Abnormal Development - Drugs
Introduction
Thalidomide is a drug that was introduced on to the market on October 1, 1957 in West Germany. Thalidomide soon became a drug prescribed to pregnant women to combat symptoms associated with morning sickness. When taken during the first trimester of pregnancy, thalidomide prevented the proper growth of the fetus resulting in horrific birth defects in thousands of children around the world.
It was the linking of newborn abnormalities with the taking of thalidomide by an Australian clinician, William McBride, that identified it as a teratogenic agent causing a "thalidomide embryopathy".[1]
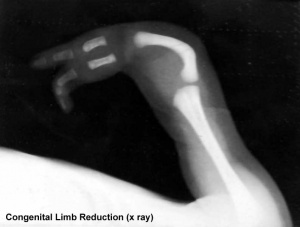
Children were born with limb and other defects, mainly in first world countries, in the late 1950's and early 1960's and became known as "Thalidomide babies".
Not all species embryos are affected by the drug in the same way, with human and rabbit being most susceptible to the teratogenic effects. In addition, the effect on human development is also dependent upon the time and dose of the drug exposure, the "critical periods".
More recent research has shown a clinical revival for thalidomide in its use in non-pregnant women during cancer chemotherapy.[2][3]
Some Recent Findings
- Identification of a primary target of thalidomide teratogenicity[4] "Here, we identified cereblon (CRBN) as a thalidomide-binding protein. CRBN forms an E3 ubiquitin ligase complex with damaged DNA binding protein 1 (DDB1) and Cul4A that is important for limb outgrowth and expression of the fibroblast growth factor Fgf8 in zebrafish and chicks. Thalidomide initiates its teratogenic effects by binding to CRBN and inhibiting the associated ubiquitin ligase activity. This study reveals a basis for thalidomide teratogenicity and may contribute to the development of new thalidomide derivatives without teratogenic activity."
- Monkey model[5] "Cynomolgus monkeys were orally administered thalidomide at 15 or 20mg/kg-d on days 26-28 of gestation, and fetuses were examined on day 100-102 of gestation. Limb defects such as micromelia/amelia, paw/foot hyperflexion, polydactyly, syndactyly, and brachydactyly were observed in seven of eight fetuses.'
- Thalidomide prevents angiogenic outgrowth during early limb formation.[6]
Cereblon
Cereblon (CRBN) was named based on its possible role in cerebral development and the presence of a Lon protease domain. Functionally it had been previously identified as being associated with neural development.
- Links: OMIM - Cereblon
Limb Reduction
Signaling Pathway
Two possible mechanisms:[4]
- cereblon dependent process
- cereblon independent process (producing reactive oxygen species)[7]
Thalidomide shown to:
- inhibits production of some cytokines (tumor necrosis factor–alpha and vascular endothelial growth factor)
- inducing apoptosis and producing reactive oxygen species
- fgf8 is a downstream target
Australia - Advisory Committee on Prescription Medicines
The Australian Drug Evaluation Committee (ADEC) was established in 1963 following the thalidomide experience and in 2010 this committee was replaced by the Advisory Committee on Prescription Medicines (ACPM). The new ACPM advises and makes recommendations to the Therapeutic Goods Administration (TGA) on prescription medicines listed on the Australian Register of Therapeutic Goods (ARTG), established under the Therapeutic Goods Act 1989. There were approximately 54,000 products on the Australian Register of Therapeutic Goods as at 23 May 2008.
Advisory Committee on Prescription Medicines
- inclusion of a prescription medicine on the Australian Register of Therapeutic Goods (the Register)
- changes to an entry of a prescription medicine on the Register
- removal or retention of a prescription medicine on the Register
Teratology
Now consider how different environmental effects during pregnancy may influence developmental outcomes. The terms listed below are often used to describe these environmental effects
- Teratogen (Greek, teraton = monster) any agent that causes a structural abnormality (congenital abnormalities) following fetal exposure during pregnancy. The overall effect depends on dosage and time of exposure. (More? Critical Periods of Development)
- Absolute risk the rate of occurrence of an abnormal phenotype among individuals exposed to the agent. (e.g. fetal alcohol syndrome)
- Relative risk the ratio of the rate of the condition among the exposed and the nonexposed. (e.g. smokers risk of having a low birth weight baby compared to non-smokers) A high relative risk may indicate a low absolute risk if the condition is rare.
