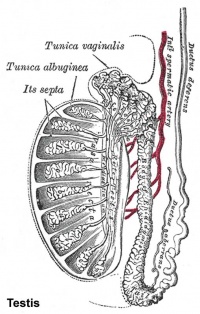
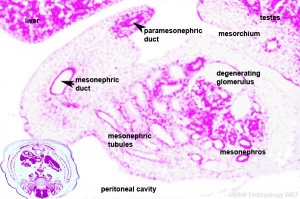
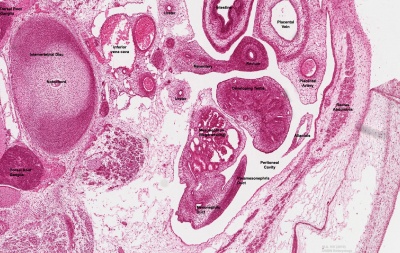
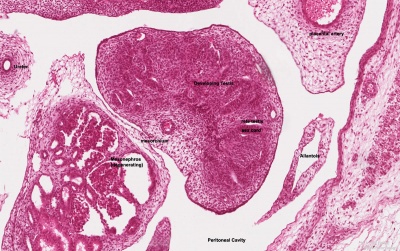
Testis Development
| Embryology - 27 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Introduction
The male gonad is the testis (pl, testes).
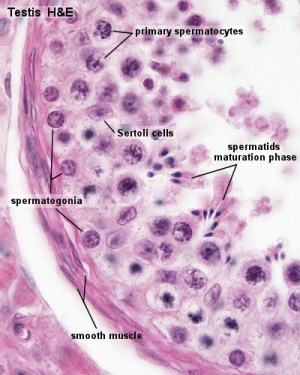
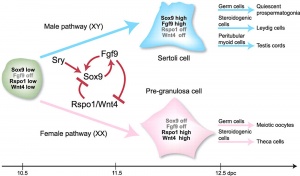
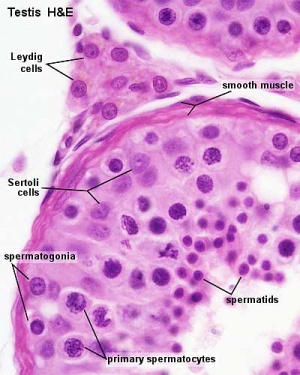
The initial difference in male and female gonad development are dependent on testis-determining factor (TDF) the protein product of the Y chromosome SRY gene. Recent studies have indicated that additional factors may also be required for full differentiation. The seminiferous tubules are considered the parenchyma of the testis. Within the developing testis the three main differentiating cell types are: gamete forming cells (spermatogonia), support cells (Sertoli cells) and hormone secreting cells (Leydig or interstitial cells).
Postnatally in humans, at 2 months of age, primordial germ cells (gonocytes) are replaced by adult dark (Ad) and pale (Ap) spermatogonia that make up the spermatogonial stem cell (SSC) population that at puberty will commence differentiation into spermatozoa. Postnatally, fetal Leydig cells are also replaced by adult cells. Development of the testis is driven at puberty by the endocrine HPG axis:
- hypothalamus - gonadotropin-releasing hormone (GnRH)
- pituitary - gonadotropins, leutenising hormone (LH) and follicle stimulating hormone (FSH)
- Gonad (testis) - testosterone
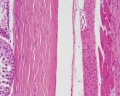
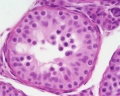
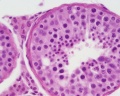
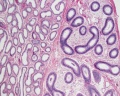
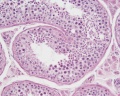
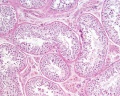
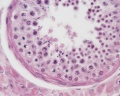
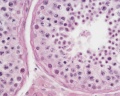
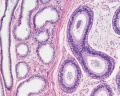
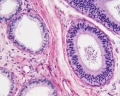
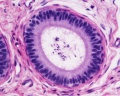
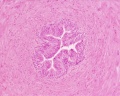
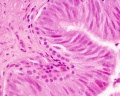
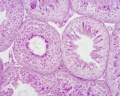
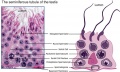
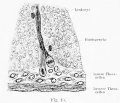
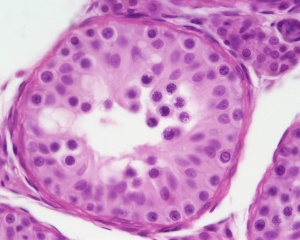
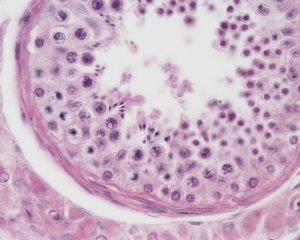
| Testis - Seminiferous Tubule | |
|---|---|
| Pre-puberty | Post-puberty |
|
|
| Cross-sectional view of the seminiferous tubule histology before and after puberty. | |
- Links: sertoli cell | Leydig cell | AMH | testosterone | Category:Testis
Some Recent Findings
|
| More recent papers |
|---|
|
This table allows an automated computer search of the external PubMed database using the listed "Search term" text link.
More? References | Discussion Page | Journal Searches | 2019 References | 2020 References Search term: Testis Embryology | Testis Development | Spermatogenesis | Leydig Cell Development | Sertoli Cell Development | Spermatogonia | Endocrine Testis | Testis Descent | Gubernaculum | Azoospermia | Anorchia | Cryptorchidism | Hydrocele |
| Older papers |
|---|
| These papers originally appeared in the Some Recent Findings table, but as that list grew in length have now been shuffled down to this collapsible table.
See also the Discussion Page for other references listed by year and References on this current page.
|
Movies
|
|
|
|
- Links: Movies
Development Overview
Sex Determination
- Humans (week 5-6)
- Germ cells migrate into gonadal ridge
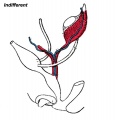
- Gonads (male/female) identical at this stage, Indifferent
Gonad Development
- dependent on sex chromosome
- Y testes
- No Y ovary
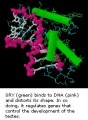
SRY
SRY protein (Testes determining factor, TDF) binds DNA Transcription factor, Bends DNA 70-80 degrees
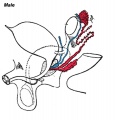
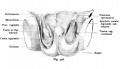
Internal Genital Organs
- All embryos form paired
- Mesonephric duct, see kidney development
- Paramesonephric duct, Humans 7th week Invagination of coelomic epithelium Cord grows and terminates on urogenital sinus
- Male Gonad (testes) secretes Mullerian duct inhibitory factor (MDIF) which causes regression of paramesonephric duct
- Male Gonad (testes) secretes Testosterone which retains mesonephric duct
External Genital Organs
- All embryos initially same (indifferent)
- Testosterone differentiates male
Week 8
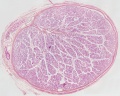
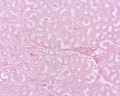
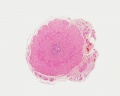
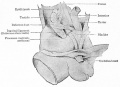
Developing testis is shown to the centre right. These images show the position, size and histological development of the testis in week 8 of human development (Carnegie stage 22, last embryonic week).
