wall with its muscles and skin, and those at first purely nephric to supply also the gut branches which persist, however, supply, as they do in the embryo, the alimentary tract, the organs derived from it (liver, pancreas), and the spleen.
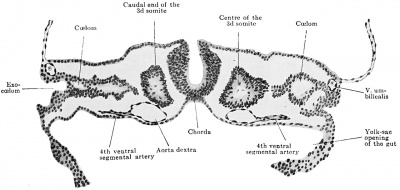
Fig. 436. Reconstruction to show the branches of the aorta in a human embryo with 23 somites (NT. 7). The reconstruction was made from six successive sections in the mid-thoracic region.
It is interesting to note that Mackay (1889) constructed a hypothetical schema classifying the branches of the aorta in a similar way, some twenty years ago. The main features of Mackay's classification are thus substantiated by development, for, though he confused some secondary with the primary characters of these vessels, he recognized that there were three kinds of them, naming them, from the influence of adult anatomy, the parietal, the intermediate, and the visceral branches.
The ventral branches arise first, owing to early importance of the vitelline circulation, the dorsal branches quickly after them, and, after an interval, the lateral branches. Although Eternod (1898) did not find any of these branches in his embryo of 1.3 mm. length, many of the ventral branches and two of the dorsal series occur in embryos with six somites (N.T. 3), while in an embryo with thirteen somites (N.T. 6) many distinct lateral branches can also be recognized. Both ventral and dorsal branches grow out before the primitive aorta? fuse, and consequently when this occurs an accurate apposition of the two aorta? permits these branches to come off in pairs from the single aorta descendens.
Fig. 437. Cross sections of injected chick embryos showing the development of the dorsal segmental vessels. A, cross section of a chick of 50 hours (24 somites), showing the loth dorsal segmental vessels; B, a chick 60 hours old; C, 78 hours old; and D, 116 hours old: all in the neighborhood of the 20th segmental vessels. S.A., dorsal segmental artery; P, C, posterior cardinal vein; S.V., dorsal segmental vein; S. C.P., spinal ganglion's capillary plexus; R.B., ventral radicular branch of the segmental artery; S., first extra-myotomal or skin branch of the segmental artery; A. C, a. centralis; S. P., superficial capillaries without the myotome; /., probable intercostal artery.
Dorsal Segmentals
(Neural Segmentals, " Segmental Arteries" (of many authors), Interprotovertebral Arteries (P. Albrecht), etc.).
The dorsal segmental branches of the aorta have often been referred to as the parietal or body wall segmentals, and, inasmuch as they furnish the large well-known intercostal and lumbar arteries, their segmental nature is preserved and recognizable in the adult. These later branches of the dorsal segmentals (i.e., aa. intercostales et lumbales) so far outstrip the primary trunks in growth that in the adult they themselves become known as the branches of the aorta, and the original dorsal segmentals merely as their posterior branches (rami posteriores). The course of development, however, shows clearly that the reverse is actually the case.
Fig. 438. Diagram of the behavior of a typical dorsal segmental artery in the human adult. (Founded on Toldt, Spalteholz, Sterzi, and Grosser.)
Fig. 439. The first dorsal segmental artery in a human embryo with 8 somites. (Collection of Professor Eternod, vide p. 594.) The endothelium is seen growing in the loose tissue of the first intersegmental cleft.
The dorsal segmentals begin to grow out from the aorta at about the time that the embryo possesses six somites (Fig. 410). The number of dorsal segmental arteries increases rapidly, and in embryos in which the extremities are recognizable, almost the whole series is present. The first pair of these vessels between the first and second somite early atrophies, although they are still clearly evident in embryos of 14 and 15 somites (N.T. 7 and embryo Graf Spee No. 52).[11]
The second pair constitute the vessels which are known as the hypoglossus arteries. These remain in embryos of five mm. in length, but shortly thereafter also atrophy, so that the first cervical pair — i.e., the arteries between the third and fourth somite, which course with the nn. cervicales 1 — are next the first of the series. As Hochstetter long ago showed for the rabbit, and as is evident for man from the Normentaf el of Keibel and Elze, the whole upper six of the cervical dorsal segmentals atrophy and the seventh only is permanent as the trunk of origin of the vertebral and subclavian arteries; this also functions as the root of origin for the eighth cervical and first (or first and second) thoracic arteries by its strong a. inter xo stalls suprema, so that the next permanent dorsal segmental behind the seventh cervical is the second or third thoracic one.
The following table shows the number of dorsal segmental arteries present in several young embryos.
| Dorsal Artery Number in Young Embryos | |||
|---|---|---|---|
| Designation of embryo | Number of somites | No. of dorsal segmental arteries. | Probable identity of the dorsal segmental arteries. |
| Pfannenstiel-Kroemer, NT. 3 | 6 | 2 | O1, O2 |
| Eternod | 8 | 4 | O1, O2; C1, C2 |
| Pfannenstiel III, NT. 6 | 13-14 | 6 | O1, O2; C1-C4 |
| Graf Spee No. 52 | 15 | 11 | O1, O2; C1-C8; T1 |
| Rob. Meyer 300, NT. 7 | 23 | 21 | - , -?; C1-C8; T1-T12; L1 |
| Broman, NT. 11 | ca 30 | 23 | - , O2; C1-C8; T1-T12; L1, L2 |
| G. 31, NT. 14 | 35 | 29 | - , O2; C1-C8; T1-T12; L1-L5; S1-S3 |
| Chr. 1, NT. 28 | 40 | 29 | - , - ; C1-C8; T1-T12; L1-L5; S1-S4 |
| Reference: Evans HM. The Development of the Vascular System - Arteries Table 1 | |||
| Keibel F. and Mall FP. Manual of Human Embryology II. (1912) J. B. Lippincott Company, Philadelphia. | |||
In their simplest form the dorsal segmental arteries consist of single capillary loops which extend from the aortas to the venae cardinales posteriores (Fig. 437, A), yet numerous other capillaries soon sprout out from these loops; and the aortic end of the original capillary loop becomes the dorsal segmental artery and the venous end the dorsal segmental vein.
Inasmuch as the dorsal segmental arteries constitute at first the arterial supply of the spinal cord, their history belongs to that of the blood supply of the cord.
With the exception of the brief account by His (1886), this subject has not been followed in detail in man; on the other hand, the main facts in the history have been ascertained for the birds (chick) and the mammalia (sheep, pig) by a series of injections, and the brief description given is based mainly on these.
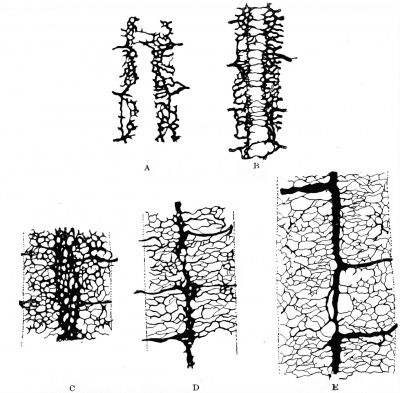
The single capillary loops which constitute the early dorsal segmentals approach the spinal cord near its ventrolateral angle and the ventral part of its lateral surface. In succeeding stages these loops give off delicate sprouts, which reach the cord at the area mentioned and anastomose with corresponding capillary sprouts given off by the adjacent segmentals, thus forming a longitudinal chain of capillaries on the lower lateral surfaces of the cord. These capillaries soon increase, growing over the spinal ganglia and forming a close plexus over the lower lateral surfaces of the cord, which extends dorsally as far as the under edges of the ganglia and their roots. Ventrally this plexus extends to the ventrolateral margin of the cord. Along the latter line sprouts begin to grow ventrally, and the earliest and more important of these, occurring near the chief trunks of the dorsal segmentals, represent the future aa. radiculares ventrales. As yet no capillaries have extended beyond the myotomes. Such are the conditions which occur in mammalian and human embryos until a body length of six or seven millimetres is reached. In the succeeding stages the blood stream in the segmental artery emphasizes in each case two main branches out of the many capillaries, an upper or dorsal and a lower or ventral branch. The upper branch courses just ventral to the spinal ganglion and the dorsal nerve roots, joining the general plexus that more intimately invests the cord just ventral to the line of emergence of the dorsal roots, — a. radicularis dorsalis; the lower branch courses ventral to the ventral roots, extending on to the ventral surface of the cord, — a. radicularis ventralis. In the next changes which occur the most striking feature is the behavior of the capillaries on the ventral surface of the cord. The. plexus which had previously begun to extend there advances from both margins until a line is reached on each side corresponding to the lateral limits of the bodenplatte; along this line they halt temporarily in their spread, thus producing a peculiar and highly characteristic vascular pattern which leaves the middle third of the ventral surface — beneath the bodenplatte — devoid of vessels but its outer thirds covered with a close net. The medial margins of this net are soon somewhat enlarged, constituting two parallel longitudinal vessels, the primitive anterior spinal arteries (tr actus arteriosi primitivi). Very soon delicate transverse capillary bridges cross the middle area which was previously non-vascular (Fig. 440). Some capillary sprouts arising from these primitive anterior spinal arteries push into the substance of the cord and course dorsally, ending usually within the gray matter of the ventral horns. These are the future aa. sulci (Adamkiewicz), or aa. centrales. This stage of double anterior spinal arteries was first seen in the human embryo by His (1886). It is probably most definite and typical for human and mammalian embryos from 9 to 11 mm. in length. His's observations showed it well marked in the human embryo of 10.9 mm. and still apparent in one of 13.8 mm.
Fig. 440. Successive stages in the development of the anterior spinal artery in the pig. The embryos were injected and the cord dissected in the region of the first three thoracic segments. A, an embryo 8.5 mm. long, B 9 mm. long, C 14 mm. long, D 15.5 mm. long, and E 28 mm. long.
The anterior radicular arteries contribute directly to the anterior spinal on each side, and the latter vessel is really to be viewed as merely a particularly prominent anastomosis between these aa. radicales ventrales. In like manner, in later stages, a strong arterial anastomosis develops between the posterior radicular arteries and is known as the posterior spinal artery.
To return now to the general development of the dorsal segmental vessels and their system of branches, we find, at the stage which we are considering, these vessels each possess two chief branches, the anterior and posterior radicular arteries, which are concerned respectively in the formation of the longitudinally coursing anterior and posterior spinal arteries, and which as development proceeds become separated more and more from the cord itself by the formation of the meninges, which (in the adult) they must pierce before reaching the cord.
But besides these two branches of the dorsal segmentals, another soon develops which sprouts out beyond into the skin. This is the representative of the trunk which later gives off both the muscular and cutaneous rami; the former do not as yet exist, so that the vessel may be said to be the ramus cutaneus dorsalis medialis (ramus posterior medialis of Grosser, Fig. 438). Below this another branch of the dorsal segmental now extends out ventral to the anlage of the rib. This, the intercostal sprout, represents the ramus anterior of the adult vessel. Its future great growth makes it the chief portion of the final vessel, but embryology shows plainly that the posterior ramus is the parent, and, again, that of the branches of this posterior ramus, the spinal branch is the primary or parent one and others (rami cutanei et museulares) secondary branches of it. From their origin to the point of division into posterior and anterior rami, then, the intercostal and lumbar arteries represent the original dorsal segmentals, but beyond the latter points they are entirely new and secondary formations. One may compare the above figures of the dorsal segmentals of embryos with the schema which I give in Fig. 438 to represent the adult.
Mall (1898) has shown that in the 16 mm. embryo anastomoses connect all the intercostal and lumbar arteries among themselves as well as with the subclavian above and the femoral below. In this way, then, arise the a. epigastrica inferior and the a. mammaria interna, and along with the rectus, nerves, and ribs shift later into the mid-ventral line (Fig. 441). He thus explains the formation of the superior intercostal artery: "The descent of the heart into the thorax on the inside with the descent of the arm over the clavicle on the outside of the body causes great tension on the upper intercostal arteries, and favors the new formation of blood-vessels in a more direct line. This is the reason why the main branch of the superior intercostal is a secondary and direct artery from the subclavian." Whereas the first two intercostals passed dorsal to the sympathetic chain originally, they now pass ventral to it.
