Penis Development
| Embryology - 27 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Introduction
The external male genitalia consists of the penis and scrotum containing the testis.
Historic: 1912 Development of the Penis and Scrotum | 1935 Prepuce
Some Recent Findings
|
| More recent papers |
|---|
|
This table allows an automated computer search of the external PubMed database using the listed "Search term" text link.
More? References | Discussion Page | Journal Searches | 2019 References | 2020 References Search term: Penis Embryology | Penis Development | Prepuce Development | Foreskin Development | Hypospadias | Epispadias | Penile Urethra Development |
| Older papers |
|---|
| These papers originally appeared in the Some Recent Findings table, but as that list grew in length have now been shuffled down to this collapsible table.
See also the Discussion Page for other references listed by year and References on this current page.
|
Movies
| <html5media height="400" width="270">File:Male_external_001.mp4</html5media> | This looped animation shows the development of external male genitalia from the indifferent external structure, covering the approximate period of week 9 to 12. (Anterior to top, Posterior to bottom)
|
Mesonephic Duct Development
The paired mesonephic ducts (Wolffian ducts) go through a series of developmental changes to form the male internal genital tract.
Prenatal Development
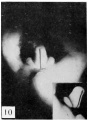
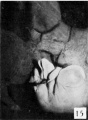
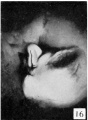
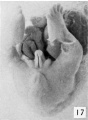
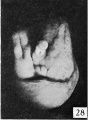
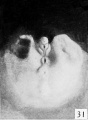
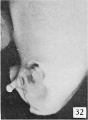
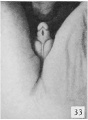
The following figures and text are from the historic 1921 study by Spaulding.[7] In this paper he defined using the Carnegie Collection embryos specific morphological "stages" (stage 1 to 10) of external genital development in both male and female embryonic and fetal periods. This is divided into the Genital-Tubercle Period (Stage 1 to 4), Phallus Period (Stage 5 to 9) and Definitive Period ((Stage 10). Note these are not the Carnegie stages, that can be identified by the Carnegie embryo number.
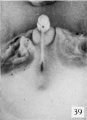
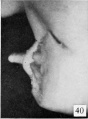
Fig. 16. Carnegie Embryo 1900-60b Male
Fig. 17. Carnegie Embryo 879c Male
Fig. 18. Carnegie Embryo 1022d Male
Fig. 22. Carnegie Embryo 1358h Male
Fig. 25. Carnegie 652K, 44 mm Male
Fig. 42. Carnegie nn34k, 75 mm Male
Development of the Male Genitalia
(Text extract from 1921 paper male summary)[7]
The genital tubercle, from which is derived the phallus and which includes the urethral and anal openings, arises as a broad conical eminence barely separated cranially from the umbilical cord, but with a well-defined caudal slope. Its rounded free end, as the primordium of the glans, is faintly separated from the basal portion. The lateral slopes, below the glans area, extend into the outlying basal tissue as the "lateral buttresses, " which I interpret as enlargements for the developing corpora cavernosa. The caudal slope is bisected by the shallow urethral groove whose margins are slightly elevated into the urethral folds. Distally, these margins merge into the glans area, while proximally they continue into the basal enlargements surrounding the anal pit. In the majority of embryos which have attained a length of 12 mm. the urethral membrane is ruptured to form the primitive urogenital opening, although a few cases have been found in which this perforation had not taken place at the stage of 16 to 17 mm. Practically from its first appearance the urethral groove shows a sex difference in its length, this difference being further accentuated upon the formation of the urogenital opening.
As development proceeds, the elongation of the tubercle and the medial migration of its lateral buttresses transform it into the somewhat cylindrical phallus, whose base is separated from the surrounding body areas by the newly formed labio-scrotal swellings (embryos 17 to 19 mm). The latter, appearing first as broad, elevated areas extending laterally from the base of the phallus, are soon transformed into swollen ridges separated, in both sexes, from the base of the phallus by the lateral phallic grooves. It may be emphasized that in the majority of specimens the labio-scrotal swellings seem to be from the first separated from the phallus. In fact, only one embryo was found which showed these areas as definitely merging into the phallus, while in all of the others of about the same age the swellings were already separated from the phallus by pronounced grooves, the lateral phallic grooves. Accompanying the elongation of the tubercle into the phallus, the urogenital opening becomes gradually more sharply outlined and the sex difference in its length correspondingly emphasized. The urethral folds which form the margins of the opening also increase in definiteness, partly through their own elevation and partly through their lateral demarcation from the cavernous portion of the shaft; the result of these combined factors being that the folds extend as plate-like caudal projections from the more cylindrical shaft. At the same time the terminal glans has increased in density so that it now forms an opaque white area in contrast to the translucent shaft, although the limiting coronary sulcus is not formed until the close of the phallus period.