- Human Stage 22: Testis - labeled overview | Testis - unlabeled overview | Testis - unlabeled detail | Testis - labeled detail | testis | Carnegie stage 22 | Movie - Urogenital stage 22
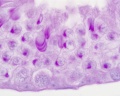
Sertoli Cells
The sertoli cell cells provide support for spermatozoa development within the seminiferous tubule. In the mouse, these cells are derived from the coelomic epithelium along with other testis somatic cells.[13] Their differentiation is regulated by the presence of a Y chromosome and in turn regulates Leydig cell differentiation. Sertoli cells direct testis morphogenesis, organizing testis cord formation, establishing testis vasculature and inducing differentiation of peritubular myoid cells and fetal Leydig cells. At puberty the immature Sertoli cells cease to proliferate and differentiate. Activin A acts upon Sertoli cells to promote their embryonic proliferation[14]
Sertoli cells express the androgen receptor and receptors for follicle stimulating hormone (FSH).
Sertoli cell functions include:
- regulation of spermatogenesis through endocrine FSH and testosterone
- regulation of the intratubular and intercellular environment adluminal to the tight junctional complexes
- meiotic and post-meiotic germ cells are sequestered by Sertoli-Sertoli junctional complexes
- generate adluminal compartment isolated from both serum and lymph
- attachment of germ cells through unique intermediate filament (desmosome-like junctions) and microfilament (actin- ectoplasmic specializations, ESs) junctions[15]
- to prevent premature sloughing of immature germ cells from the seminiferous epithelium
- desmosome-like junctions are initially present (up to step 8 spermatids)
- ectoplasmic specializations then replace this junction (in step 8 spermatids)
(see also review[16])
Ultrastructural description of human Sertoli cells[17]
- 7 weeks - first morphologically recognised in testicular cords, organised as primordial germ cells surrounded by pre-Sertoli cells.
- 7 to 8 weeks - basal lamina of the cords becomes distinguishable, pre-Sertoli cells the rough endoplasmic reticulum develops.
- 14 to 20 weeks - pre-Sertoli cells maintain their general morphology whereas the most significant change is the maximum development of Leydig cells.
Molecular factors:
- Follicle Stimulating Hormone (FSH) -> Krüppel-like factor 4 (KLF4)
- Krüppel-like factor 4 (KLF4) - zinc finger transcription factor, terminal differentiation of epithelial cells.
- Epidermal Growth Factor (EGF)
- Transforming Growth Factor-beta (TGFbeta)
- Links: sertoli cell
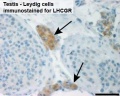
Leydig Cells
Interstitial or Leydig cell, named after German zoologist Franz von Leydig (1821 - 1908) who first histologically described them in 1850.[18] Their initial development appears to be influenced by Sertoli cell differentiation.
These cells produce the male testicular androgens and have a role during life prenatally (fetal) and postnatally during puberty onward.
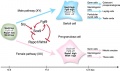
Fetal Leydig cells are mesenchymal cells developing from coelomic epithelium and undifferentiated perivascular cells in the gonad–mesonephros border region.
Adult Leydig cells appear after birth from stem/progenitor cells among peritubular and peri-vascular cells.
In the mouse both fetal and adult Leydig cells appear to arise from a common progenitor population.[19]
A recent study[20] has looked at the postnatal development of Leydig cells from stem cells:
- early postnatal testis - interstitial compartment undifferentiated mesenchymal-like stem cells
- adult testis - peritubular and perivascular locations quiescent stem cells (nestin, PDGFRα, COUP-TFII, CD51 and CD90)
Factors regulating division
- proliferation - DHH (Desert hedgehog), FGF2, PDGFBB, activin and PDGFAA
- suppression of proliferation - TGFβ, androgen and PKA signaling.
A study using Template:Induced pluripotent stem cells[21] shows "differentiation of hiPSCs into either human Leydig-like (hLLCs) or adrenal-like cells (hALCs) using chemically defined culture conditions. Factors critical for the development of LCs were added to both culture systems. hLLCs expressed all steroidogenic genes and proteins important for T biosynthesis, synthesized T rather than cortisol, secreted steroid hormones in response to dibutyryl-cAMP and 22(R)-hydroxycholesterol, and displayed ultrastructural features resembling LCs."
Fetal Leydig Cells
Fetal Leydig Cells (FLCs) have a hormonal role in male genitalia differentiation and are lost postnatally. The cells arise approximately at 6 weeks (human) and mouse E12.5 and there are differences in hormonal sensitivity between these two species. The cell initial differentiation requires both luteinizing hormone (LH) and adrenocorticotrophic hormone (ACTH) and therefore normal pituitary development.
Fetal Leydig Cells produce androstenedione but lack 17β-hydroxysteroid dehydrogenase (17β-HSD), to but produce testosterone. Fetal Sertoli cells do express 17β-HSD and can therefore convert androstenedione to testosterone.[22]
- Links: pituitary
Adult Leydig Cells
Adult Leydig cells (ALCs) have a hormonal role in puberty, secondary sex characteristics and sexual maturation. Their initial differentiation from peritubular mesenchymal cells does not require gonadotropin, but development and function are dependent upon luteinizing hormone (LH).
The cells differentiate with three discrete stages (newly formed, immature, mature) leading to a decrease in proliferation and increasing testosterone biosynthetic capacity. Insulin-like growth factor I (IGF-I) stimulates proliferation of immature cells and promotes their maturation. Testosterone and estrogen inhibit the process of precursor cell differentiation and may be responsible for the cessation of proliferation in the adult Leydig cells.