Fig. 441. Arteries of the trunk in a human embryo 16 mm. long, showing the formation of the internal mammary and deep epigastric arteries. (Mall collection, 43.) (After Mall, Johns Hopkins Hospital Bulletin", 1898.)
Concerning the development of the muscular rami which belong to the dorsal segmentals little is known.
The cutaneous rami, though at one time thought to develop equally and symmetrically (Manchot, 1889), do not do so, as Grosser (1905) has recently been able to show. In fact, the segmental symmetry of these vessels is quite completely destroyed in the adult.
It is entirely probable tbat in tbe early stages of development tbe twigs which represent the blood supply of the skin are arranged perfectly symmetrically and segmentally. They doubtless correspond accurately with the segmental cutaneous nerve branches. Both, passing out from their source, find their territory of distribution opposite them and at the same level. But the skin does not keep its relation with the skeleton, but shifts over it, dragging, as it were, its nerves and vessels with it. Thus it happens that hi the adult the segmental vessels and nerves no longer supply the skin area opposite them. Since in the thoracic region this shifting is chiefly caudalward, the cutaneous nerves all supply territories lying below their points of emergence from the intervertebral foramina. The arteries, however, though tending to follow the same law, also acquire new connections with the skin territories secondarily opposite them, and accordingly also supply besides their own proper segmental area territory which originally belonged to the adjoining more cranial segments. Such a departure probably does not obtain in the nervous system, where we may perhaps rely on the innervation of a skin territory to reveal its primary segmental position. In the case of the vascular system the departure is doubtless due to the tendency of a blood current to take the shortest possible path — a fundamental law in the development of the vessels. Some others accomplish this shorter path by the employment of anastomoses normally existing between the various cutaneous rami, and so come to course not only downward with the nerve of their own original segment, but also directly outward with the cutaneous nerves of contiguous upper segments and emerge with the latter into the skin. The original segmental skin arteries of these more cranial segments thus vicariously supplied may no longer play any role in the supply of the skin and in this way the number of actual skin vessels is reduced. Another cause, besides this shifting and secondary assumption of a shorter path, operates to disturb a primary segmental symmetry in the skin vessels. This also is fundamental in the development of the vascular system — the tendency of favored vascular channels to annex contiguous ones. Such a tendency is shown to a remarkable degree in cases of certain twin embryos, where we appear to have a contest between the two hearts. In the skin plexus the favored channels supplying this net enlarge at the expense of others, and this may result in the complete assumption of the territories of some three original skin rami by the vessel originally belonging to only one. It is probable that this tendency would operate in the absence of any shifting of the skin even though it is encouraged by the latter, for it is unlikely that exactly equal conditions should obtain in the case of supply of all the segmental skin areas, and a disproportion once established is rapidly exaggerated. This is without doubt the reason why both the posterior rami (it. cutanei dorsales mediales et laterales) of a particular vessel seldom persist, usually the medial rami alone persisting in the upper segments and the lateral rami in the lower ones.
The further history of the anterior spinal artery may be briefly given here.[12]
His (1886) had noticed that in the human embryo of 18 mm. the single anterior spinal artery of the adult was finally present, and indicated that its definitive singleness was attained by a medial dislocation and fusion of the two primitive trunks, a process typified, for instance, by the well-known fusion of the two aortse. This view has never rested on any embryological evidence, Kadyi (1889), Hoffmann (1900), and others merely accepting it tentatively, following His. Although such a fusion seems to be actually the case in the elasmobranchs (Sterzi, 1904), in the higher vertebrates, and especially in all the mammalia, a series of more elaborate changes must occur before the single vessel is formed. These changes do not involve a fusion process, but consist essentially in the selection of one of the possible paths offered by the primitive vessels and a plexus which has sprung up between them. The single definitive vessel may thus be unilateral, median, or even oblique in origin (Sterzi, 1904, Evans, 1909). In the first case the adult vessel represents one of the original primitive paired vessels, in the other cases it is formed from the median plexus which connects the two primitive vessels.[13]
8 The single anterior spinal begins to be formed in human embryos when a length of about 15 to 16 mm. is attained. The irregular, " vacuolated " character of the young primitive trunk (Fig. 440, E) betrays its origin from the original plexus, as elsewhere in the developing vascular system.
Variations
The studies of Kadyi (1889), Burrows,[14] and others show that the form of the adult anterior spinal artery often bears the stamp of its method of origin, being median in some areas but in very many others truly right or left sided. In some areas it even retains its original plexus character (circuli arteriosi medullares), and in others consists of two strong parallel trunks which again unite, — e.g., Kadyi (1889), Taf. 3, Fig. 11.
His stated that the double aa. sulci were later shifted together in the midline, but this does not rest on evidence differing from that for his statement of the fusion of the anterior spinals. Usually, indeed, the aa. sulci or centrales are distinctly separate in man, even in the adult (Kadyi), thus disclosing their original paired origin from the primitive anterior spinals : a thing which Kadyi first discovered in man, Hoche (1899) in the rabbit and dog, and Sterzi has recently shown from many other instances to be the general mammalian plan.
Even in those rare instances in which some of the aa. centrales have a common trunk, this does not arise from fusion of the two original ones, but from the development of an anastomosis between these and the persistence of only one of the two penetrating trunks below the level of the anastomosis, as is normally the case in the birds (Sterzi). (Vide Sterzi's figure, page 311.) The aa. centrales are evident in chick embryos of the 96th honr and in sheep embryos of about 6 mm. In human embryos of 10-11 mm. they form two distinct rows of delicate vessels which enter the cord at the margin of the primitive ventral sulcus and, anastomosing on each side among themselves, produce two vertical or dorso-ventral planes of capillaries. These two rigid planes of capillaries form a striking picture of the internal circulation of the cord at this time.
This is the earliest method of blood supply of the cord in all the higher vertebrates, a sole exception being made for the urodelous amphibia, in which the first cord vessels penetrate from the lateral surfaces (Sterzi).
The further development of the cord vessels is as follows: Some time after the entrance of the aa. centrales into the cord, other vessels also penetrate it from the lower lateral surfaces opposite the level of the dorsal margins of the anlagen of the ventral gray columns (aa. periphericce). For a while, although both these ventral and lateral penetrating vessels exist, the dorsal two-thirds of the spinal marrow is still non-vascular. The whole lateral sides of the cord and its ganglia are quickly covered with the capillary plexus, but few if any sprouts have ventured on to the dorsal surface ( 7 mm. pig embryos) . Thus the cord presents the remarkable condition of a close capillary investment everywhere save on its upper surface, which is as yet non-vascular. However, this surface is now rapidly covered, at first by delicate transerse capillaries which bridge the gap just as they do at first between the primitive anterior spinals. Gradually then a close mesh is formed here. The gray matter of the cord is better and better supplied by secondarily arising penetrating arteries, which may arise as far dorsally as just beneath the posterior nerve roots (sheep embryos of 10^ mm.). Eventually the aa. periphericae exceed in importance the original aa. sulci, an event which occurs not only in man, but also in the rodents, artiodactyls, perisodactyls, and carnivores, in all of which the peripheral penetrating arteries come ultimately to supply the greater part of the cord substance. In the chiroptera and insectivores, on the other hand, the original ventral segmentals remain always the : chief arterial supply of the cord. The white matter of the cord is always supplied late, it remaining practically non-vascular in sheep embryos until a body length of almost 50 mm. is reached. Gradually there develop on each lateral half of the cord four longitudinal anastomotic chains; the first to arise and more important of these forms at or just medial to the line of exit of the posterior roots (sheep, 50 mm.). This is the posterior spinal artery of descriptive anatomy (tractus arteriosus postero-lateralis of Kadyi), and corresponds to the tractus arteriosus lateralis of most mammals. Next, a similar but weaker anastomosis develops along the line of exit of the ventral nerve-roots (tractus arter. ventro-lateralis) (tractus arteriosus antero-lateralis, Kadyi). Finally, anastomotic arterial chains are established dorsal to the dorsal roots (tractus arteriosus posterior, Kadyi), and opposite the ligamenta denticula (tractus arteriosus lateralis), the latter being peculiar to man and the apes. Of the various longitudinal venous trunks which develop, the order of establishment is similar to that for the arteries, the ventral, lateral, and finally dorsal appearing successively.
Anomalies of the Dorsal Segmental Arteries
As regards their manner of origin from the aorta, the dorsal segmental arteries show two main types of anomaly. They may (1) either disappear completely on one or both sides, their branches being taken over by the adjacent cranial or caudal segmentals, or they may (2) fuse with the vessel of the opposite side into a single median stem, a process normal to the ventral segmentals (vide infra).
Examples of the first type of anomaly are not infrequent in man, Krause having recorded cases in which as many as four interstitia intercostaliawere supplied by a single intercostal artery. It is interesting to note that such a condition occurs on one or both sides in the normal development of certain fish, amphibia, and birds. The second type of anomaly in which the two dorsal segmentals of one and the same segment fuse to a common stem is also common in man. Ernst has recorded a remarkable case hi which all the intercostal and lumbar arteries arose in this way, — i.e., for each segment from a single median trunk. Broman has found this second type of anomaly occurring in instances in the early embryo (13 mm.), and advances the notion that it occurs through an actual fusion rather than through the atrophy of one of the pair.[15] Many years ago Krause emphasized that the two places in which this anomaly was commonest were in the lowest intercostal and lowest lumbar regions, and Broman suggests that this is connected with the fact that the aorta? first fuse in the lower thoracic region and that a marked fusion process, normally bringing the roots of the two common iliacs together, occurs in the lower lumbar region. Common stems are normally produced in the ease of some or all the dorsal segmental pairs in some mammals, — Lepus (Ernst), Halichoerus (Hepburn).
Fig. 442. Reconstruction of the aorta and its branches in a human embryo 3.4 mm. long. (After Broman, 1908.)
The Ventral, Segmental Arteries
(Gut Segmentals, Yolk Segmentals, "Visceral Circle" Mackay)
The first branches to be given off by the aortae, if we except the precocious and immense umbilical arteries, are those which course on to the primitive gut and the yolk-sac. Here the primitive aa. vitellinae were first seen in the human embryo by Mall (1897).
Bischoff (1842) has usually been given credit for the discovery of the row of yolk arteries given off by either aorta; his observations were made on the rabbit.
Von Baer (1827), however, had preceded him, for in his " de ovi ruammalium et hominis genesi epistola " (Fig. VII a) he shows some six or seven pairs of yolksac arteries in a young dog embryo.
When the two aortae have met and fused, opposite ventral arteries are quite accurately matched, as is always the case with the dorsal segmental arteries, so that from the now single aortic tube there go off at many places pairs of ventral or gut arteries which are also often accurately segmentally (i.e., intersegmental^) placed.
It should be mentioned, though, that, while this is the case for most of the aorta's length, in its most cranial portion the ventral branches have perished before the aortic fusion has taken place, so that a condition of paired ventral vessels from the single aorta does not ever come about in this region, — i.e., in the territory of the occipital and six upper cerical segments.
The most cranial lying ventral branches are very transitory, and the very first of them have entirely escaped notice until recently. In the Mall embryo No. 391 (Dandy, 1910) possessing seven somites, the ventral or gut branches extend as far forward as the first intersegmental cleft (Fig. 408). By the time the embryo possesses fourteen somites (2.1 mm., Mall, 1897, Pfannenstiel III, N.T. 6) the most cranial ventral branches appear in the region of the fourth and fifth somites. In the embryo with twenty-three somites (Robert Meyer, No. 300, N.T. 7) the ventral vessels opposite the next three caudally lying somites are also in degeneration, so that the vessels near the beginning of the eighth somites constitute the first of the functioning series.
In the Broman embryo of 3 mm. (N.T. 11) (Fig. 422) the ventral vessels opposite the 7th cervical dorsal pair constitute the most cephalic of the series, and this pair is probably the most cranial of the ventral branches to persist long enough for fusion of the aortae to occur in their neighborhood.[16] By the time the embryo attains a length of five millimetres, all of these ventral pairs have given place to single median stems (Fig. 443). Broman (1908) believes this to take place first in the middle of the unpaired aorta and to have proceeded cranially and caudally from this point. In a human embryo of five millimetres which Tandler (1903) has described, all of the ventral pairs have "fused" and there exists a complete series of unpaired or median ventral segmentals from the seventh cervical to the second lumbar segments inclusive. Broman (1908) describes these vessels as representing in each case a fusion of the original segmental pairs, and not, as has been supposed (Thane, 1892, and others), persisting right or left members of the original pairs ; but it is possible, as Felix remarks from his study of the embryo of 23 somites, that this is often not the case, since here occasionally right members of the ventral pairs were already larger. The question is an open one.