As a result of these combined changes the male phallus at this time is markedly different from that of the female. The length of the urogenital opening is still, however, the chief diagnostic feature in the two sexes. In the male it consists of a long sUt extending from the base to the apex of the phallus, whereas in the female it extends only to the base of the glans. In the male it is more open distally, the apposition of the urethral folds reducing it proximally to a mere slit. This is in marked contrast to the condition in the female, where the opening (restricted to the shaft) has exactly the opposite shape, being broadest basally, while the terminal portion is narrowed into a slit.
No further pronounced morphological changes take place in the male genitalia until after the embryo has reached a CR length of 38 mm. At this time a series of changes begins which result in the transformation of the male genitalia into approximately their final form (about 45 mm.) and in the complete separation of the urogenital opening from the anus. The gutter-like urogenital sinus is transformed into the tubular urethra by the merging of the basal portions of the urethral folds into the raphe, reducing the primitive urogenital orifice to an irregularly shaped opening on the distal portion of the shaft. At the same time the glans becomes sharply defined by the formation of a wide coronary sulcus and the labio-scrotal swellings assume their final position caudal to the penis to form the scrotum, the halves of which become more or less closely approximated to the raphe in the midventral line, although they never entirely lose their bilateral character.
This period (45 mm. CR length) marks the completion of the definite' sex differentiation, characterized by the development of definite structural features in the male in sharp contrast to the lack of these characters in the female.
As growth continues, the gradual constriction of the urethral opening synchronous with the formation of the prepuce, to be described later, is accompanied by an outgrowth of its rim which, in embryos 60 to 85 mm. CR length, results in the formation of a decided cup in the bottom of which the opening is located. With the later stages of prepuce formation the urethral opening is shifted to a more terminal position in the frenular notch of the glans. It then becomes entirely closed and eventually there is formed the new, permanent terminal urethral opening on the apex of the glans.
The first evidence of the prepuce is found in embryos of about 65 mm. CR length, at which time it can be recognized as a pair of swellings on each side of the urethral opening. Gradually these swelUngs fuse together over the dorsum of the shaft to form a flattened ridge of skin whose distal margin has enveloped the proximal margin (corona glandis) of the glans (embryos 75 mm. CR length). Subsequent growth results in the progressive inclosure of the glans by this distally migrating fold of skin until at about 100 mm. CR length the originally naked glans is entirely covered. It must be emphasized that these observations upon the formation of the prepuce are based only upon the external examination of the genitalia of these older embryos and have not been confirmed by histological study. For this reason they must be considered merely as suggestions of what apparently takes place and may later be corroborated or contradicted when it becomes possible to make a study of sectioned embryos showing this development.
Initiation
Invagination
Elongation
- Links:
Foreskin Development
The foreskin (prepuce) is a protective double-layered fold covering the glans (head) the penis formed by smooth muscle tissue, blood vessels, nerves, integument, and a mucous membrane. The foreskin is retractable in only about 1 in 20 boys. Circumcision describes the medical removal of the foreskin from the human penis.
Innervation includes: somatosensory (penis dorsal nerve and branches of perineal nerve(, autonomic (pelvic plexus), and somatosensory receptors (mechanoreceptors and nociceptors).
Foreskin development occurs during the second trimester.[8]
- GA week 12 (Week 10) - ectoderm circular invagination at the glandular periphery now grows ventrally.
- GA week 13 (Week 12) - glans partially covered by the foreskin.
- GA week 16-17 (Week 14-15) - glans almost completely covered by the foreskin.
- GA week 18-19 (Week 16-17) - complete foreskin was formed.
- GA week 20 (Week 18) - entirely involves the glans.