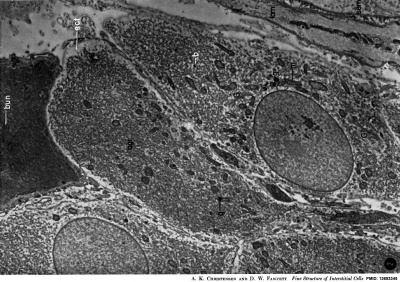
| Leydig Cell Electron Micrographs | |
|---|---|
|
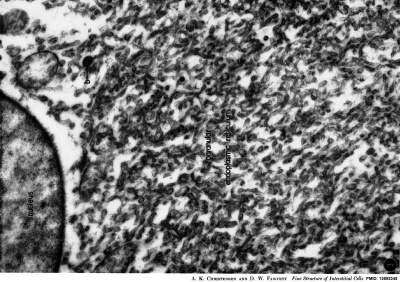
|
| Low power EM | High power EM |
EM images above from historic study of the opossum testis.[23]
Testosterone
Leydig cells produce testosterone. Historically in 1935, Ernst Laqueur (Amsterdam, Netherlands) isolated testosterone, and Adolf Butenandt (Gdansk. Poland) and Leopold Ruzicka (Zürich, Switzerland) synthesised testosterone.[24]
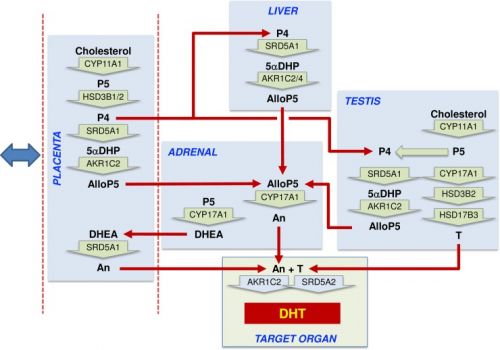
|
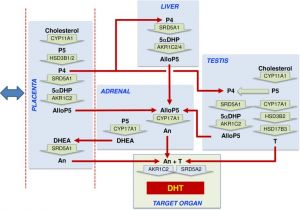
Proposed steroidogenic pathways leading to androsterone synthesis and masculinization in the second trimester human male fetus.[1]
Steroid hormone conversion is shown by wide green arrows, with the converting enzymes written within the arrow. Red arrows show potential transport between organs in the fetal circulation. The blue double-headed arrow indicates that exchange is also taking place between the placenta and the maternal circulation. Most circulating progesterone in the fetal circulation is likely to come from the placenta, and this will be reduced to 5αDHP by SRD5A1 in the placenta, fetal liver, and fetal testis, with the fetal liver likely to be the major site. Allopregnanolone (AlloP5) production by AKR1C2 is also most likely to occur in the placenta and fetal liver because the substrate is present in those tissues, and they express the highest total levels of enzyme transcript. Some conversion may also occur in the testis. Significant levels of androsterone are only detectable in the placenta and adrenal, and thus they are a likely source of the circulating steroid, although, given sex differences, other tissues are probably involved. The adrenal lacks other intermediates in the backdoor pathway, and thus AlloP5 must come from other tissues. The placenta lacks CYP17A1, so androsterone production is likely to depend on adrenal DHEA as substrate. Testosterone from the fetal testes also acts as an essential substrate for DHT synthesis at the external genitalia. AlloP5, allopregnanolone; An, androsterone; DHEA, dehydroepiandrosterone; DHT, 5α-dihydrotestosterone; P4, progesterone; P5, pregnenolone; T, testosterone; 5αDHP, 5α-dihydroprogesterone. |
The adult testes produce about 6-10 mg /day in males (~0.5 mg / day in females) carried in circulation by a specific carrier globulin.
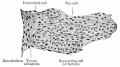
Peritubular Myoid Cells
The peritubular myoid cell surrounds the seminiferous tubule and have smooth muscle properties. Contraction of theses cells aids spermatozoa movement within the seminiferous tubule towards the epididymis for storage.
Tunica Albuginea
The capsule surrounding the testis is a multilayered fibrous structure. The main component of the capsule is the tunica albuginea composed dense collagen fibres, fibroblasts and smooth muscle cells.
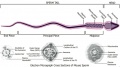
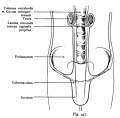
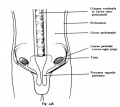
Epididymis
Both the ductus epididymis and ductus deferens differentiate from the mesonephric duct (Wollfian duct) elongation (cell proliferation). In the case of the epididymis, elongation also is associated with extensive coiling, the adult human epididymis about 6 metres in length (mouse 1m, rat 3m). Embryonic growth is regulated by androgens, members of the PCP pathway, and inhibin beta A. While postnatally androgens and other growth factors may have roles in final maturation. (see review[25]) The ductus epididymis is lined by a very tall pseudostratified columnar epithelium, consisting of principal cells with long stereo cilia.
Following puberty, the epididymis is involved in maturation of the spermatozoa released from the seminiferous tubules and their storage.
- middle segment - site of final functional maturation of the spermatozoa.
- terminal segment - site of storage of the mature spermatozoa.
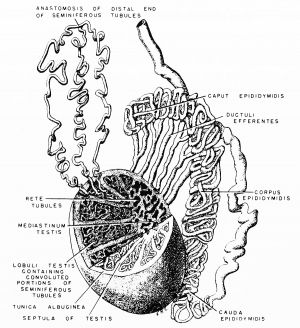
|
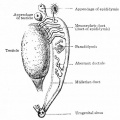
Anatomically the adult epididymis is about 30 cm in length and consists of 3 regions:
|
The head is intimately connected with the upper end of the testis by means of the efferent ductules of the gland; the tail is connected with the lower end by cellular tissue, and a reflection of the tunica vaginalis (tunica vaginalis propria testis) the serous covering of the testis.
The lateral surface, head and tail of the epididymis are free and covered by the serous membrane; the body is also completely invested by it, excepting along its posterior border; while between the body and the testis is a pouch, named the sinus of the epididymis (digital fossa). The epididymis is connected to the back of the testis by a fold of the serous membrane.
Caput
In humans, the majority of the caput (head, globus major) is primarily efferent ducts and not epididymal duct, with multiple efferent ducts connecting to the main epididymal duct.[26]
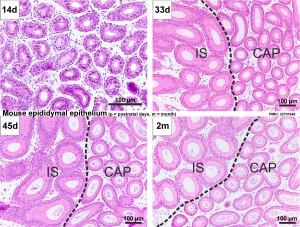
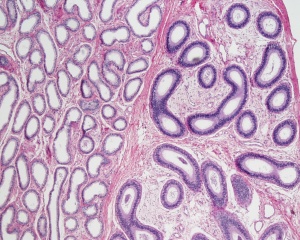
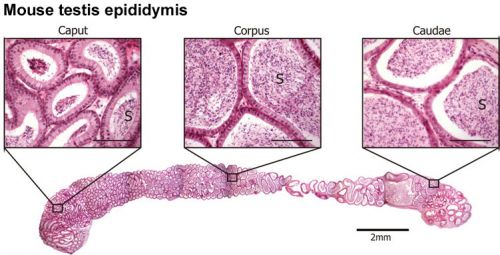
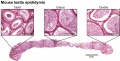
Adult Mouse Epididymis Histology[28]
Testis Descent
The research data on human testis descent timing has been highly variable. Testis descent is thought to have 2 phases:
- transabdominal descent - dependent on insulin-like hormone 3 (INSL3).