Broman has attempted to explain the normal fusion of the ventral segmentals, in contrast to the persistence of the paired condition which the dorsal segmentals exhibit, by affirming that the ventral vessels are from the very beginning placed nearer each other than are the two dorsal stems. This statement, of course, will not hold, as can be seen from the study of younger embryos than were at his disposal (Fig. 444). The coalescence of the ventral segmentals is doubtless connected with those forces which pull the intestine farther away from the aortic wall to produce the dorsal mesentery.
Fig. 443. Reconstruction model of the aorta and its branches in a human embryo 5 mm. long. (After Broman, 1908.) The cranial end of the right mesonephros and the position of the metanephric anlage are indicated by dotted lines.
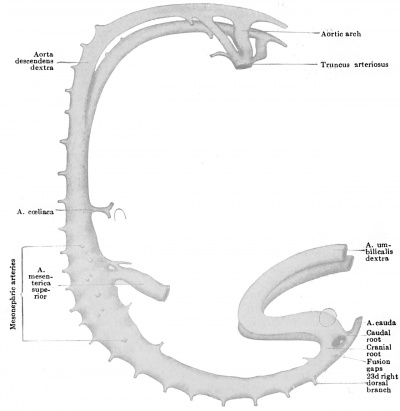
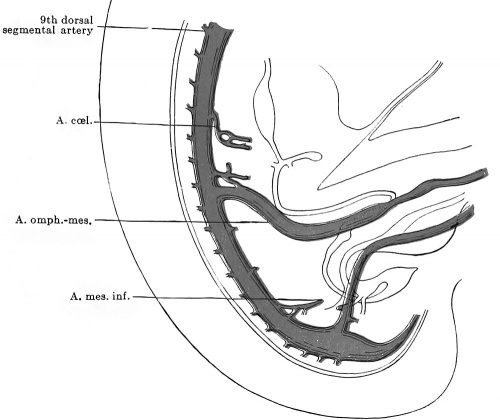
It is quite possible that the seventh pair of ventral segmentals remain longer than those above them just because they function as one of the roots of the cceliac artery. At the stage of five millimetres, although the series of mid-ventral segmentals may be uninterrupted, some of the members of the series are already much exaggerated over the remainder and enable us to recognize them as forming the cceliac and omphalomesenteric arteries respectively (Fig. 445). The former vessel arises by two roots from the seventh and eighth ventral segmentals and, coursing ventrally, forks, the two branches being traceable forward toward the portion of the alimentary canal from which later the stomach and liver are respectively derived. The omphalomesenteric artery is by far the largest of the ventral series, and, while its main trunk is the continuation of the thirteenth segmental vessel, the four ventral segmentals cranial to this also share in giving origin to it, for they are connected with this artery by a series of longitudinal anastomoses. As can be seen from Fig. 445, the omphalomesenteric artery splits on reaching the intestine and surrounds the latter at its junction with the ductus omphalo-entericus, with an arterial ring, before proceeding on its way to its final field of distribution on the yolk-sac. Fig. 446 shows conclusively that the left limb of this ring has atrophied, since the artery now passes entirely on the right side of the gut.
Fig. 444. Cross section of a human embryo of 7 somites, showing the primitive ventral (segmental) branches of the aorta. The yolk-sac is so spread out that these branches appear as lateral derivatives of the aorta, although later ventral. (After a drawing kindly placed at my disposal by Dr. Walter E. Dandy.)
Anomalies
Sometimes a considerable part of the old omphalomesenteric artery persists in those rare cases of the most primitive type of Meckel's diverticulum. In such cases what is undoubtedly the original artery courses beyond the gait and it? diverticulum to the umbilicus, and a dotprmination of nu which side of the gut the vessel courses will disclose whether the right or left limb of the early arterial ring has persisted. All of the more advanced types of the diverticulum, in which the process is merely supplied by an unusually strong vessel but in which the old trunk cannot be identified with certainty, must be inadmissible for the determination of this point, for the diverticulum is a healthy functioning pocket of the bowel and as such could have secondarily attracted for its supply branches from the vessels of either contiguous wall of the intestine.[17]
Fig. 445. Sagittal reconstruction showing the aorta and its branches in a human embryo of 5 mm. (After Tandler, Anat. Hefte, Bd. 23, p. 192, Fig. 1.)
Opposite the lower colon, no one of the ventral segmental arteries is especially enlarged above its fellows, and the equal part which all of them play in the nourishment of this part of the bowel prevents us from identifying any one of them as the a. mesenterica inferior. Nevertheless, in an 8 mm. embryo the latter artery is apparent as the 20th ventral segmental (Broman, 1907).
In the succeeding stages in the life of the embryo, the vessels which we must recognize as the cceliac, superior mesenteric, and inferior mesenteric respectively are all found at successively lower levels on the aortic wall, a fact which is to be correlated with the descent of the intestinal viscera (their territories of distribution) into the abdomen. This highly interesting phenomenon, the so called "caudal wandering" of the visceral arteries, was first discovered by Mall (1891), and has since been abundantly confirmed and extended by the studies of Tandler (1903) and Broman (1908). The subjoined table shows the position of these vessels in a number of human embryos during the time of their migration (p. 648).
Fig. 446. Sagittal reconstruction showing the aorta and its branches in a human embryo measuring 9 mm. (After Tandler, Anat. Hefte, Bd. 23, p. 197, Fig. 2.)
The coeliac artery thus wanders from the seventh cervical to the twelfth thoracic segments, a displacement of some eleven segments, and the superior mesenteric artery almost equally as far (ten segments, second thoracic to first lumbar) ; whereas the inferior mesenteric artery wanders through but three segments (twelfth thoracic to third lumbar). The great change which the levels of origin of the first two vessels undergo, in contrast to the slight one of the third, is readily intelligible from the proportionately great dislocation which the upper part of the alimentary tract undergoes. All of these vessels usually attain their adult levels by the time the embryo is 17 mm long.
This shifting of the intestinal arteries is not produced by a displacement of the aorta on the vertebral column, but is an actual shifting of these ventral branches when compared with the dorsal branches of the same trunk.
| Dorsal Segmental Artery Position | |||||
|---|---|---|---|---|---|
| Length of embryo | Position of a. coeliaca | Position of a. mes. sup. | Position of a. mes. inf. | Observer | |
| 1 | 4.9 mm | C. 7 | T. 1, 2, 3, 4. | Ingalls | |
| 2 | 4.5 mm | Betw. C. 8 and T. 11 | T. 2 and T. 3 | Broman | |
| 3 | 5 mm | C. 7 and C. 8 | T. 1, 2, 3, 4, 5 | Tandler | |
| 4 | 5 mm | C. 8 and T. 1 | T. 4 and 5 | Broman | |
| 5 | 6.75 mm | T. 2 | T. 5 and 7 | Keibel and Elze | |
| 6 | 7 mm | T. 5 | Betw. T. 5 and 7. | L. 1 | Elze |
| 7 | 8 mm | T. 2 | T. 4, 5, 6 | T. 12 | Broman |
| 8 | 9 mm | T. 4 | T. 5, 6, 7 | T. 12 | Tandler |
| 9 | 9 mm | T. 4 | T. 6, 7 | L. 1, 2 | Tandler |
| 10 | 10 mm | T. 8 | T.9, 10 | L.2 | Broman |
| 11 | 10.3 mm | Betw. T. 7 and T.8 | T.9, 10 | Betw. L. 1 and 2 | Broman |
| 12 | 11 mm | T. 6, 7, 8 | T.8, 9 | L.3 | Broman |
| 13 | 11.7 mm | Betw. T. 7 and 8. | T. 9 | Betw. L. 1 and 2 | Broman |
| 14 | 11.7 mm | T. 9 | T. 10 | L.2 | Broman |
| 15 | 12.5 mm | T. 8 | T. 10 | L.2 | Tandler |
| 16 | 13.2 mm | T. 8, 9 | T. 10, 11 | L. 2 | Broman |
| 17 | 14 mm | T. 10 | T. 10, 11 | L.2 | Broman |
| 18 | 14.5 mm | T. 9, 10 | T. 11 | L.2 | Tandler |
| 19 | 14 mm | T. 10 | T. 11 | Betw. L. 1 and 2 | Evans |
| 20 | 14 mm | T. 11 | T. 12 | L.2 | Tandler |
| 21 | 15.5 mm | T. 11 | T. 12 | L.2 | Evans |
| 22 | 16 mm | T. 12 | T. 12 | L.3 | Broman |
| 23 | 16.2 mm | T. 11 | T. 12 | Betw. L. 2 and 3 | Broman |
| 24 | 16 mm | T. 11 (lower part) | T. 12 (upper part) | L. 2 | Evans |
| 25 | 16 mm | T. 12 (upper part) | T. 12 (lower part) | L. 2 (lower part) | Evans |
| 26 | 17 mm | T. 12 | L. 1 | L. 3 | Tandler |
| 27 | 19 mm | T. 12 | L. 1 | L. 3 | Broman |
| 28 | 19 mm | T. 12 (lower part) | L. 1 | L. 3 | Evans |
| Ingalls - Zwischen dem f iinf ten und seehsten Rumfganglion findet sich ein bis an den Darm verfolgbares Gefass, das vielleieht als a. mes. inf. anzusehen ist. | |||||
| Reference: Evans HM. The Development of the Vascular System - Arteries Table 2 | |||||
| Keibel F. and Mall FP. Manual of Human Embryology II. (1912) J. B. Lippincott Company, Philadelphia. | |||||
43 The exact manner in which this wandering of the gastro-intestinal vessels is accomplished has not as yet been established. Undoubtedly one possible method in early stages is by means of the anastomoses which connect the ventral vessels. This, however, will only account for very early shiftings, for the studies hitherto made show that very soon there may not be a single other vessel between the points of origin of the three chief vessels (e.g., Tandler's embryo K.S.). Consequently other methods have been called on to explain this caudal wandering. These are — 1. That it takes place through the formation of special non-segmental anastomoses between the wandering arteries and the aortic wall below them, with the ensuing atrophy of the older roots. The chief evidence in favor of this view consists in the frequent presence of non-segmental roots of origin for these vessels. The original roots being all supposedly segmental, any non-segmental position for the vessel is explained by the acquirement of secondary non-segmental roots. Such a view overlooks the fact that even in the beginning non-segmental ventral branches are present (see, for instance, the vessels in Broman's Fig. 1, page 646).
Regarding the development of the peripheral branches of these arteries in man almost nothing is as yet known.[18] Tandler has identified the a. pancreatico-duodenalis superior in an embryo 13 mm. long (N.T. 57). At 15.5 mm. (Mall's collection, 390) the coeliac axis possesses the following branches: a. phrenica inferior, a. gastrica sinistra with oesophageal rami, a. hepatica with its a. cystica (strongly developed), a. pancreatico-duodenalis superior, and a. lienalis (Fig. 447).
Interest attaches to the development of the ventral branches which the adult aorta is known to send to the (esophagus, especially as to whether these also are descended from the early segmental branches. Some of these aa. oesophageales have moreover been identified in relatively early stages, but they are apparently new formations.[19]
2. That it takes place through an active ventral wandering, by which it is understood that the caudal wall at its junction with the aorta bulges itself out, while the cranial wall at a corresponding place is taken up by the aortic wall. There is no evidence for this view.
In discussing the subject it is to be pointed out that the cceliac and superior mesenteric arteries have their roots in an uninterrupted chain of anastomosing vessels, and there is no a priori reason why the vessel functioning as the superior mesenteric in one stage may not subsequently be used as the coeliac channel. As the area of distribution of one of these vessels shifted caudally, the blood stream could adapt itself to a more direct path by the employment of these anastomoses which enable it to come from successively lower segments of the aortic wall.