Historic
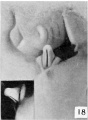
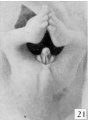
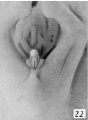
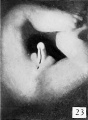
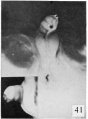
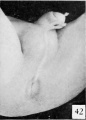
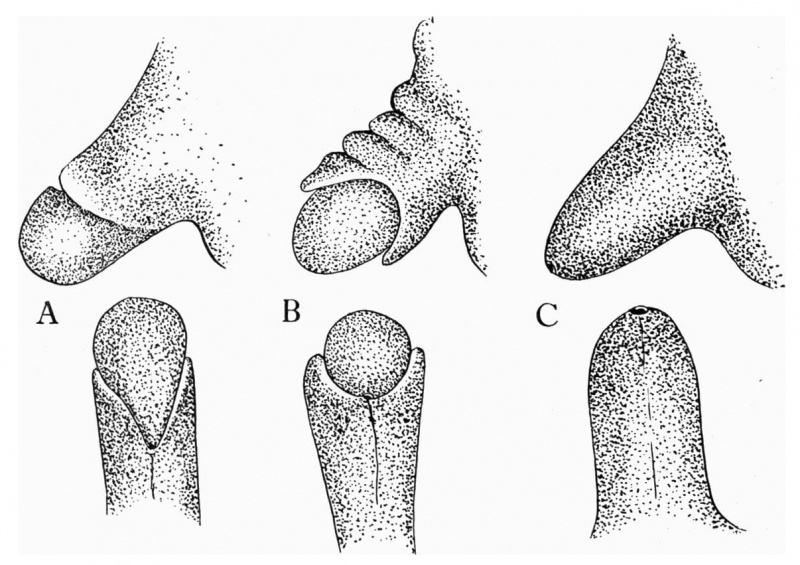
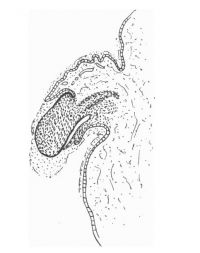
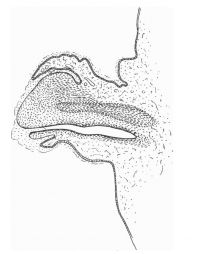
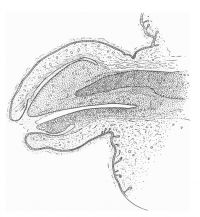
The figures below are from a paper by Hunter (1935)[9]

|
|
|
|
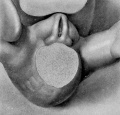
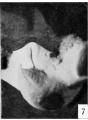
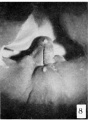
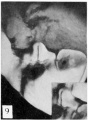
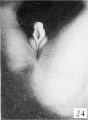
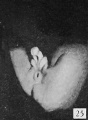
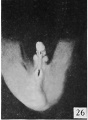
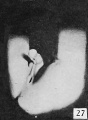
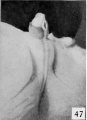
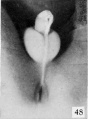
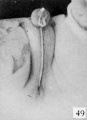
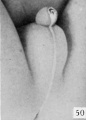
| Penis of human foetus 40 mm CRL. Note the ridge of ectoderm behind the corona and the glans, and the thin layer of desquamating cells on the surface. | Penis of human foetus 70 mm CRL. Note the dorsal ectodermal tissues thrown into loose folds, and the distribution of the desquamating cells. | Penis of human foetus 100 mm CRL. The prepuce is here seen “overflowing” the glans and enclosing a layer of desquamating cells between it and the glans. | Penis of human foetus 170 mm CRL. This section shows the prepuce covering the whole of the glans, with the layer of desquamating cells connecting the two. Note the “plug” of desquamating cells closing the opening of the urethra. |
Figure shows the lateral and ventral aspects of wax-plate reconstructions of three stages in the development of the prepuce. A, 40 mm CRL.; B, 70 mm. CRL.; C, 170 mm CRL.
Postnatal Development
Abnormalities
Hypospadias
| ICD-11 |
|---|
Hypospadias
Hypospadias, coronal - abnormally placed urinary meatus that opens in the ventral portion of the coronal sulcus. |
External urethral opening on the ventral surface of penis, can extend back into scrotum. Abnormality due to failure of genital fold fusion during fetal development of the male external genitalia.
| Hypospadia Classification | Meatus Opening |
| Anterior | on inferior surface of glans penis |
| Coronal | in balanopenile furrow |
| Distal | on distal third of shaft |
| Penoscrotal | at base of shaft in front of scrotum |
| Scrotal | on scrotum or between the genital swellings |
| Perineal | behind scrotum or genital swellings |
| Links: Genital Abnormalities | Penis Development | |
- Links: hypospadias
Epispadias
Uncommon abnormality associated with the penis, 1 in 30,000 infant males, external urethral opening on the dorsal surface of penis.
| ICD-11 LB55 Epispadias - Epispadias is a congenital genitourinary malformation belonging to the spectrum of the exstrophy-epispadias complex and is characterized in males by an ectopic meatus or a mucosal strip in place of the urethra on the penile dorsum and in females by bifid clitoris and a variable cleft of the urethra. |
- Search PubMed: Epispadias
Aphallia
Congenital absence of the penis (aphallia) is associated with early failure of genital tubercle development.[10][11][12]
- Search PubMed: Aphallia
Endocrine Disruptors
Endocrine disruptors in female reproductive tract development and carcinogenesis.[13]
Additional Images
Historic
References
- ↑ Shen J, Isaacson D, Cao M, Sinclair A, Cunha GR & Baskin L. (2018). Immunohistochemical expression analysis of the human fetal lower urogenital tract. Differentiation , 103, 100-119. PMID: 30287094 DOI.