- inguinoscrotal descent - dependent on androgens.
Other suggested factors with a role in descent include: gonadotropin-releasing hormone, fibroblast growth factors, Müllerian inhibiting substance, and inhibin B[29]
The regulation of testis descent is still being investigated and several different factors have been identified that may have roles in descent. The first stage of testicular descent occurs 10–15 weeks of gestation with the testes moving to the inguinal region. The second inguino-scrotal phase occurs between 25-35 weeks.[30]
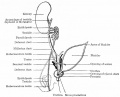
Gubernaculum
The gubernaculum (gubernaculum Hunteri) is the caudal inguinoscrotal ligament that connects the testis to the lower abdomen. The cranial suspensory ligament (mesonephric ligament) is the cranial ligament that connects the tesitis to the posterior abdominal wall.
- Insulin-like factor 3 (INSL3, relaxin-like factor) from fetal leydig cells acting through its receptor (Rxfp2) and BMP and WNT signaling pathways to promote testis descent.
- Calcitonin gene-related peptide (CGRP) from genitofemoral nerve suggested to mediate the inguinoscrotal testicular descent.
- Epidermal growth factor (EGF) may promote by activating the androgen responsive systems.
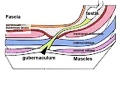
|
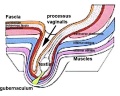
|
| |||
| Before Descent | End of Descent | Testis Descent Movie |
Puberty
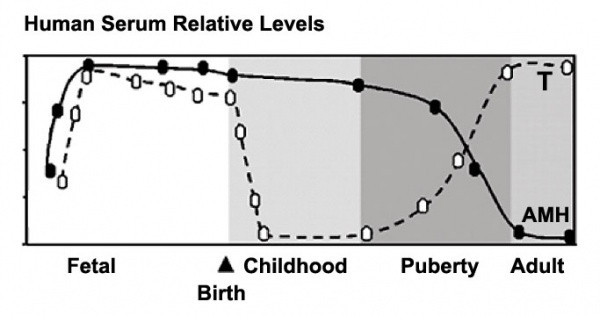
Human Male Testosterone and Anti-Müllerian Hormone (AMH) relative levels[31]
- AMH production by Sertoli cells .
- Testosterone production by Leydig cells.
In humans at puberty, hormonal and morphological changes occur within the gonad and other systems (secondary sex characteristics). Within the testis the immature Sertoli cells cease to proliferate and differentiate. Spermatogonium proliferate and spermatogenesis begins, and it takes about 70 days for cells to mature from the diploid spermatogonium to a primary spermatocyte. This maturation occurs in waves along the seminiferous tubules.
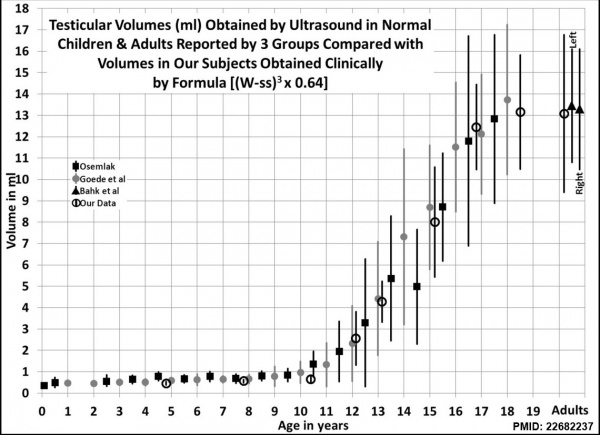
Male puberty testicular volume graph[32]
Testicular volume increase at puberty can be measured by: including orchidometry, rulers, calipers, and ultrasonography. There is also an earlier empirical formula developed by Lambert[33] used to estimate testicular volume.
Links: puberty
Orchidometer
- (orchiometer) A simple clinical instrument used to measure postnatal testis volume using 12 beads ranging from 1 to 25 millilitres. Developed by Dr. Andrea Prader (1919 – 2001) an endocrinologist who also discovered the Prader-Willi syndrome and developed the Prader scale, a second clinical classification used to describe CAH virilization of female genitalia.
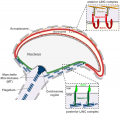
Blood-Testis Barrier
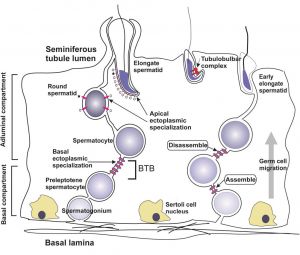
Within the testis seminiferous tubules the Sertoli cells located near the basement membrane act as an initial cellular barrier with many functions, but often described as forming a "blood-testis barrier". (see review[35]
Functions:
- prevent substances reaching the developing spermatozoa (through drug transporters)
- establish a basal and adluminal (apical) compartment (specialized microenvironment)
- provide an immunological privilege status of the testis (anti-sperm antibodies are not developed)
| Tight Junctions | Adherens Junctions |
|---|---|
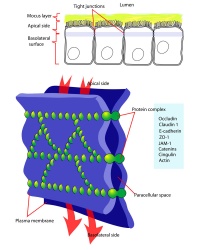
|
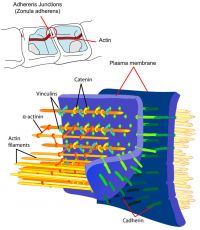
|
| Germ cells first have to break through the tight junctions at the BTB with minimal disruptions. | Progression of germ cells then relies on series of transient adherens junctions at the Sertoli–germ cell interface. |
| Assembly and disassembly of junctional complexes at the basal and apical ectoplasmic specialization occurs continuously. | |
Abnormalities
Male Infertility Genes
| Gene abbreviation | Name | Gene Location | Online Mendelian Inheritance in Man (OMIM) |
HUGO Gene Nomenclature Committee (HGNC) |
GeneCards (GCID) | Diagnosis |
|---|---|---|---|---|---|---|
| AURKC | Aurora kinase C | 19q13.43 | 603495 | 11391 | GC19P057230 | Macrozoospermia |
| CATSPER1 | Cation channel sperm-associated 1 | 11q13.1 | 606389 | 17116 | GC11M066034 | Asthenozoospermia |
| CFTR | Cystic fibrosis transmembrane conductance regulator | 7q31.2 | 602421 | 1884 | GC07P117465 | Obstructive azoospermia |
| DNAH1 | Dynein axonemal heavy chain 1 | 3p21.1 | 603332 | 2940 | GC03P052350 | Asthenozoospermia |
| DPY19L2 | Dpy-19-like 2 gene | 12q14.2 | 613893 | 19414 | GC12M063558 | Globozoospermia |
| GALNTL5 | Polypeptide N-acetylgalactosaminyltransferase-like 5 | 7q36.1 | 615133 | 21725 | GC07P151956 | Asthenozoospermia |
| MAGEB4 | MAGE family member B4 | Xp21.2 | 300153 | 6811 | GC0XP030260 | Azoospermia |
| NANOS1 | Nanos C2HC-type zinc finger 1 | 10q26.11 | 608226 | 23044 | GC10P119029 | Azoospermia |
| NR0B1 | Nuclear receptor subfamily 0 group B member 1 | Xp21.2 | 300473 | 7960 | GC0XM030322 | Azoospermia |
| NR5A1 | Nuclear receptor subfamily 5 group A member 1 | 9q33.3 | 184757 | 7983 | GC09M124481 | Azoospermia |
| SOHLH1 | Spermatogenesis and oogenesis-specific basic helix–loop–helix 1 | 9q34.3 | 610224 | 27845 | C09M135693 | Azoospermia |
| vSPATA16 | Spermatogenesis-associated 16 | 3q26.31 | 609856 | 29935 | GC03M172889 | Globozoospermia |