It seems to me most probable, however, that the identity of the three main vessels is established permanently very early, and that the great shifting is due to an entirely different -phnomenon, — namely, to the unequal growth of dorsal and ventral walls of the aorta. Attention may be called here to the remarkable shifting undergone by the fourth aortic arch, for instance, compared with the dorsal segmental vessels, and yet the arches have not been thought to climb down by special secondary roots, etc.
Fig. 447 Graphic reconstruction of the arterial system of a human embryo 15.5 mm. long, which had been injected while the heart was still beating by Mr. Broedel. The embryo was subsequently cut into a series of sagittal sections. (No. 390, Mall collection.)
As far as I know, nothing has been ascertained concerning the development of the bronchial arteries. In the embryo of 15.5 mm. (Fig. 447) three ventral branches of the aorta are seen to constitute aortic vasa vasorum.
The main branches of the mesenteric arteries are formed very early and can be identified in mammalian embryos well under 10 mm. in length. From the time of the earliest existence of the ventral segmentals, the gut is supplied with capillaries, and in the early embryo these form a close plexus in the tissues of the simple intestinal tube.
The earliest capillaries plexify in a fairly definite plane which corresponds to the future submucosa. This tunic — the so-called " tunica vasculosa " of the older anatomists — contains, as is well known, the chief plexus of intestinal vessels in the adult; there the chief vessels of the intestinal wall are found, and it is from them chiefly that the muscular rami and all of the mucosal rami are derived. This fact finds a better comprehension from the history of the vascularization of the gut wall, for in the submucosa the earliest and hence oldest vessels are found. From this layer of vessels, with the progressive development of the muscularis and the mucosa, there sprout out the rami which nourish these tunics. When the first villi are formed they receive simple capillary loops and sprouts; from the capillary plexus of the older villi, the villous arteries and veins are formed. The increase in complexity of the proper intestinal vessels proceeds from above downward, just as does the development of the intestinal walls and especially the villi; the vessels of the small intestine much precede in complexity those of the large bowel, and the latter portion, for a long time smaller in girth, remains supplied only with a single, simple, submucosal net at a time when the small gut has manifold muscular and mucosal rami.
Anomalies
The coeliac and superior mesenteric arteries sometimes arise from a common trunk — the so-called " cceliaco-mesenterica," Rathke. This is an entirely normal condition in the Anura, some of the Chelonia and Lacertilia, and some of the Mammalia (PhocaBna [Cuvier], Talpa [Tandler], Echidna [Hyrtl], etc.). The formation of such a trunk has been interpreted as due to the approach and fusion of the cceliac and superior mesenteric arteries (Howes, Klaatseh, Fransen, etc.). Tandler (1904), however, has studied the embryonic development of Talpa, in which this occurs as a part of normal development. He finds a strong longitudinal anastomosis between the various early segmentals of the cceliac and superior mesenteric group. Only one of these early segmentals remains as the permanent trunk, and it has as its chief cranial branch a longitudinally coursing vessel, which is doubtless the old longitudinal anastomosis between the segmental series, the cranial members of which have now degenerated. From this longitudinal vessel the gastric (sinistra), hepatic, and splenic arteries are later distinguished as arising. The main part of the permanent trunk is the omphalomesenteric channel; in this way, then, the anastomosis enables the latter vessel to take over the branches which usually belong to the cceliac. Tandler has applied these findings to explain also the anomalous occurrence of an a. cceliaco-mesenterica in man. If his schemata are interpreted liberally as signifying any mesenteric anastomoses by virtue of which one vessel can take over the whole or part of its neighbor, they deserve to stand as the most reasonable and plausible explanation for these anomalies. It is significant that it is always the stronger vessel — the a. mesenterica superior — and never the weaker cceliac which performs the annexation, a fact in conformity with our general ideas of the method of development of the vascular system. Tandler in fact recognizes a general anastomosis between the branches of aorta in this region, constituting, as it were, a general cceliaco -mesenteric complex. Normally there occurs a later separation of the cceliac and mesenteric systems. Broman, on the other hand, thinks that from the earliest time at which they can be recognized these two vessels with their multiple roots are entirely separate; this is because the human material hitherto explored has not revealed a complete chain of anastomoses between the two vessels, as it has in Talpa. The limitations of method of attack here make it probable that these vessels can not always be seen and that future researches will show them present. If they are not present, another method of formation of a truncus cceliaco-mesentericus may be the correct one; this is the active outgrowth of a wandering root from the cceliac which attaches itself to the superior mesenteric rather than the aorta (Broman).
The rather commoner, longer anastomoses between the cceliac and upper mesenteric arteries are doubtless more secondary developments from the plexus in the primitive mesentery. (In this category are to be placed the cases reported by Aeby, Biihler, Fawcett, Tandler, Thane, Toldt, and others.) The superior mesenteric artery has also been reported as taking over the field of the inferior mesenteric (Fleischmann, 1815), but this is doubtless an anomaly of the greatest possible rarity, because the lower vessel is initially so far removed from the superior one as to be from the beginning a far more effective supply for the bowel which is opposite it.
Lateral Branches
(Nepkric Segmentals, Intermediate Arteries (Mackay), etc.)
Mention has already been made of the occurrence of primitive lateral branches of the aorta in human embryos of 15 and 23 somites (see Fig. 436). The relation of these vessels to the lateral branches of the aorta present in embryos of 4 to 5 mm., and which are now clearly concerned in the supply of the Wolffian body, is not clear, and will not be so until intermediate stages are possessed. I shall discuss here only the latter arteries, which we may designate simply as lateral branches of the aorta or the mesonephric arteries.
His (1880) first observed multiple branches of the aorta supplying the mesonephros in a seven millimetre embryo, and Mall (1891) emphasized the tendency of these to be segmentally arranged in early stages.[20]Broman has recently given a more extended account of them and their fate in a series of embryos, and I follow him.
At first, when the "Wolffian bodies are relatively small, the number of mesonephric vessels is correspondingly small and these come from only the middle portion of the aorta (2d to 8th thoracic segments) ; but when, at the end of the first month, the mesonephros reaches its greatest development, it receives many direct branches from the aorta at levels cranial as well as caudal to the original ones. The following table will indicate this :
| Mesonephric Arteries in Young Embryos | |||
|---|---|---|---|
| Length of embryo | Level of origin of mesonephric arteries | No. of mesonephric arteries on each side | Observer |
| 5 mm | 2d to 8th th. segments | 7 | Broman (1908) |
| 5 mm | 1st to 12th th. segments | 13 | Tandler (1903) |
| 7 mm | 8th cerv. to 12th th. segments | 14 | Mall (1891) |
| 8 mm | 8th cerv. to 12th th. segments | 20 | Broman (1908) |
| Reference: Evans HM. The Development of the Vascular System - Arteries Table 3 | |||
| Keibel F. and Mall FP. Manual of Human Embryology II. (1912) J. B. Lippincott Company, Philadelphia. | |||
The last vessels added to the series appear to grow out from the level of the first lumbar to second lumbar segments in embryos of 10 millimetres. These indeed are destined to persist in the adult representatives of these arteries, for all the remainder atrophy by the time the embryo is from 16 to 19 mm. long. When the sex glands and the adrenal arise, they are supplied by branches from many of the neighboring mesonephric arteries.
Gradually the sex gland loses all but a single one of its many arteries, and this is the branch from the mesonephric vessel opposite the second lumbar segment. The atrophy of the Wolffian body permits the entire blood stream in this artery now to supply the sex gland, and thus the a. spermatica interna appears to be a direct branch of the aorta (Hochstetter for mammals, Broman for man).[21]
The branches of the mesonephric arteries to the adrenal gland are originally many (6 at least), and come off from the higher members of the series, — e.g., in a 10 mm. embryo, from the mesonephric arteries arising from the sixth to eighth thoracic segments. But eventually, with the relative descent of the adrenal, it acquires branches from the Wolffian body arteries at lower and lower levels. At last in 16 mm. embryos the adrenal arteries are branches of three mesonephric vessels near the first and second lumbar segments.[22] With the atrophy of the Wolffian body, these three adrenal arteries persist and consequently take over the entire blood current, thus appearing as three independent branches of the aorta. Before the adult state is reached, the upper and lower members of the series of three adrenal vessels acquire important secondary connections, for the latter comes to supply the permanent kidney (a. renalis),[23] and the former the diaphragm (a. phrenica inferior). These secondary fields for the upper and lower adrenal vessels soon exceed in importance their adrenal territory, and so, in the adult, we only speak of the upper and lower adrenal arteries as small branches of the large renal and inferior phrenic vessels, though embryologically the reverse is the case. The a. renalis soon takes a descending course, and only in the second half of fetal life does it appear transverse.[24] Luna (1908) has shown that the a. phrenica inferior does not surpass its adrenal branch in size until about the seventh embryonal month.
As a result of all observations hitherto made, it may be stated that the permanent kidney in mammalian embryos certainly does not receive any large and readily appreciable arterial supply until its definitive position is reached. Hochstetter has stated that the vv. renales also wait such a time for their development. These facts, however, can not be taken to mean that the renal anlage possesses no circulation during the important early period of its development. For it can be shown, even from ordinary histological sections, that the kidney during this time possesses many small vessels in its walls, and Broman (1907) has recently traced connections between these and the posterior cardinal veins, on the one hand, and with the efferent Wolffian body veins, on the other.[25] This is not the only source of blood for the early metanephros, for injections of mammalian embryos (pig) indicate that its capillaries receive arterial blood from the a. sacralis media (Fig. 448) and inferior mesenteric artery. (See Jeidell, 1911.) Variations. — Supernumerary renal arteries have been known for a long time (Macalister [18831 records a case of seven), but until the embryology is accurately known explanations for their occurrence will be highly speculative, as they have been in the past. From the time of Meckel onward, there have been observers willing to postulate a hypothetical " splitting " of the usual single renal artery to explain this! (e.g., Kolster, recently). However, other observers have stated their belief in the derivation of supernumerary aa. renales from Wolffian body vessels, and Bromans derivation of the normal a. renalis from this source makes this explanation of multiple renals the most plausible. The abnormal origin of the renal artery in common .with other trunks is of some interest, inasmuch as we can now explain a large number of these embryologically. The inferior phrenic, adrenal, and sexgland arteries being derivatives of the original mesonephric vessels, all combinations in the origin of the former vessels may be expected. Thus the origin of the a. spermatica interna from the a. renalis is not uncommon, the common origin of a. renalis and a. suprarenalis inferior is normal, and the other adrenal vessels may likewise come from the renal. It is now possible, in view of new observations on the earliest blood supply of the metanephros, that certain types of origin of the renal artery from lower sources — e.g., from the a. mesenterica inferior or a. sacralis media — represent the retention of its first vascular connections when the gland was pelvic in position. There still remain, of course, many remarkable anomalies of all these arteries which indicate entirely secondary shiftings or connections, — e.g., the origin of the a. spermatica interna from certain lumbar arteries. Broman has emphasized that the mesonephric arteries may come off at variable points from the lateral aortic circumference, many of them, in fact, being ventrolateral derivatives. It is easy to understand how, in the latter cases, in further growth the mesonephric artery may come to be incorporated with a contiguous ventral branch of the aorta. The most common instance of this is afforded by the common origin of coeliac and inferior phrenic arteries.
Fig. 448. Arteries to the permanent kidney in a pig embryo 14 mm. long, after an injection of the living embryo. The arteries in question are the small upwardly-directed branches which arise from the a. sacralis media and the lateral plexus formed by the a. sacralis media. The same plexus is seen to give rise to the aa. segmentales dorsales on each side.
End Branches of the Aorta
(Caudal, Lower Limb, and Pelvic Arteries)
In all vertebrates in which the hind limbs are important, the aorta does not appear to go over insensibly into the a. caudalis, but is rather drained of most of its blood by the mighty iliac branches, which we have come to speak of, in addition to the caudal vessel, as the end branches of the aorta. The simplest arrangement of the aortic end branches is that seen in man, and involves merely a tripartite division" into the two common iliacs and the a. caudalis (a. sacralis media).[26] In many mammals, including man, later shifting makes the sacralis media appear as a dorsal derivative of the aorta and not as its direct continuation, — e.g., in the human adult it almost constantly arises cranialward from the "bifurcation place" of the aorta.[27]
In the human embryo we have seen that the tremendous importance of an early placental circulation has "pushed forward" the development of the umbilical arteries so that they much precede of course the appearance of limb arteries.