- Mutagen a chemical or agent that can cause permanent damage to the deoxyribonucleic acid (DNA) in a cell. DNA damage in the human egg or sperm may lead to reduced fertility, spontaneous abortion (miscarriage), birth defects and heritable diseases.
- Fetotoxicant is a chemical that adversely affects the developing fetus, resulting in low birth weight, symptoms of poisoning at birth or stillbirth (fetus dies before it is born).
- Synergism when the combined effect of exposure to more than one chemical at one time, or to a chemical in combination with other hazards (heat, radiation, infection) results in effects of such exposure to be greater than the sum of the individual effects of each hazard by itself.
- Toxicogenomics the interaction between the genome, chemicals in the environment, and disease. Cells exposed to a stress, drug or toxicant respond by altering the pattern of expression of genes within their chromosomes. Based on new genetic and microarray technologies.
References
Reviews
- Saunders EJ, Saunders JA. Drug therapy in pregnancy: the lessons of diethylstilbestrol, thalidomide, and bendectin. Health Care Women Int. 1990;11(4):423-32.
- Stephens TD. Proposed mechanisms of action in thalidomide embryopathy. Teratology. 1988 Sep;38(3):229-39.
- Newman CG The thalidomide syndrome: risks of exposure and spectrum of malformations. Clin Perinatol. 1986 Sep;13(3):555-73.
- Fletcher I. Review of the treatment of thalidomide children with limb defeciency in Great Britain. Clin Orthop Relat Res. 1980 May;(148):18-25.
Articles
- Benegbi M. 45 years later...where do we stand? Can J Clin Pharmacol. 2007 Winter;14(1):e37-9. "The Thalidomide Victims Association of Canada (TVAC) was founded in 1988 and is the only organization in North America to work with and for Thalidomide victims. Our mission is to empower our members and to improve their quality of life through various programs and customized services. With the return of Thalidomide on the market, TVAC also took on the mandate of informing the public on the devastating effects of this medication and to promote awareness and caution when using any teratogenic products currently available".
- McBride WG. Prescription drugs in the first trimester and congenital malformations. Aust N Z J Obstet Gynaecol. 1992 Nov;32(4):386.
Books on Thalidomide
A selection of recent general public information books on Thalidomide, available from various internet commercial suppliers (search using the book title). Please note that this listing does not reflect an endorsement of the book or its content and is provided for educational purposes only.
- Thalidomide Kid (Paperback) by Kate, Rigby (Author)
- Thalidomide - A Medical Dictionary, Bibliography, and Annotated Research Guide to Internet References (Paperback) by ICON Health Publications (Author) Internet supplier link: Amazon
Search Pubmed
June 2010 "thalidomide teratogenicity" All (147) Review (57) Free Full Text (11)
Search Pubmed: thalidomide teratogenicity | Thalidomide
UNSW Embryology Links
- Abnormal Development
- Abnormal Development- Australian Statistics | Abnormalities by Systems
- Prenatal Diagnosis
- Genetic Abnormalities | Down Syndrome | Edwards Syndrome | Fragile X | Lesch-Nyhan Syndrome
- Maternal Factors | Maternal Diabetes | Maternal Hyperthermia | Neural Tube Defects | Fetal Alcohol Syndrome | Smoking | Chemical | Drugs | Illegal Drugs | Radiation | Heavy Metal | Iodine Deficiency | Viral Infection | Rubella | Polio | Parvovirus | Varicella | Bacterial Infection | Malaria | Toxoplasmosis | Autism
- Fetal Origins Hypothesis
- Intrauterine Growth Retardation
- Twinning
External Links
- United Nations International Drug Control Programme
- Australian Drug Foundation (ADF)
- Centre for Education and Information on Drugs and Alcohol (CEIDA) (Australia)
- Child Health and Safety (Australia)
- NIDA (USA)- Consequences of Prenatal Drug Exposure
- Australian Medicines Handbook (no electronic version yet)
- Australian Congenital Anomalies Monitoring System (ACAMS)
- Australian Institute of Health and Welfare (AIHW)
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2024, April 26) Embryology Abnormal Development - Drugs. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Abnormal_Development_-_Drugs
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G