| SYCE1 | Synaptonemal complex central element protein 1 | 10q26.3 | 611486 | 28852 | GC10M133553 | Azoospermia |
| TAF4B | TATA-box binding protein-associated factor 4b | 18q11.2 | 601689 | 11538 | GC18P026225 | Azoospermia |
| TEX11 | Testis expressed 11 | Xq13.1 | 300311 | 11733 | GC0XM070528 | Azoospermia |
| TEX15 | Testis expressed 15, meiosis and synapsis associated | 8p12 | 605795 | 11738 | GC08M030808 | Azoospermia |
| WT1 | Wilms tumour 1 | 8p12 | 607102 | 12796 | GC11M032365 | Azoospermia |
| ZMYND15 | Zinc-finger MYND-type containing 15 | 17p13.2 | 614312 | 20997 | GC17P004740 | Azoospermia |
| Table data source[36] (table 1) Links: fertilization | spermatozoa | testis | Male Infertility Genes | Female Infertility Genes | oocyte | ovary | Genetic Abnormalities | ART Asthenozoospermia - (asthenospermia) term for reduced spermatozoa motility. Azoospermia - term for no spermatozoa located in the ejaculate. Globozoospermia - term for spermatozoa with a round head and no acrosome. | ||||||
International Classification of Diseases
| ICD-11 5A81 Testicular dysfunction or testosterone-related disorders |
|---|
|
| genital abnormalities | ICD-11 |
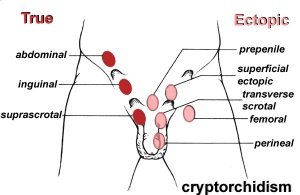
Cryptorchidism
| ICD-11 LB52 Cryptorchidism - A disorder affecting males, caused by an abnormality occurring in sex development during the antenatal period. This disorder is characterized by the absence of one or both testes from the scrotum. This disorder may also present with reduced fertility, psychological implications, or increased risk of testicular germ cell tumours. Confirmation is by imaging, karyotyping, or identification of male sex hormones in a blood sample. |
The external location of the testes in the scrotum acts as a local thermo-regulator and provides a temperature environment below that of the general body temperature.[38] This thermal function is essential for normal spermatogenesis and cryptorchidism therefore affects fertility.
- abnormality of either unilateral or bilateral testicular descent, occurring in up to 30% premature and 3-4% term males.
- Descent may complete postnatally in the first year, failure to descend can result in sterility.
Testis descent is thought to have 2 phases:
- transabdominal descent - dependent on insulin-like hormone 3 (INSL3).
- inguinoscrotal descent - dependent on androgens.
Management of cryptorchidism in children: guidelines.[39] "Cryptorchidism is best diagnosed clinically, and treated by surgical orchiopexy at age 6-12 months, without a routine biopsy. If no testis is palpable, or if other signs of hypovirilisation such as hypospadias are present, the chromosomal sex and hormonal status must be assessed. Laparoscopy is the best way of diagnosing and managing intra-abdominal testes."
- Links: cryptorchidism
Anorchia
| ICD-11 LB51 Anorchia or microorchidia - A disorder affecting males, caused by an abnormality occurring in sex development during the antenatal period. This disorder is characterized by individuals who are born with absence of the testes, or with testes that are deficient in size and function. Confirmation is by physical examination, identification of low testosterone levels but elevated follicle stimulating hormone and luteinizing hormone levels in a blood sample, or imaging. |
Clinical term for (embryonic testicular regression, vanishing testis syndrome) the absence of testes in a 46,XY individual with a male phenotype. Rare abnormality with an incidence of about 1 in 20,000 male births, and occurs more frequently with cryptorchidism (1 in 177 cases).
A recent study has identified undetectable plasma concentrations of anti-Müllerian hormone (AMH) and inhibin B and an elevated plasma FSH, together with 46,XY complement are sufficient for diagnosis of anorchia. Genetic analysis showed that NR5A1 and other genes (INSL3, SRY, LGR8 , MAMLD1) implicated in gonadal development and testicle descent were also not mutated.[40]
Myotonic dystrophy type 1
Postnatal muscular dystrophy resulting in myotonia, muscle weakness, abnormalities of heart, lungs, eye, brain and endocrine system. There is an associated progressive testicular atrophy (about 80% affected males) leading to Leydig cell hyperproliferation and elevated basal levels of follicle stimulating hormone (FSH).[41]
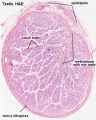
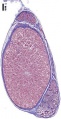
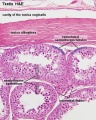
Histology
Testis Histology Links: Testis Development | Spermatozoa Development | Histology
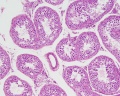
- Human (young): overview labeled | overview unlabeled | convoluted seminiferous tubules x10 | x40 | x40 | tunica albuginea x20
- Human (adult): overview x2 | convoluted seminiferous tubules labeled | x10 | x20 | x40 | x40 | epididymis ductulus efferens | ductus epididymidis | epithelium | overview x4 | x10 | x20 | x40 | ductus deferens labeled overview | epithelium | overview x2 | x10 | x40
- Human Stage 22: Testis - labeled overview | Testis - unlabeled overview | Testis - unlabeled detail | Testis - labeled detail | testis | Carnegie stage 22 | Movie - Urogenital stage 22
- Mouse: postnatal epididymis | 14 days postnatal | 33 days postnatal | 45 days postnatal | 2 months postnatal
| Spermatozoa Development (expand to see terms) | ||
|---|---|---|
|
Note there are additional glossaries associated with genital, spermatozoa, oocyte and renal.