Studies on early embryos show that the umbilical artery is relatively farther cranial in position than it subsequently comes to be, — i.e., it appears to wander caudally. We have seen that thp primitive umbilical arteries possess many roots of origin from the aorta which are in fact only the caudal members of the general vitelline series (aa. vitelline).[28]
In very early stages this caudal migration of the umbilical artery is unquestionably brought about by the caudal growth of the aorta itself together with its intestinal branches, the whole forming a plexus with which the umbilical arteries are constantly in relation and by means of which the blood to them gradually flows in more and more caudally placed ventral branches. Thus, in the Kroemer-Pfannenstiel embryo of 6 somites (N.T. 3), these vessels arise at about the level of the future seventh or eighth segment, — i.e., the fourth cervical somite. In embryos measuring less than 4 mm. the artery is almost at the level of the first lumbar vessels. It is probable that the single or at most double roots which the a. umbilicalis possesses at this stage are its final ones which belong to the original vitelline series. These roots, however, are themselves displaced or "wander" caudally, so that in embryos of 5 mm. they are found at or slightly below the level of the third lumbar vessels.[29]They do not, however, constitute the definitive roots of these arteries, for, as Hochstetter (1890) some years ago showed for rabbits, the umbilical arteries of mammals next gain a more laterally placed root of origin from the aorta by the development of an anastomosis with the posterior limb arteries, whose origin from the aorta now becomes the root trunk of the umbilical artery, the original ventral umbilical root now atrophying. Hochstetter showed clearly that both ventral and lateral roots for the umbilical may exist for a short time coincidently {e.g., in rabbits of eleven days, two hours), and so form an arterial ring enclosing the Wolffian duct and ccelomic cavity, lateral to which the secondary roots and medial to which the primary roots course. Such a condition can be seen in human embryos of about 5 mm. (N.T. 16) as Keibel and Elze (1908) first reported and as may be seen from Felix 's Fig. 449, drawn from the Keibel embryo. In the embryo of 7 mm. (N.T. 28) only the secondary root is found, and the vessels are at their permanent location (at or slightly below the level of the fourth lumbar dorsal segmentals).[30]In embryos of this age, then, the strong umbilical arteries are found giving off, shortly after their origin from the aorta, a distinct branch, which courses somewhat downward and outward to the posterior limb, where it goes over into a capillary plexus. This is the primary artery of the limb, the a. ischiadica, and, while originally reaching the limb tissue without piercing the lumbar-sacral nerve plexus, at length the ventral growth of the latter makes this necessary in embryos of 9.5 mm. (Elze, 1907).
Fig. 449. Reconstruction of the a. umbilicalis in a human embryo 5.3 mm. long. (Collection of Professor Keibel, No. 1420.) The umbilical artery is seen to arise from the aorta by three roots, a visceral and two parietal. (After Felix, 1910.)
Soon there also arises, from the upper side of the umbilical artery, the second vessel to the limb, a. femoralis, and we may now designate the umbilical trunk from the aorta to the femoral branch, the common iliac, for what is at first merely a femoral soon gives off the a. epigastrica inferior and other branches and consequently comes to be the a. iliaca externa of the adult. The remainder of the umbilical trunk together with its a. ischiadica constitutes the definitive a. hypogastrica. Now the ischiadica is soon not merely that vessel, for in the 15.5 mm. embryo it gives off a prominent a. pudenda interna (Fig. 447). The root portion of the ischiadica from umbilical to this division place is consequently probably the great anterior division of the a. hypogastrica in the adult, and after the origin of the internal pudic comes to be the a. glutea inferior before at last the a. comes nervi ischiadici is reached. We are still without any series of observations on the development of the pelvic vessels.
Extremity Vessels
For no portion of the vascular system do we stand in such need of a series of well-verified observations as we do in the case of the embryology of the extremity vessels. This field is of profound interest, too, from two stand-points : first, because the developmental history ought to furnish us with a key for the explanation of the manv anomalies which the limb vessels show and which have formed the basis for classic studies on the variation of the vascular system {e.g., Baader 1866, Ruge 1883, etc.) ; and, secondly, because enough has already been learned to indicate that the first arterial tree in the limb recapitulates in a striking way the simpler conditions which are definitive for some of the lower vertebrates (Zuckerkandl, 1894). The subject gains added interest also from another aspect, for from a closer study of the extremity vessels, Miiller (1903) and De Vriese (1902) in recent years have been led to advocate the idea of a capillary plexus ancestry for vascular trunks, in contrast to notions which had previously prevailed. Subsequent research has confirmed this general idea, extending it in some places and restricting it in others, as has already been mentioned. However our exact knowledge of the development of the subclavian tree is still scanty, and there is an even greater dearth of observations in the case of the lower limb.
Arm
The earliest channels of an arterial source into the anterior limb buds are doubtless capillaries which arise directly from the lateral aortic wall at many points and anastomose profusely in the early limb tissue.
This stage has yet to he described for man, but may be shown clearly by injections of embryos of the chick and duck (Fig. 391). That it also obtains in the mammalia has been recently indicated by the reconstructions made by Goppert (1908) for the early subclavians in white mice (Fig. 450). Thus, as many as eleven of these earliest subclavians have been seen in the birds and five in the mammalia (Goppert). The capillary plexus which is formed by the anastomoses and further extension of these delicate vessels into the tissue of the limb is uniformly distributed in the blastema of the latter, save in a definite marginal zone which constitutes a narrow non-vascular shell of mesenchyma lying beneath the ectoderm. The plexus is drained into the posterior cardinal and umbilical veins through a series of small venules, and later, after the survival of only a single subclavian artery, the well-known marginal vein of Hoehstetter is established.
Very soon after the outgrowth of these early multiple subclavians, changes occur which involve a disappearance of those vessels not arising at intersegmental points, so that the arrangement retained consists of two or more subclavian arteries which are located exactly opposite the dorsal segmental vessels in this neighborhood and are hence "segmental subclavians. " In the mammalia, including man, one of these segmental subclavians is always opposite the seventh cervical dorsal segmental vessel (according to Hoehstetter 's method of counting, the sixth), and others may have persisted at segmental points above or below this.[31] Very soon after the establishment of strictly segmental subclavians, these vessels are incorporated in common stems of origin with the dorsal segmental vessels, so that they no longer appear as direct lateral derivatives of the aorta, as was the case earlier, but become strong side branches of the dorsal segmentals.
Fig. 450. Reconstructions of the arterial system in the arm buds of embryos of the white mouse, S days old. (After Goppert, Verb., d. anat. Gessell., Vers. 22, Anat. Anz., 1908, p. 94, Figs, la and lb.)
All the stages just mentioned, however, are passed over by the time the human embryo attains a length of five millimetres, for at this stage only a single member of the early subclavian series remains to constitute the definitive subclavian artery, the vessel of the seventh segment. It forms now the sole supply of the capillary plexus formerly nourished by multiple vessels and, after a short course to the root of the extremity, is soon resolved into a "spray" of many capillaries. As Muller (1903) has shown, the main stem of the artery at this stage, while tending to be a fairly strong trunk, centrally located, often shows island-formations in its course, and eventually, before the true capillaries arise, becomes quite plexiform in character (Fig. 451).[32]
Fig. 451. Arm bud of a human embryo 5 mm. long, showing central arterial net. (After Miiller, Anat. Hefte, Bd. 22, Taf. 25-26, Fig. 1.)
In the next stage which has been described, that of a 7 mm. embryo in the excellent study by Elze, but little change has occurred. No inselbildungen happen to occur into the proximal course of the artery, nor, apparently, is there any plexiform condition of the artery before the capillaries are given off.
In an embryo of 8 mm. Miiller found the subclavian giving off a branch just before the ventral nerve mass was penetrated; this branch continued for a short distance still medial to the ventral nerve, eventually plunging obliquely through the latter and joining the main stem, which has kept along the lateral side of the nerve; from this arterial loop, the main stem continues along the lateral side of the nerve, and other fine vessels are given off to course just ventral to the dorsal nerve mass of the limb, as well as ventral to the ventral nerve.
This is evidently the condition occurring still in the 9.5 mm. embryo which Elze (1907) has carefully reconstructed, although the branch of the subclavian given off to continue medially along the ventral nerve does not anastomose with the main vessel, which, as in the previous stage, continues along the lateral side of the ventral mass, especially along the m. medianus; a more dorsally directed branch can be followed along the radial nerve (Figs. 452 and 453).
Fig. 452. Reconstruction of the arteries and nerves of the right arm of a human embryo 9.5 mm. long, viewed ventrally. (After Elze, Anat. Hefte, Bd. 35, Taf. 17-18, Fig. 3.)
Some years ago Leboucq (1893) reported that in human embryos of about this age (7 to 11 mm.) the primary vessel of the limb coursed in the forearm between the anlagen of the radius and ulna, and represented the a. interossea volaris. Zuekerkandl had been led to expect this fact by comparative-anatomical studies which indicated that the interossea volaris was the oldest trunk in the lower arm, as well as by embryological observations on other mammalian embryos. His studies, constituting the first researches on the development of these vessels, remain of fundamental value.
Comparative
Zuekerkandl (1894) thus described the condition of the vessels in the fore limb of rabbit embryos 8.9 mm. long, in which the skeleton was just indicated by mesodermal thickenings. The brachial artery, after accompanying the median nerve in a typical way in the upper ami, is continued in the forearm as a stem lying next the skeletal anlagen, covered by the flexor pre-muscle mass. Just below the elbow, the artery gives off a branch which goes through to the dorsal side of the forearm (a. interossea dorsalis). As the main artery continues to go distally, the median nerve turns away from it to become superficial, leaving its internal interosseus branch to accompany the axial vessel, which may thus now be called the a. interossea volaris. As the main part of the median nerve leaves the stem artery it is supplied by the latter with an accompaniment of fine vessels which continue with it along its entire superficial course to the palm, where they constitute a superficial palmar plexus. The axial artery itself divides at the distal end of the forearm into a ramus volaris, which breaks up to constitute a delicate deep volar plexus next the skeletal anlagen, and a strong ramus dorsalis which supplies the back of the hand. In rabbits somewhat older the fine vessels along the main median nerve constitute an artery large enough to begin to dispute the field with the interossea volaris, the a. mediana, while the ulnar nerve has a small accompanying vessel, a. ulnaris. Essentially the same conditions are shown in an 11 mm. cat embryo.
Fig. 453. Vessels and nerves of the same arm (Fig. 452) shown from above. (After Elze, Anat. Hefte, Bd. 35, Taf. 17-18, Fig. 4.)
De Vriese has found that in the human embryo of 10 mm. the chief nerve trunks are all accompanied by capillary vessels,[33] and has chosen to represent these as already constituting arterial pathways. It is doubtful whether these should all be given the valuation which she has set on them, and it must be left to future research to modify or confirm the conception of an already quite complicated arterial system which her description gives us.
The author recognizes at this early stage the a. n. interossea dorsalis, a. n. radialis, a. n. ulnaris, a. n. mediani, and a, n. interossea volaris, the last of which constitutes the continuation of the- axial stem and divides just above the carpus into dorsal and palmar branches, which are themselves in communication by means of a small a. perforans carpi. Four vascular planes are distinguished in the hand, two palmar and two dorsal.
In an embryo measuring 11.7 mm. Müller has reconstructed the chief arterial system of the limb, and, inasmuch as the nerves and the anlagen of the humerus, radius, and ulna were evident, homologized the vessels present with those occurring in the adult (Fig. 454).
Fig. 454. Reconstruction of the arterial system of the arm in a human embryo 11.7 mm. long. After Müller, Anat. Hefte, Bd. 22, Taf. 25-26, Fig. 9.) a. a., a. axillaris; a.b.s.s., a. brachialis superficialis superior; a.b.s.m., a. brachialis superficialis media; a.b.s.i., a. brachialis superficialis inferior; S-, widening of the arterial tube after it has passed through the ventral plexus plate; a.b.s., distal part of the a. brachialis superficialis; a.b.p., a. brachialis profunda; a.r., a. radialis; a.i., a. interossea; a.m., a. mediana; a.u., a. ulnaris; a.a.b.s., a. antibrachii superficialis.