- ↑ Baskin L, Shen J, Sinclair A, Cao M, Liu X, Liu G, Isaacson D, Overland M, Li Y & Cunha GR. (2018). Development of the human penis and clitoris. Differentiation , 103, 74-85. PMID: 30249413 DOI.
- ↑ Liu G, Liu X, Shen J, Sinclair A, Baskin L & Cunha GR. (2018). Contrasting mechanisms of penile urethral formation in mouse and human. Differentiation , 101, 46-64. PMID: 29859371 DOI.
- ↑ Shen J, Overland M, Sinclair A, Cao M, Yue X, Cunha G & Baskin L. (2016). Complex epithelial remodeling underlie the fusion event in early fetal development of the human penile urethra. Differentiation , 92, 169-182. PMID: 27397682 DOI.
- ↑ Ishiguro T, Tamagawa S & Ogawa H. (1992). [Changes of pupil size in brain death patients]. Seishin Shinkeigaku Zasshi , 94, 864-73. PMID: 1484906
- ↑ Gallo CB, Costa WS, Furriel A, Bastos AL & Sampaio FJ. (2014). Modifications of erectile tissue components in the penis during the fetal period. PLoS ONE , 9, e106409. PMID: 25170760 DOI.
- ↑ 7.0 7.1 Spaulding MH. The development of the external genitalia in the human embryo. (1921) Contrib. Embryol., Carnegie Inst. Wash. Publ. 81, 13: 69 – 88.
- ↑ Favorito LA, Balassiano CM, Costa WS & Sampaio FJ. (2012). Development of the human foreskin during the fetal period. Histol. Histopathol. , 27, 1041-5. PMID: 22763876 DOI.
- ↑ Hunter RH. Notes on the development of the prepuce. (1935) J Anat. 70: 68-75. PMID 17104576
- ↑ Qiang S, Li FY, Zhou Y, Yuan Y & Li Q. (2019). Congenital absence of the penis (aphallia): A rare case report. Medicine (Baltimore) , 98, e15129. PMID: 30985678 DOI.
- ↑ Gabler T, Charlton R, Loveland J & Mapunda E. (2018). Aphallia: a review to standardize management. Pediatr. Surg. Int. , 34, 813-821. PMID: 29679134 DOI.
- ↑ Joshi A, Gross J & Thomalla JV. (2015). Congenital Aphallia: Review of Pathogenesis and Current Treatment Guidelines. Urology , 86, 384-7. PMID: 26194292 DOI.
- ↑ Ma L. (2009). Endocrine disruptors in female reproductive tract development and carcinogenesis. Trends Endocrinol. Metab. , 20, 357-63. PMID: 19709900 DOI.
Reviews
Cunha GR & Baskin L. (2018). Development of human male and female urogenital tracts. Differentiation , 103, 1-4. PMID: 30262219 DOI.
Articles
Ramaswamy VV, Rao GV, Suryanarayana N & Gummadapu S. (2019). Unusual finding in the karyotype of a neonate with glandular hypospadias with chordee. BMJ Case Rep , 12, . PMID: 30898940 DOI.
Liu X, Liu G, Shen J, Yue A, Isaacson D, Sinclair A, Cao M, Liaw A, Cunha GR & Baskin L. (2018). Human glans and preputial development. Differentiation , 103, 86-99. PMID: 30245194 DOI.
Isaacson D, Shen J, Overland M, Li Y, Sinclair A, Cao M, McCreedy D, Calvert M, McDevitt T, Cunha GR & Baskin L. (2018). Three-dimensional imaging of the developing human fetal urogenital-genital tract: Indifferent stage to male and female differentiation. Differentiation , 103, 14-23. PMID: 30262218 DOI.
Liu G, Liu X, Shen J, Sinclair A, Baskin L & Cunha GR. (2018). Contrasting mechanisms of penile urethral formation in mouse and human. Differentiation , 101, 46-64. PMID: 29859371 DOI.
Wisniewski H & Terry RD. (1968). Further studies on experimental neurofibrillary tangles. J. Neuropathol. Exp. Neurol. , 27, 149-50. PMID: 5690451
Genital Links: genital | Lecture - Medicine | Lecture - Science | Lecture Movie | Medicine - Practical | primordial germ cell | meiosis | endocrine gonad | Genital Movies | genital abnormalities | Assisted Reproductive Technology | puberty | Category:Genital
| ||||
|
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2024, April 27) Embryology Penis Development. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Penis_Development
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G