See also: Spermatozoa Terms collapse table
|
Genital Links: genital | Lecture - Medicine | Lecture - Science | Lecture Movie | Medicine - Practical | primordial germ cell | meiosis | endocrine gonad | Genital Movies | genital abnormalities | Assisted Reproductive Technology | puberty | Category:Genital
| ||||
|
Molecular
Sry
- Y chromosome gene for a transcription factor
- member of the high mobility group (HMG)-box family of DNA binding proteins
- human - 204 amino acid protein[42]
- Links: OMIM - Sry
Sox9
Recent studies have identified upstream SOX9 enhancers that when duplicated or deleted result in 46,XX or 46,XY sex reversal, respectively.[43][44]
- autosomal transcription factor
- Development of XY females - presence of only a single functional copy of the transcription factor encoding genes SOX9, SF1, or WT1 (Note- not all XY humans are sex-reversed if only a single copy of a normal SF1 or WT1 allele is present)
- A nuclear export signal within the high mobility group domain regulates the nucleocytoplasmic translocation of SOX9 during sexual determination[45]
Other roles
- Cartilage - essential for chondrocyte differentiation
- Hearing - otic placode formation, maintenance of progenitors in the otic epithelium
Fog2
- transcription factor, named Friend of Gata2
- human - (8q23) 1,151 amino acid nuclear protein that contains 8 zinc finger motifs[46]
- dosage critical for fetal testis development in mice[47]
- Links: OMIM - Fog2
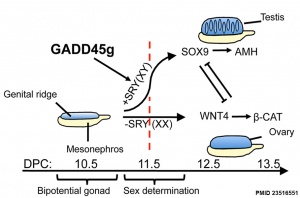
Gadd45g

Growth Arrest- And Dna Damage-Inducible Gene (GADD45, GAMMA; GADD45G)
A Recent mouse study[2] has shown that Gadd45g protein has a role in primary sex differentiation. Knockout mice (Gadd45g(-/-) XY gonads) resulted in a a sex reversal.
- Links: OMIM - Gadd45g
Gata4
- transcription factor
- dosage critical for fetal testis development in mice[47]
Eif2s3y
References
- ↑ 1.0 1.1 O'Shaughnessy PJ, Antignac JP, Le Bizec B, Morvan ML, Svechnikov K, Söder O, Savchuk I, Monteiro A, Soffientini U, Johnston ZC, Bellingham M, Hough D, Walker N, Filis P & Fowler PA. (2019). Alternative (backdoor) androgen production and masculinization in the human fetus. PLoS Biol. , 17, e3000002. PMID: 30763313 DOI.
- ↑ 2.0 2.1 2.2 2.3 Johnen H, González-Silva L, Carramolino L, Flores JM, Torres M & Salvador JM. (2013). Gadd45g is essential for primary sex determination, male fertility and testis development. PLoS ONE , 8, e58751. PMID: 23516551 DOI.
- ↑ Sakashita A, Wakai T, Kawabata Y, Nishimura C, Sotomaru Y, Alavattam KG, Namekawa SH & Kono T. (2019). XY oocytes of sex-reversed females with a Sry mutation deviate from the normal developmental process beyond the mitotic stage†. Biol. Reprod. , 100, 697-710. PMID: 30289439 DOI.
- ↑ Chimote BN & Chimote NM. (2018). Dehydroepiandrosterone (DHEA) and Its Sulfate (DHEA-S) in Mammalian Reproduction: Known Roles and Novel Paradigms. Vitam. Horm. , 108, 223-250. PMID: 30029728 DOI.
- ↑ Hoshi M, Reginensi A, Joens MS, Fitzpatrick JAJ, McNeill H & Jain S. (2018). Reciprocal Spatiotemporally Controlled Apoptosis Regulates Wolffian Duct Cloaca Fusion. J. Am. Soc. Nephrol. , 29, 775-783. PMID: 29326158 DOI.
- ↑ Wen Q, Wang Y, Tang J, Cheng CY & Liu YX. (2016). Sertoli Cell Wt1 Regulates Peritubular Myoid Cell and Fetal Leydig Cell Differentiation during Fetal Testis Development. PLoS ONE , 11, e0167920. PMID: 28036337 DOI.
- ↑ Caruso M, Ferranti F, Corano Scheri K, Dobrowolny G, Ciccarone F, Grammatico P, Catizone A & Ricci G. (2015). R-spondin 1/dickkopf-1/beta-catenin machinery is involved in testicular embryonic angiogenesis. PLoS ONE , 10, e0124213. PMID: 25910078 DOI.
- ↑ Bormann CL, Smith GD, Padmanabhan V & Lee TM. (2011). Prenatal testosterone and dihydrotestosterone exposure disrupts ovine testicular development. Reproduction , 142, 167-73. PMID: 21493716 DOI.
- ↑ Pask AJ, Calatayud NE, Shaw G, Wood WM & Renfree MB. (2010). Oestrogen blocks the nuclear entry of SOX9 in the developing gonad of a marsupial mammal. BMC Biol. , 8, 113. PMID: 20807406 DOI.
- ↑ Kleisner K, Ivell R & Flegr J. (2010). The evolutionary history of testicular externalization and the origin of the scrotum. J. Biosci. , 35, 27-37. PMID: 20413907
- ↑ Hiramatsu R, Harikae K, Tsunekawa N, Kurohmaru M, Matsuo I & Kanai Y. (2010). FGF signaling directs a center-to-pole expansion of tubulogenesis in mouse testis differentiation. Development , 137, 303-12. PMID: 20040496 DOI.
- ↑ Childs AJ, Cowan G, Kinnell HL, Anderson RA & Saunders PT. (2011). Retinoic Acid signalling and the control of meiotic entry in the human fetal gonad. PLoS ONE , 6, e20249. PMID: 21674038 DOI.
- ↑ Karl J & Capel B. (1998). Sertoli cells of the mouse testis originate from the coelomic epithelium. Dev. Biol. , 203, 323-33. PMID: 9808783 DOI.
- ↑ Archambeault DR & Yao HH. (2010). Activin A, a product of fetal Leydig cells, is a unique paracrine regulator of Sertoli cell proliferation and fetal testis cord expansion. Proc. Natl. Acad. Sci. U.S.A. , 107, 10526-31. PMID: 20498064 DOI.
- ↑ Kopera IA, Bilinska B, Cheng CY & Mruk DD. (2010). Sertoli-germ cell junctions in the testis: a review of recent data. Philos. Trans. R. Soc. Lond., B, Biol. Sci. , 365, 1593-605. PMID: 20403872 DOI.
- ↑ Griswold MD. (1995). Interactions between germ cells and Sertoli cells in the testis. Biol. Reprod. , 52, 211-6. PMID: 7711190
- ↑ Heyn R, Makabe S & Motta PM. (2001). Ultrastructural morphodynamics of human Sertoli cells during testicular differentiation. Ital J Anat Embryol , 106, 163-71. PMID: 11732573
- ↑ Zur Anatomie der männlichen Geschlechtsorgane und Analdrüsen der Säugetiere (On the anatomy of the male sexual organs and anal glands of the mammals). (1850) Zeitschr. f. wiss. Zoologie.
- ↑ Shima Y & Morohashi KI. (2017). Leydig progenitor cells in fetal testis. Mol. Cell. Endocrinol. , 445, 55-64. PMID: 27940302 DOI.
- ↑ Chen H, Wang Y, Ge R & Zirkin BR. (2017). Leydig cell stem cells: Identification, proliferation and differentiation. Mol. Cell. Endocrinol. , 445, 65-73. PMID: 27743991 DOI.
- ↑ Li L, Li Y, Sottas C, Culty M, Fan J, Hu Y, Cheung G, Chemes HE & Papadopoulos V. (2019). Directing differentiation of human induced pluripotent stem cells toward androgen-producing Leydig cells rather than adrenal cells. Proc. Natl. Acad. Sci. U.S.A. , 116, 23274-23283. PMID: 31591190 DOI.
- ↑ Wen Q, Cheng CY & Liu YX. (2016). Development, function and fate of fetal Leydig cells. Semin. Cell Dev. Biol. , 59, 89-98. PMID: 26968934 DOI.
- ↑ CHRISTENSEN AK & FAWCETT DW. (1961). The normal fine structure of opossum testicular interstitial cells. J Biophys Biochem Cytol , 9, 653-70. PMID: 13693345
- ↑ Nieschlag E & Nieschlag S. (2019). ENDOCRINE HISTORY: The history of discovery, synthesis and development of testosterone for clinical use. Eur. J. Endocrinol. , 180, R201-R212. PMID: 30959485 DOI.
- ↑ Hinton BT, Galdamez MM, Sutherland A, Bomgardner D, Xu B, Abdel-Fattah R & Yang L. (2011). How do you get six meters of epididymis inside a human scrotum?. J. Androl. , 32, 558-64. PMID: 21441421 DOI.
- ↑ Ilio KY & Hess RA. (1994). Structure and function of the ductuli efferentes: a review. Microsc. Res. Tech. , 29, 432-67. PMID: 7873793 DOI.
- ↑ Björkgren I, Saastamoinen L, Krutskikh A, Huhtaniemi I, Poutanen M & Sipilä P. (2012). Dicer1 ablation in the mouse epididymis causes dedifferentiation of the epithelium and imbalance in sex steroid signaling. PLoS ONE , 7, e38457. PMID: 22701646 DOI.
- ↑ Borg CL, Wolski KM, Gibbs GM & O'Bryan MK. (2010). Phenotyping male infertility in the mouse: how to get the most out of a 'non-performer'. Hum. Reprod. Update , 16, 205-24. PMID: 19758979 DOI.
- ↑ Hadziselimovic F. (2017). On the descent of the epididymo-testicular unit, cryptorchidism, and prevention of infertility. Basic Clin Androl , 27, 21. PMID: 29163975 DOI.
- ↑ Favorito LA, Bernardo FO, Costa SF & Sampaio FJ. (2016). Is there a trans-abdominal testicular descent during the second gestational trimester? Study in human fetuses between 13 and 23 weeks post conception. Int Braz J Urol , 42, 558-63. PMID: 27286121
- ↑ Rey R. (2005). Anti-Müllerian hormone in disorders of sex determination and differentiation. Arq Bras Endocrinol Metabol , 49, 26-36. PMID: 16544032 DOI.
- ↑ Sotos JF & Tokar NJ. (2012). Testicular volumes revisited: A proposal for a simple clinical method that can closely match the volumes obtained by ultrasound and its clinical application. Int J Pediatr Endocrinol , 2012, 17. PMID: 22682237 DOI.
- ↑ LAMBERT B. (1951). The frequency of mumps and of mumps orchitis and the consequences for sexuality and fertility. Acta Genet Stat Med , 2, 1-166. PMID: 15444009
- ↑ Wang CQ & Cheng CY. (2007). A seamless trespass: germ cell migration across the seminiferous epithelium during spermatogenesis. J. Cell Biol. , 178, 549-56. PMID: 17698604 DOI.
- ↑ Su L, Mruk DD & Cheng CY. (2011). Drug transporters, the blood-testis barrier, and spermatogenesis. J. Endocrinol. , 208, 207-23. PMID: 21134990 DOI.
- ↑ Harper JC, Aittomäki K, Borry P, Cornel MC, de Wert G, Dondorp W, Geraedts J, Gianaroli L, Ketterson K, Liebaers I, Lundin K, Mertes H, Morris M, Pennings G, Sermon K, Spits C, Soini S, van Montfoort APA, Veiga A, Vermeesch JR, Viville S & Macek M. (2018). Recent developments in genetics and medically assisted reproduction: from research to clinical applications. Eur. J. Hum. Genet. , 26, 12-33. PMID: 29199274 DOI.
- ↑ Virtanen HE & Toppari J. (2008). Epidemiology and pathogenesis of cryptorchidism. Hum. Reprod. Update , 14, 49-58. PMID: 18032558 DOI.
- ↑ Moore CR. (1924). THE BEHAVIOR OF THE GERMINAL EPITHELIUM IN TESTIS GRAFTS AND IN EXPERIMENTAL CRYPTORCHID TESTES (RAT AND GUINEA PIG). Science , 59, 41-4. PMID: 17839834 DOI.
- ↑ Gapany C, Frey P, Cachat F, Gudinchet F, Jichlinski P, Meyrat BJ, Ramseyer P, Theintz G & Burnand B. (2008). Management of cryptorchidism in children: guidelines. Swiss Med Wkly , 138, 492-8. PMID: 18726735 DOI.
- ↑ Brauner R, Neve M, Allali S, Trivin C, Lottmann H, Bashamboo A & McElreavey K. (2011). Clinical, biological and genetic analysis of anorchia in 26 boys. PLoS ONE , 6, e23292. PMID: 21853106 DOI.
- ↑ Ergoli M, Venditti M, Dotolo R, Picillo E, Minucci S & Politano L. (2017). Study of anti-Müllerian hormone levels in patients with Myotonic Dystrophy Type 1. Preliminary results. Acta Myol , 36, 199-202. PMID: 29770362
- ↑ Su H & Lau YF. (1993). Identification of the transcriptional unit, structural organization, and promoter sequence of the human sex-determining region Y (SRY) gene, using a reverse genetic approach. Am. J. Hum. Genet. , 52, 24-38. PMID: 8434602
- ↑ Gonen N, Futtner CR, Wood S, Garcia-Moreno SA, Salamone IM, Samson SC, Sekido R, Poulat F, Maatouk DM & Lovell-Badge R. (2018). Sex reversal following deletion of a single distal enhancer of Sox9. Science , 360, 1469-1473. PMID: 29903884 DOI.
- ↑ Croft B, Ohnesorg T, Hewitt J, Bowles J, Quinn A, Tan J, Corbin V, Pelosi E, van den Bergen J, Sreenivasan R, Knarston I, Robevska G, Vu DC, Hutson J, Harley V, Ayers K, Koopman P & Sinclair A. (2018). Human sex reversal is caused by duplication or deletion of core enhancers upstream of SOX9. Nat Commun , 9, 5319. PMID: 30552336 DOI.
- ↑ Gasca S, Canizares J, De Santa Barbara P, Mejean C, Poulat F, Berta P & Boizet-Bonhoure B. (2002). A nuclear export signal within the high mobility group domain regulates the nucleocytoplasmic translocation of SOX9 during sexual determination. Proc. Natl. Acad. Sci. U.S.A. , 99, 11199-204. PMID: 12169669 DOI.
- ↑ Holmes M, Turner J, Fox A, Chisholm O, Crossley M & Chong B. (1999). hFOG-2, a novel zinc finger protein, binds the co-repressor mCtBP2 and modulates GATA-mediated activation. J. Biol. Chem. , 274, 23491-8. PMID: 10438528
- ↑ 47.0 47.1 Bouma GJ, Washburn LL, Albrecht KH & Eicher EM. (2007). Correct dosage of Fog2 and Gata4 transcription factors is critical for fetal testis development in mice. Proc. Natl. Acad. Sci. U.S.A. , 104, 14994-9. PMID: 17848526 DOI.
Reviews
de Mello Santos T & Hinton BT. (2019). We, the developing rete testis, efferent ducts, and Wolffian duct, all hereby agree that we need to connect. Andrology , , . PMID: 31033257 DOI.
Harpelunde Poulsen K & Jorgensen A. (2019). Role of Nodal signalling in testis development and initiation of testicular cancer. Reproduction , , . PMID: 30999282 DOI.
Stévant I & Nef S. (2019). Genetic Control of Gonadal Sex Determination and Development. Trends Genet. , 35, 346-358. PMID: 30902461 DOI.
Rotgers E, Jørgensen A & Yao HH. (2018). At the crossroads of fate - somatic cell lineage specification in the fetal gonad. Endocr. Rev. , , . PMID: 29771299 DOI.
Stévant I & Nef S. (2018). Single cell transcriptome sequencing: A new approach for the study of mammalian sex determination. Mol. Cell. Endocrinol. , 468, 11-18. PMID: 29371022 DOI.
Barton LJ, LeBlanc MG & Lehmann R. (2016). Finding their way: themes in germ cell migration. Curr. Opin. Cell Biol. , 42, 128-137. PMID: 27484857 DOI.
De Felici M. (2016). The Formation and Migration of Primordial Germ Cells in Mouse and Man. Results Probl Cell Differ , 58, 23-46. PMID: 27300174 DOI.
Virtanen HE & Toppari J. (2014). Embryology and physiology of testicular development and descent. Pediatr Endocrinol Rev , 11 Suppl 2, 206-13. PMID: 24683945
Svingen T & Koopman P. (2013). Building the mammalian testis: origins, differentiation, and assembly of the component cell populations. Genes Dev. , 27, 2409-26. PMID: 24240231 DOI.
Hinton BT, Galdamez MM, Sutherland A, Bomgardner D, Xu B, Abdel-Fattah R & Yang L. (2011). How do you get six meters of epididymis inside a human scrotum?. J. Androl. , 32, 558-64. PMID: 21441421 DOI.
Griswold SL & Behringer RR. (2009). Fetal Leydig cell origin and development. Sex Dev , 3, 1-15. PMID: 19339813 DOI.
Articles
Nef S & Wilhelm D. (2018). The impact of new technologies in our understanding of testis formation and function. Mol. Cell. Endocrinol. , 468, 1-2. PMID: 29807571 DOI.
Kaftanovskaya EM, Feng S, Huang Z, Tan Y, Barbara AM, Kaur S, Truong A, Gorlov IP & Agoulnik AI. (2011). Suppression of insulin-like3 receptor reveals the role of β-catenin and Notch signaling in gubernaculum development. Mol. Endocrinol. , 25, 170-83. PMID: 21147849 DOI.
Stukenborg JB, Colón E & Söder O. (2010). Ontogenesis of testis development and function in humans. Sex Dev , 4, 199-212. PMID: 20664245 DOI.
Hutson JM, Balic A, Nation T & Southwell B. (2010). Cryptorchidism. Semin. Pediatr. Surg. , 19, 215-24. PMID: 20610195 DOI.
Adham IM & Agoulnik AI. (2004). Insulin-like 3 signalling in testicular descent. Int. J. Androl. , 27, 257-65. PMID: 15379965 DOI.
Heyns CF. (1987). The gubernaculum during testicular descent in the human fetus. J. Anat. , 153, 93-112. PMID: 2892824
Books
De Groot LJ, Chrousos G, Dungan K, Feingold KR, Grossman A, Hershman JM, Koch C, Korbonits M, McLachlan R, New M, Purnell J, Rebar R, Singer F, Vinik A & Hutson JM. (2000). Cryptorchidism and Hypospadias. , , . PMID: 25905331
Search PubMed
Search Pubmed: Testis Development | Epididymis Development | Sry | Sox9 |
Additional Images
Historic Images
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
External Links
External Links Notice - The dynamic nature of the internet may mean that some of these listed links may no longer function. If the link no longer works search the web with the link text or name. Links to any external commercial sites are provided for information purposes only and should never be considered an endorsement. UNSW Embryology is provided as an educational resource with no clinical information or commercial affiliation.
- Blue Histology - Male Reproductive System
Genital Links: genital | Lecture - Medicine | Lecture - Science | Lecture Movie | Medicine - Practical | primordial germ cell | meiosis | endocrine gonad | Genital Movies | genital abnormalities | Assisted Reproductive Technology | puberty | Category:Genital
| ||||
|
| Y Chromosome | Week 1 - Spermatogenesis | Ovary | Puberty
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2024, April 27) Embryology Testis Development. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Testis_Development
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G