The subclavian perforates the brachial plexus in the usual manner from its ventral side, but the strong branch which, as in previous stages, is given off just before the penetration of the plexus to continue on the medial side of the latter, sends an anastomosing branch through the ventral nerves to join the main vessel. This anastomosing branch joins the main stem at or near the origin of the radial artery from the latter, and on its course toward the chief trunk splits into other branches, as the figure shows. Two of these branches also run into the main trunk, one by piercing the median nerve, the other by going under the same, while another branch courses along volar to the median to anastomose eventually with the median artery branch of the main stem again. In the other limb of the same embryo three strong perforating branches join the part of the main artery medial to the ventral nerve mass with the stem lying lateral to the same. So that in both limbs we are dealing with a rather plexiform condition of the axillary artery.[34] The main vessel pursues a general course along the radial border of the median nerve to become in the forearm the a. interossea volaris. In its upper-arm portion it gives off, in addition to the a. mediana, a small vessel which joins a chain of capillaries lying in front of the radius anlage (identified by Miiller as the a. radialis). Another branch of the main stem joins correspondingly small vessels lying along the ulnar nerve, and constitutes the a. ulnaris.
In his embryos of 14 mm. Müller has identified the a. profunda brachii, the a. mediana, a. interossea vol., a. radialis, and a. ulnaris.[35] The main vessels in a 16.2 mm. embryo may be seen at a glance from Fig. 455, in which the lower-arm and hand areas have been omitted.
Mention has already been made of the fact that if, for example, the point of union of the sixth aortic arch with the aorta dorsalis be taken as a fixed point, the subclavian artery appears to wander upward. "Whereas in the embryo of 4.9 mm. the a. subclavia is some eight segmental spaces below this point, in the embryo of 7 mm. it is but six spaces below it, and in the embryo of 11.5 mm. it is opposite the sixth arch.
The a. mammaria interna and the a. thyreo-cervicalis are conspicuous stems in the embryo of 15.5 mm. (Fig. 447). The a. thyreo-cervicalis courses for some distance in the wall of the jugular lymph-sac in this embryo, and, as McClure has observed the same thing in embryos of the cat, the relation is probably of general significance.
In reviewing the facts hitherto acquired concerning the history of the arm vessels, one must be struck with the need of more careful studies here.[36] The reconstructions of Müller and Elze are our sole possessions in this field. Viewed from a more general stand-point, however, the history of the arm vessels in man certainly confirms the conclusions arrived at some years ago by Zuckerkandl in his studies on the general morphology of these vessels, for in man also the primary artery is an axial stem from shoulder to hand and in the forearm constitutes the later a. interossea volaris. There is also a very general agreement among all observers in the important role played by the embryonic a. mediana.[37] However, there is as complete an agreement in the recognition that at first the volar interosseus is the chief lower-arm vessel, and no support whatever for the idea of Janosik who speaks of the mediana in that primary role.
Fig. 455. Reconstruction of the nerves and arteries of the arm in a human embryo 16 mm. long. (After Müller, Anat. Hefte, Bd. 22, Taf. 27-28, Fig. 6.) The vessel accompanying the radial nerve is the a. profunda brachii (a. nervi radialis of De Vriese). A. a., a. axillaris; A.i., a. interrossea; A.m., a. mediana; A. r., a. radialis; A. u., a. ulnaris; A", m., n. medianus; A", m. c, n. museulocutaneus; N. r., n. radialis; A', u., n. ulnaris.
Comparative
The a. interrossea volaris with a. perforans carpi is the chief vessel of the forearm in the adult in amphibia, reptilia, and in Ornithorhynchus among the mammalia (Zuckerkandl). It is also apparently the plan in the embryos of all the mammals. (Zuckerkandl, rabbit, cat; Hochstetter, Echidna; Grosser, bats; De Vriese, calf.) In very many mammals the chief definitive arterial stem is the a. mediana (marsupials, edentates, most carnivores, bats, etc.). In the primates its territory is taken over by the ulnar. A vessel accompanying the ulnar nerve, hence an a. nervi ulnaris, occurs in adult amphibia and reptiles and apparently constantly in the embryos of mammals. In the adults of the latter class the artery is not, as a rule, important and may be lacking entirely (most ungulates). A vessel which can be designated the radialis is not of general occurrence till the mammals are reached, and in the majority of these is a superficial radial. It comes to possess its deep volar territory in the higher mammals, but its proximal end is still superficial (really the superficial brachial here) in many of the primates, as Bayer has well shown.
Variations
Many of the variations of the arm vessels must remain uncertain in origin until we possess a well verified series of observations on their embryology. There can be no doubt, however, that Miiller has demonstrated the manner in which a superficial brachial may arise, for arterial channels are retained on the ventral side of the median nerve in most of his specimens. It may be pointed out, also, that eases of persistence of great median or even volar interosseus arteries (Baader) are unquestionably survivals of embryonic conditions, and we may have all possible degrees of variation in the part taken by these vessels in the supply of the hand (Schwalbe and others). Krause pointed out that high origins of the radial or ulnar arteries usually involved a superficial course for the proximal part of these vessels, a fact which may be explained by the retention of a brachialis superficialis inferior. Attention may also be called to the very ingenious series of schemata which Miiller lias constructed to explain the lower-arm arterial anomalies, but until more is learned of the normal history here, we can not venture to present satisfactorily founded diagrams for anomalies.
Arteries of the Lower Limb
In human embryos measuring from 5.5 to 7 mm. and shortly after the umbilical arteries have acquired their secondary, more lateral, stems of origin from the aorta in the neighborhood of the fourth or fifth lumbar segments, there can be seen going out from these vessels on either side, a small artery which penetrates the tissues of the posterior limb bud (Fig. 420). When the nerve-plate for the lower limb grows out farther, it surrounds this vessel, so that the extremity artery appears now to pass through it, just as is the history with the subclavian artery and the brachial plexus. Later the ischiadic nerve joins this vessel and it may consequently be identified as the a. ischiadica.[38]
Probably injections of earliest stages here would show that the a. ischiadica is really only the exaggerated member of a series of vessels, which originally supply the limb tissue, as is the case with the upper limbs.
This vessel (a. ischiadica) forms a central or axial nourishing channel for the early leg bud, just as is the case with the subclavian and early arm bud. Leboucq (1893) first called attention to the fact that the primitive blood supply of the hind limb consisted in a single axially-coursing artery, the a. ischiadica, which, as soon as skeletal elements could be recognized, continued to course in the lower-leg region between the anlagen of tibia and fibula, and ended chiefly as a strong branch which perforated the interspace between the elements of the first tarsal row, to reach the dorsum of the foot. Lately De Vriese[39] has confirmed this.
It may be well to refer here to the important previous observations of Zuckerkandl (1894-95), who described the leg arteries in a rabbit embryo of 7.7 mm. somewhat as follows : The a. ischiadica is continued in the lower leg as a strong axial vessel next to the skeleton. It sends two perforating branches towards the side of the limb, the upper of which probably corresponds to the a. tibialis ant., while the lower supplies the dorsum of the foot. The distal end of the axial vessel supplies the depths of the sole. Fine vessels accompany the posterior tibial nerve in its lower course, and in embryos of 13.5 mm. these constitute a distinct artery, a branch of the axial vessel. The further history in this animal disclosed the a. saphena (from the a. femoralis) taking over the posterior tibial trunk.
The supremacy of the a. ischiadica in the supply of the extremity is soon disputed by the appearance of a new vessel, the a. femoralis, which in the embryo of 15.5 mm. (Fig. 447) is already the chief vessel in the limb. The femoral soon gains all the branches of the a. ischiadica in the territory of the lower leg (e.g., tibialis posterior et anterior), by anastomosing with the ischiadica near the knee; we know the a. ischiadica of the adult only as the stem portion of the a. glutea inferior.[40]
This ontogenetic history of primary and secondary vessels for the human leg is closely paralleled by the vessels found in an ascending vertebrate series, as Zuckerkandl showed.
Comparative
The a. ischiadica is the chief vessel of the thigh in the adult for amphibia, reptilia, and the birds, yet the femoral in the latter class may attain quite an area of distribution, and in some (e.g., Spheniscus.) even behaves as in mammals by taking over the chief rami of the a. ischiadica and constituting the chief limb vessel (Hochstetter). On the other hand, among the mammalia the atrophy of the ischiadica is the ride. Yet it may persist in part, as, for example, forming the a. tibialis anterior of bats (Grosser, 1901).
The appearance of an a. ischiadica in the embryos of all mammals was indicated by the observations of Hochstetter and Zuckerkandl. The chief lower-leg portion of the a. ischiadica in adults in the amphibia, reptiles, and birds behaves exactly as it does in the early stages of the embryo of man, namely, courses between the tibia and fibula and supplies the dorsum of the foot by means of a large perforans tarsi. Other stages in the ontogeny of man's leg vessels are found definitive in various mammals, e.g., the stage in which a distinct superficial plantar arch or plexus exists, as well as a deep one. This is the case in most apes, as Popowsky has shown, and is lost in the anthropoids, where, as in man, the lateral plantar artery is larger than the medial and the deep plexus practically the only one present.
Much interest attaches to the saphenous artery. The earlier work of Zuckerkandl emphasized the very general occurrence of this vessel in all the mammalia,[41] and led him to declare it the oldest (phylogenetically speaking) branch of the femoral. In its lower portion this artery usually takes over the dorsalis pedis artery, or the primary tibialis posterior, or both, in which latter case it constitutes the chief or 011I3- vessel for the supply of the foot. The vessel retains its importance in the primates, — e.g., in Cebus, where it supplies the entire foot. Popowsky has recalled the anomalous occurrence of this vessel in man and reported two interesting cases in which the a. saphena was large, in both cases anastomosing with the posterior tibial artery and in one case in addition with the dorsalis pedis. He has again called attention to the frequent great development of this vessel in the monkeys, where, even in the anthropoids, it supplies the dorsalis pedis. Popowsky, evidently much influenced by this, states his belief that this vessel must play a prominent role in the development of the leg arteries of man. There is no evidence, however, that such is the case. The work of De Vriese indicates there is apparently no necessity for the recapitulation of a stage in which the saphenous functions as the chief artery of the lower leg. In the reworking of this field, nevertheless, great interest will attach to the re-examination of the embryonic importance of this vessel, for the reasons above given.
Variations
Dubrueil, Krause, Ruge and others have described cases in which the a. ischiadica was the chief vessel of the limb in man, which is quite evidently a survival of embryonic conditions. The occurrence of an a. saphena magna, following the saphenous nerve, has already been mentioned, the first case having been observed by Zagorsky in 1809. Normally this vessel probably reaches the lower third of the leg, for in well-injected subjects I have traced it this far, as Hyrtl first did. Krause and more recently Salvi report cases where an artery accompanies the n. cutan. suras lat. This corresponds to an embryonic vessel seen by De Vriese at the peroneal side of the leg in the 13 mm. embryo, but it usually disappears entirely. Cases in which the a. peronaea instead of the tibialis ant. supplies the dorsum of the foot are not rare, and represent again the embryonic picture where the axial artery behaves normally thus. Most interesting are cases in which a perforans tarsi persists, joining the dorsalis pedis with the deep plantar vessels. In the adult also a superficial plantar arch occasionally occurs, as Krause, Gegenbaur and others mention.
Footnotes
Note - these footnotes appear throughout the chapter text and the arrows below should be used to return to the original text.
- ↑ In 1886 Van Bemmelen for the first time described a transitory aortic arch found between the systemic and pulmonic arches in embryos of birds and reptiles. A year later Boas (1887), in welcoming this discovery, pointed out its importance in the comparative anatomy of vertebrates. He recalled (Morph. Jahrb., Bd. 7, 1882: Bd. 8, 18S3) that in amphibian larvae, as well as in Ceratodus, Polypterus, and Amia, the four aortic art-lies which occur occupy the third to the sixth visceral arches, the pulmonic artery being given off in each ease by the last pair, i.e., by the aortic arch of the sixth gill-bar. It hence appeared evident that the pulmonic arch was the sixth and not the fifth of the series in all vertebrates, and Boas now predicted the discovery of a transitory fifth arch in the embryos of mammalia, the only remaining class in which it had not as yet been seen. Two years later Zimmermann, as is well known, reported the presence of a fifth arch in embryos of man, the rabbit, and the sheep. Tandler's careful paper, following in 1902, reported traces of the arch in the rat and two very clear examples of it in man, for which he furnished the first figures published. Lehman has described what were interpreted as vestiges of the arch in the rabbit and gave a distinct instance of it in the pig. It is important that in some of these cases — e.g., in Tandler's (man) and in Lehman's (pig) — we had also to do with what was apparently a fifth pharyngeal pouch. This structure, the so-called postbranchial body, is not new. It had been known to appear behind the fourth pouch but soon to grow into the latter, with which it had a common opening into the pharynx and of which it now appeared to be a diverticulum. It seemed highly significant also that in the cases which have just been enumerated the fourth pouch pointed towards the ectoderm between the fourth and fifth arches, while the postbranchial body occurred between the fifth arch and the sixth. The whole picture of these interrelations, in short, pointed strongly to their all being serial true branchial structures. In 1906 F. T. Lewis indicated that the very general acceptance of this interpretation was probably caused by the weight of comparative considerations. He called attention to the ordinary conception of a vessel which could be called an aortic arch having a definite course from the ventral to the dorsal aorta, and emphasized that a fifth arch of this completeness had never been seen, except by Zimmerrnann in the rabbit, where subsequent investigators (Lehman, Lewis) have not been able to confirm him. This fact must be admitted, for even in Tandler's clear cases the accessory arch does not join the dorsal aorta, but instead fuses with the pulmonary arch before this ends dorsally. Locy has emphasized that it seems generally true that the fifth arch is in some way connected with the last pair, in some of the lower classes, in fact, the pulmonic arch appears to have split, — e.g., Lacerta (Peter). Other reports have recently come in affirming fifth aortic arches in other mammals, — Soulie and Bonne (1908) in the mole, Coulter (1910) for the cat, and Reinke for the pig, — (Note on the Presence of the Fifth Aortic Arch in a 6 mm. Pig Embryo, Anat. Record, vol. 4. No. 12, December, 1910) and the evidence is too unanimous to cause doubt that vascular rudiments in the position of a fifth arch occur generally in the mammalia.
- ↑ Ramus posttrematicus der IV Schlundtasche," "E. posttrematicus V," or " R, posttrematicus II des Vagus," given off by the N. laryngeus superior shortly after its departure from the ganglion nodosum and described by Elze (1907) and Tandler (1909) in embryos from 7 to 9 mm. in length. It is usually absent in later stages, although Grosser (1910) believes he has identified it in an embryo 19.75 mm. (crown-rump), passing through the foramen thyreoideum.
- ↑ This subject forms one of the few instances in which a correction is necessary in the conception originally given us by Rathke in his epoch-making monograph on the " Aortenwurzeln und die von ihnen ausgehenden Arterien der Saurier." Rathke, as is well known, represented the right and left pulmonary arteries both coming each from its respective arch in lizards and birds, but for the snakes among the reptilia and for the mammalia he showed the two vessels arising from only a single arch, in the snakes the right one and in the mammalia the left one. Rathke's ideas were founded on appearances given by embryos which have passed the earliest stages of origin of the pulmonary artery. His first showed that the earliest human puhnonaries came each from its respective arch, as in the lacertilia, and Bremer has proved that this is a general fact for all the mammalia, and suggested the high probability of its general occurrence, at least in earlier stages, in all air-breathing vertebrates. Bremer's studies have included man, the rabbit, cai, dog, pig, opossum, sheep, guinea-pig, cow, and deer, and, as a result of them, he distinguishes two methods for the formation of the adult pulmonary stem in mammals. In the method occurring most generally (man, cat, dog, rabbit, sheep, cow, deer, and opossum) the original symmetry is disturbed by an absorption of the proximal part of the left arch into the truncus pulmonalis, so that the left pulmonary, artery now rises from the bifurcation place into left and right arches while the right artery comes off its usual distance from this bifurcation place. With the destruction of the distal part of the right arch up to the point of origin of the a. pul. dextra and the eventual atrophy of the corresponding part of the left arch, the adult plan is reached, and this therefore means that we must consider the left pulmonary artery as representing only the original embryonic one, but the right pulmonary vessel has also as its most proximal part the right pulmonic arch. A second method followed in the evolution of the adult mammalian pulmonaries is exemplified by the pig and guinea-pig, in both of which forms the two early pulmonary arteries are joined in a general capillary plexus, the anastomosis enabling one root to serve as a common stem, which in the pig happens to be the left original artery and in the guinea-pig the right. Consequently, in the former animal the blood to both lungs must first traverse the proximal parts of the left arch and left original artery, and in the latter animal the corresponding parts on the right side. Sakurai (1904) has declared that the left artery in the deer moves toward the right past the bifurcation of the truncus pulmonalis to the right arch, but Bremer questions his interpretation, and the case here must rest till an examination of more abundant material in the stages implicated. In the meanwhile, Bremer's work has shown the incorrectness of the conventional diagram in which both definitive pulmonaries are shown as sharing equally the proximal parts of the sixth arches, for in no mammal is this true. Man and most of the other members of the class have a right a. pulmonalis which is of this nature, but an a. pul. sinistra, which is merely the original pulmonary artery of that side, the corresponding proximal part of its arch having been assimilated in the truncus pulmonalis.
- ↑ Injections of very early bird and mammalian embryos show that the trunk of the internal carotid arterv extending from the first arch distalward is represented at first by the outgrowth of a plexus of capillaries from that arch (Figs. 393, 394). This plexus spreads over the sides of the early mid-brain first, then over the fore and hind-brains (Fig. 395). Soon out of the several capillary stems of origin from the first aortic arch, one is chosen to become the artery and the remainder perish. Gradually the plexus of capillaries invades the ventral surface of the brain and tends to halt there on either side of a narrow mid-ventral non-vascular strip. In the meanwhile the continuation of the main arterial stem is being evolved out of this plexus in such a way that the carotid, after giving off an a. ophthalmica, appears to have two terminal branches anterior and posterior. The latter connects up with the medial ventral margin of the capillary mesh on each side, and so there come to be in the midventral region two long parallel vessels, the continuation of the posterior terminal branches of the two carotids. This conversion of the medial margins of the ventral capillary mesh here is analogous to the formation of the aorta? from the medial margins of the vitelline capillary plexus. It will be shown that in the spread of the capillary plexus over the spinal cord an exactly similar phenomenon takes place, — that is, the capillaries halt along two parallel lines on either side of the midventral plane. In the cord region also these two plexus margins are converted into two transitory longitudinal arteries, furnished at every segmental point by blood from the segmental arteries and connected headward with the same vessels supplied by the carotid arteries. The whole structure from head to tail has been called by Sterzi the tractus arteriosus primitivus; by De Vriese the primitive anterior spinal arteries. It will be evident from all this that this primitive midventral vessel is the earliest arterial anastomosis between the carotids and dorsal segmental vessels. The second of the dorsal segmentals is the hypoglossal artery, and that part of the anastomosis between it and the carotid is of the greatest importance, for it, according to De Vriese, is the a. vertebralis cerebralis of His; at any rate it is destined to form the basilar artery in the region beneath the hind-brain. Thus the basilar artery is primitively paired and gets its chief supply of blood from the carotids, for the hypoglossal artery cannot figure greatly. Gradually the double basilar is replaced by a single vessel, which is really formed through the development of anastomoses between the two parallel trunks permitting the original left vessel to persist in some areas and the right one in others. This is also what happens as regards the anterior spinal artery. Very soon after the unpaired basilar is produced, its lower source of blood exceeds its upper in importance, and when the cerebral vertebrals are taken over by the cervical vertebrals, the latter vessels are the main supply of the basilar.
- ↑ These pre-segmental branches of the aorta have, of course, another interest, inasmuch as we may be dealing with evidences of a segmentation of the head in front of the occipital somites. Be this as it may, Tandler (1902) has seen a remarkable row of these vessels in the rat. where they seem to arise at regular intervals.(Compare his Fig. 8, p. 302.) De Vriese (1905) mentions their appearance in the rabbit (see her Fig. 28, planehe 16), and for the area in front of the hypoglossus vessel mentions two as being more constant, one at the level of the otic vesicle and the other near the gasserian ganglion. In the human embryo KroemerPfannenstiel (N.T. 3), with six somites, I have found two of these vessels on the left in front of the first somite, and in the Eternod embryo, with eight somites, one behind the region of the first aortic arch, just in front of the second pharyngeal outpocketing ; whereas in the Spee embryo No. 52 there are on each side, although not paired, four of these pre-segmental dorsal branches of the aorta. Finally, Ingalls in his 4.9 mm. embryo distinguished clearly four of these vessels on the left side. The relation of these vessels to the cranial nerves or visceral arches awaits demonstration.
- ↑ Although the recognition of an embryonic artery piercing the mammalian stapes dates back some thirty years (Salensky, 1880), no one had before established the relation of this vessel to the aortic arches.
- ↑ It has been known since the time of Rathke that in many adult reptiles an artery exists which pierces the columella. The same reptiles possess another artery which supplies the upper jaw and courses with the vidian nerve. It would be of the greatest interest if ernbryologieal observations here should establish the origin of the vidian -aceomjimnying artery from the first aortic arch and the columella-piercing vessel from the second arch, like the stapedial of mammals. Evidence that this may be true is furnished by an interesting variation found by Grosser (1901) in a young mammalian embryo (bat). Here the infra-orbital branch of the stapedial artery was not a member of the usual trunk, but an independent branch of the carotis interna, having a definite relation to the vidian nerve, just median to which it coursed. If these homologies, which were suggested by Tandler, are established, then 1. The territory of the first aortic arches in all the higher vertebrates is supplied at first by vessels corning from that arch. The stem for these vessels or one of them may course with the vidian nerve. 2. The territory of the second arch possesses a vessel normally related to the columella (or stapes). 3. The second vessel (stapedial) remains in its original state in the reptiles mentioned, but in the mammals usually annexes the branches developed from the first arch. 4. In adult mammals the stapedial artery secondarily surrenders these branches to the external carotid and atrophies, or, in the cases where is persists, at least loses its mandibular ramus to the external carotid artery (rat), and in some cases also its infraorbital one (bat).
- ↑ Since the above went to press I note that Pensa has given us reconstructions of the pulmonary arteries in two human embryos, 11.5 and 25 mm. long respectively. Antonio Pensa, " Osservazioni sulla morfologia e sullo sviluppo della ai'teria pulmonalis nell' uomo." Boll, della Soc. Med. Chir. di. Pavia Comunicazione fatta nella seduta del 8 Aprile, 1910. Pavia, 1910.
- ↑ I am aware that Broman, for instance, bases much of bis discussion of the position of the ventral branches and their changes on the supposition of their being primarily segmentally arranged. This, however, is not the case, as my experience with embryos of from six to twenty-three somites clearly proves. Many of the ventral branches are unquestionably as far as possible from a segmental alignment, so that the most which can be said here is that a segmental influence is evident, but expresses itself imperfectly. Later, however, there is a marked agreement with the segmental plan, so that we have conditions analogous to what occurs in the limb buds where stages of a more irregular row of primitive limb arteries are succeeded by those in which these vessels are segmentally arranged.
- ↑ Felix (1910), chiefly on comparative grounds, assigns the primitive function of the intestinal arteries to the supply of the pronephros. There seems, however, little evidence for this in human ontogeny, where these arteries are from the first truly intestinal vessels and where the pronephric rudiments are not in relation with these but with the primitive lateral branches of the aorta.
- ↑ The first pair of the dorsal segmental arteries is not generally referred to. Hochstetter (1903), for instance, states that the first pair of these arteries courses with the hypoglossus nerve, as a result of the embryos which he, Zimmermann (1890), and Piper (1900) had studied. These embryos were so old that the first pair of the segmental arteries had already atrophied.
- ↑ Sterzi has pointed out that the condition of paired anterior spinal arteries or a " tractus arteriosi primitivi," is never developed in the mammals to the degree seen in the birds. In the latter class they form large, much stronger and less transient trunks, — e.g., lasting from the third to the twelfth day in the chick. It is interesting to note that this condition is definitive in the cyclostomes.
- ↑ Sterzi was the first to show that the anterior spinal artery usually seen in the adult is only formed after the appearance of a series of anastomoses between the two parallel primitive trunks. The final vessel, according to him, may in some regions be derived from the left primitive vessel and in other regions from the right one, according to chance. The development of the anastomoses between the two primitive vessels permits the branches of that one destined to perish to be taken over by its more successful neighbor. Probably the usual anterior spinal is thus really unilateral in origin. At the same time, however, another plan may be followed in some areas. The anastomoses between the two primitive anterior spinals may become so large and numerous as to completely destroy in places the paired character of the arterial channels of the ventral cord surface and in such areas the cord is nourished by a rather wide median arterial plexus, from which later an exactly median vessel can emerge (Evans).
- ↑ Burrows, M. T.. unpublished observations.
- ↑ But see Hochstetter, 1911.
- ↑ Whether the oesophageal arteries which Broman and I have seen in quite young embryos are remains of these cephalically lying original vitelline vessels or entirely new sprouts does not permit of determination.
- ↑ It is of interest to note that Allen (1883) some years ago pointed out that remnants of both the a. and v. omphalomesenterica are normally found in the newborn of the cat, dog, and guinea-pig in a strand of tissue which reached the navel.
- ↑ Fransen has studied the branches of the a. mesenterica inferior in two human fetuses between the eighth and ninth month. The six chief branches which he finds going off from this artery he interprets not as the usual branches of the third order, but as original ventral segmentals from the aorta and sacralis media, which subsequently became united through a longitudinal anastomosis (the ascending and descending rami of the a. mesenterica inferior), whereas the root portions die. There is nothing embryologically to establish this claim. These lower ventral segmentals do not exist long enough to leave a permanent trace in the mesenteric plexus. Like the earliest limb vessels they are usually of a capillary nature. The establishment of the inferior mesenteric artery rearranges the whole vascular pattern of its territory of distribution, and the six branches to which Fransen refers came out of this plexus.
- ↑ In an embryo of 4.5 mm. Broman has identified three of these vessels rising from the right aorta opposite the third and fourth dorsal segmental vessels, and reports them in embryos of 10.3 and 14 mm. I have myself seen them in embryos of 10 and 14 mm. In the embryo of 15.5 mm. shown in Fig. 447 they are shown as delicate twigs opposite the sixth and seventh thoracic segments, and have been seen in this location or slightly lower in five other embryos measuring from 19 to 23 mm. (Numbers 229/368, 108, 57, and 382. Mall's collection). In the older of these embryos they were represented by a fairly strong vessel opposite the eighth thoracic segment.
- ↑ Tandler has confirmed this tendency for a segmental arrangement of the mesonephric arteries, but the studies of Broman, Ingalls, Elze, etc., show that many non-segmental arteries exist either from the beginning or as a result of shifting of original ones and we must admit that the metameric arrangement of the Wolffian body arteries is soon completely lost. Hochstetter has called attention to the fact that the mesonephric vessels in Selachians are described as coming from the segmental body wall arteries (Dohrn), and in Amphibia as being true segmental offshoots of the aorta (Semon). He is of the opinion that the corresponding vessels in amniotes were also segmentally arranged in correspondence with segmental mesonephric glomeruli, each of which had its own artery. Actual observations on amniote embryos which will support this have not yet been made.
- ↑ Along with this goes the fact that the recognizable rudiments of the Wolffian body in the adult — the epididymis or epoophoron — are naturally supplied by the sex gland artery.
- ↑ It is to be noted that at this stage these are at last the only mesonephric arteries existing, with the exception of the very last member of the series — that of the second lumbar segment — which sends a branch to the sex gland.
- ↑ Hochstetter declares the a. renalis of other mammals to be a direct secondary outgrowth of the aorta, and the same history was described by Hill for the pig. The subject is worthy of reinvestigation in very early injected embryos.
- ↑ Broman explains this by a descending course of the a. suprarenalis inferior at the time the a. renalis supplants it. Formerly these vessels were transverse, but after the closure of the diaphragm he thinks the latter successfully prevents any upward extension of the adrenal, and that adrenal growth from now on consequently pushes down its lower pole together with the a. suprarenalis attached there.
- ↑ From such a finding Broman concludes that the post-cardinal venous blood flows to the kidney and is drained out again into the vv. revehentes of the Wolffian body; this would furnish a renal-portal system for the early metanephros comparable with the renal-portal system so well known in the ease of the mesonephros, and in accord with similar observations made some years ago by Hochstetter on the metanephros of reptiles.
- ↑ Hoehstetter has shown that in some mammals, although this plan is originally followed, there subsequently occurs a disappearance of the common iliac vessels, so that the external and internal iliacs arise separately from the aorta (cat). Hoehstetter in 1903 felt inclined to explain this as due to a splitting of the aa. iliaea communes, Broman (1908), by a fusion of the umbilicals down to the point of origin of the external iliacs. Very recently now, Hoehstetter (1911) has subjected the matter to a careful restudy, and comes to the conclusion that the cat's truncus hypogastricosacralis comes through a, wandering upward of the origin of the a. iliacae externas from the wall of the a. iliacae communes to that of the aorta ; a similar thing apparently occurs as regards the a. iliolumbales which wander from the external iliacs to the aorta.
- ↑ Young has attempted to maintain that the umbilical arteries really represent the original continuations of the aorta? which have fused only down to the point of origin of these vessels. He goes over into a hypothetical and poorly founded realm in declaring that the aortae thus bend around into caudal arches comparable with the aortic arches. He explains the a. sacralis media as a secondary branch, being much impressed with its dorsal origin from the aorta at a point cranial to the iliacs rather than at the exact division place. Nevertheless in development the sacralis media goes off at the point of origin of the a. umbilicales, and in addition behaves like the aorta in its position, dorsal segmental branches, etc. Broman explains the definitive origin of this artery cranial to the division of the aorta into its iliacs by assuming that the last part of the aortic stem is formed by a secondary fusion of the aa. umbilicales for a short space at their proximal ends. No evidence exists for this view, and if relative growth differences cannot completely account for the apparent cranial shifting of the sacral artery, we must assume a true wandering to have taken place.
- ↑ In this connection it is of interest that in some mammals the omphalomesenteric artery first appears to take origin from the umbilical by a stem which leaves the latter vessel and courses cranially toward the place where later strong arterial connections with the aorta enable the proper omphalomesenteric artery to displace it. Those are the conditions seen by Ravn (1894) in the rat and mouse, and I can report an almost similar phenomenon in early embryos of the pig. Here injections show that there exists for a time (7.5 to 9 mm.) a strong arterial route for the omphalomesenteric artery which arises from the a. umbilicalis and courses cranially to join the former vessel. These phenomena were quite unintelligible before we were aware, as we now are, that the entire vitelline-umbilical complex of vessels is originally one and the same system.
- ↑ Broman has explained this caudal " migration " of the umbilical arteries by the successive development of " wandering " roots by virtue of which the artery acquires lower and lower connections with the aorta. As evidence of this he points to the double-rooted condition in which the artery may be found. This coincides with his explanation for the descent of the gut arteries. It is to be pointed out that many embryos do not show these multiple roots, and the appearance, even when found, is possibly merely an instance of inselbildungen. Disproportionate growth of the two aortic walls may again be responsible for this dislocation, or we may have to do with an actual active caudal migration of an individual trunk.
- ↑ According to the investigations of Hochstetter (1911), they have, indeed, wandered to a position farther caudalward than that in which they are normally found in the adult, for in embryos of this age (6.5 to 10 mm.) the fifth lumbar arteries still usually arise from the division place of the aorta, whereas in later embryos, as in the adult, the aortic bifurcation is " pushed up " to lie opposite the fourth lumbar vessels, so that the aa. lumbales V can no longer be found coming from the aorta directly but are given off by the a, sacralis media. The latter vessel appears to wander cranialward independently, and so comes to arise from the dorsal wall of the aorta rather than at its exact division place; it may even be found giving rise to the aa. lumbales IV.
- ↑ Thus, three segmental subclavians have been seen in the rabbit and mouse, and several cases of two segmental subclavians reported for man. The human embryos 16 and 17 of the N. T. possess subclavians from the sixth and seventh segments. Embryo 148 in Mall's collection has segmental subclavians from the seventh cervical and first thoracic segments. These observations show the possibility of four subclavians in man, - i.e., from the last three cervical and first thoracic segment
- ↑ Within the tissues of the limb, then, the central arterial channel is not everywhere in the form of a single tube, but is rather constituted by an axial arterial plexus from which the capillaries are given off. The same character for this central nourishing channel of the early limb can be demonstrated by injections of the vessels in other mammalian embryos, and may be correctly taken to indicate that for a time the arterial current employs several instead of a single channel out of many available channels open to it by reason of the pre-existing general capillary mesh.
- ↑ Injected mammalian embryos show that this is partially true, although the poor material with which De Vriese worked has justly led to the conviction that perineural spaces were confused with true capillaries, a fact which the illustrations accompanying her research leads us to suppose.
- ↑ Great interest attaches to the arterial plexus formed by the three vessels which penetrate the brachial plexus in this embryo, for it again indicates the employment by the arterial stream of several rather than a single channel from the pre-existing capillary plexus. This transitory plexus axillaris arteriosus of Miiller has also been seen in other mammals (Goppert in the mouse). It by no means constitutes an invariable intermediate stage in the development of the arm vessels, but, as Müller himself has shown, may occur on one side of the embryo, while the opposite axillary is composed of but a single trunk. Such phenomena may be expected to occur somewhat more frequently in the developing vascular system than in the adult, inasmuch as the increasing blood current exercises a more definite choice to the elimination of multiple paths, but that they may persist even here is shown by the occasional presence of islands in the course of adult arterial trunks.
- ↑ In two 14 mm. embryos Müller has found the main artery splitting into two branches which surround the median nerve and fuse again, and is consequently of the opinion that we have here a retention of the arterial paths ventral to the median nerve shown in the previous stage. That this has been the case is strengthened by the fact that the two limbs of the brachialis meet again at the place of origin of the a. radialis which is quite an exact correspondence with the place of opening of one of the anastomosing channels shown in Fig. 454. In one of these embryos the artery lying ventral to the median nerve is the larger of the two, while in an older embryo (W. P. 20.5 mm.) it is the persisting one. It seems reasonable to suppose that we are dealing here with instances of a so-called superficial brachial artery, which, as is well known, lies on the volar side of the median nerve, while the normal brachial is dorsal to the latter.
- ↑ Very recently Goppert (1910) has supplied us with the history of the development of the arteries in the arm of the white mouse, an account which is by far the most complete we possess for any mammal.
- ↑ The observations of De Vriese even indicate that this vessel is not finally displaced from the hand until the embryo reaches almost 30 mm. in length.
- ↑ It is to be noted that in mammalian embryos, where the history of the leg vessels has been followed more carefully, the a. ischiadica is primarily a branch of the aorta, and its proximal portion serves later as the stem of origin for the umbilical arteiy when the latter abandons its ventral roots (Hochstetter, 1890). The origin of the a. ischiadica from the aorta has not yet been observed in man.
- ↑ De Vriese has considered the history of the leg vessels in man. I will not, however, detail her account, for reasons given above in the account of the arm vessels.
- ↑ According to Hochstetter's (1S91) investigations on mammals the a. comes n. ischiadici does not appear to be a relic of the old ischiadica, although this assumption is made by most authors. De Yriese describes the lower leg portion of the original axial artery (a. n. interossei cruris) as becoming the a. peronea of the adult, giving over all of its important branches in the territory of the foot to the a. tibialis anterior.
- ↑ With the exception of Bradypus bidaetylus, Lemur catta, and man, in which it is much atrophied.
| Embryology - 26 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Keibel F. and Mall FP. Manual of Human Embryology II. (1912) J. B. Lippincott Company, Philadelphia.
- XVIII. Development of Blood, Vascular System and Spleen: Introduction | Origin of the Angioblast and Development of the Blood | Development of the Heart | The Development of the Vascular System | General | Special Development of the Blood-vessels | Origin of the Blood-vascular System | Blood-vascular System in Series of Human Embryos | Arteries | Veins | Development of the Lymphatic System | Development of the Spleen
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |