Paper - Two Early Human Embryos
| Embryology - 27 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Morton WRM. Two early human embryos. (1949) J. Anat., 83: 308-314.
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
Two Early Human Embryos
By W.R.M. Morton
Department of Anatomy, Queen's University, Belfast
Introduction
The two embryos described here were given to me by Prof. J. H. Biggart, who had recovered them from curettage material removed by Prof. C. H. G. Macafee; I have named the older specimen the Biggart Ovum and the younger the Macafee Ovum. The material was fixed in formol-saline and later transferred to formol-Zenker; it was embedded in parafiin, cut at 6 /4 and stained with haematoxylin and eosin.
The Biggart Ovum
The Biggart ovum was recovered from a woman aged 24 years; married 7 years; one child 4 years 8 months; no miscarriages; menstruation began at 15, 7 ~day loss recurring very regularly every 21 days; last menstruation stated to have ended 29 June; admitted to hospital 12 July and curetted 16 July; coitus said to have occurred only once between the end of menstruation and admission, probably on 2 July and certainly between 1 and 5 July.
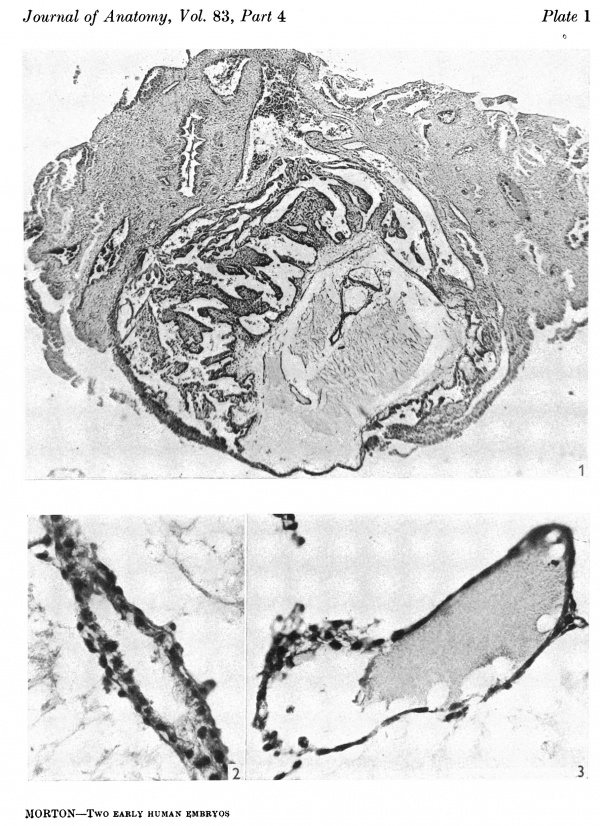
The specimen consists of a piece of endometrium in which the ovum is very superficially implanted (Pl. 1, fig. 1). Examination shows the preservation to be good and that only some peripheral villous material and one section containing embryonic material proper are missing; but some of the sections are a little distorted. It was found to be cut almost vertically to the embryonic shield and obliquely at 13° to its sagittal plane. The primary measurements in millimetres of the ovum and its parts are as follows.
| Region | Antero-posterior | Vertical | Transverse |
|---|---|---|---|
| Trophoblast, external, including the villi | 2.53 | 2.10 | 1.68 |
| Trophoblast cavity | 1.53 | 1.05 | 0.83 |
| Shield ectoderm | 0.27 | 0.027 | 0.16 |
| Amniotic cavity | 0.22 | 0.10 | 0.16 |
| Yolk-sac cavity | 0.19 | 0.82 | 0.18 |
- Shield ectoderm antero-posterior measurement taken diagonally from the graphic reconstruction.
The endometrium shows early decidual reaction and contains peripheral syncytium beyond the limits of the trophoblastic shell; there is no zone of necrosis. The glands are dilated and in the saw-tooth phase; they contain extravasated blood and secretion. Large blood sinuses are present beneath the ovum and seem to have limited the villus formation over one part of the vesicle. The site of entry is wide and filled with the thin ab-embryonic wall of the chorionic vesicle covered by a thin layer of schlusscoagulum; there is no operculum.
The trophoblast, which is lined with a chorionic mesoderm, consists of cytotrophoblast and syncytiotrophoblast, and villi are present over most of it, but are absent over the superficial surface and over one part of the deep surface near a large venous sinus (Pl. 1, fig. 1). Many of the villi contain a mesodermal core and some have begun to branch.
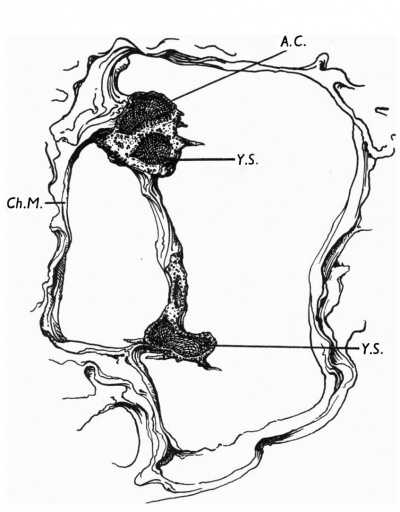
The chorionic vesicle contains a granular coagulum surrounding the embryonic rudiment, which consists of an embryonic shield, a small anmiotic cavity, and a larger yolk-sac which is continued by a. long narrow tubular duct into a large irregular expansion (Text-fig. 1). The embryonic rudiment is joined to the chorionic mesoderm first by a broad connecting stalk attached to the amnion, and secondly by mesoderm at the distal expansion of the yolk—sac (Text-fig. 1).
|
|
| Text-fig. 1. Drawing of wax model cut to show the relations of the embryonic mass. A.C. Amniotic cavity. C'h.M. Chorionic mesoderm. Y.S. Yolk-sac. |
Text-fig. 2. Scale diagram of surface view of the embryonic shield. |
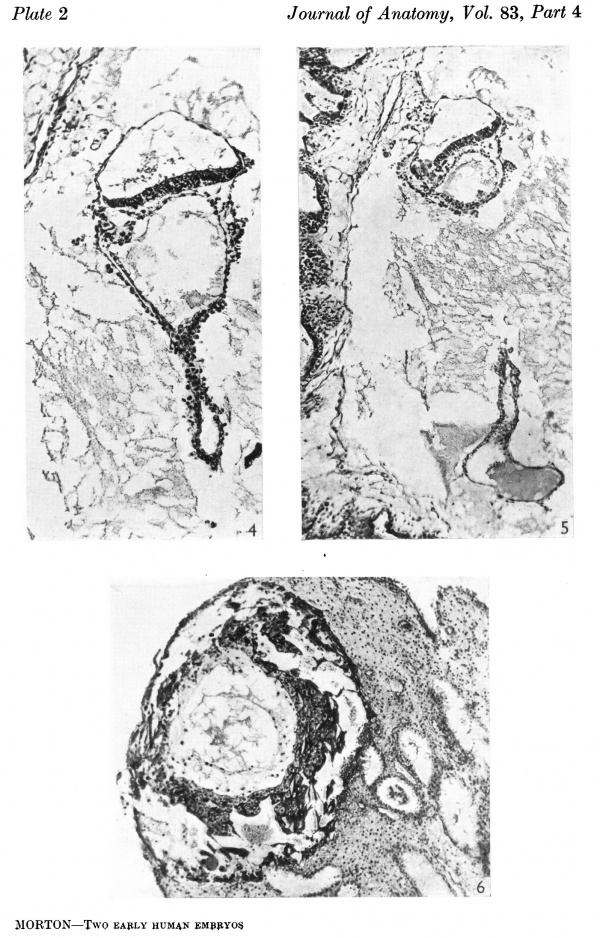
The embryonic shield is a broad oval in dorsal View and is not quite symmetrical about its long axis (Text-fig. 2). It is slightly convex towards the amniotic cavity. Its margins are continuous with the thinner amniotic ectoderm, the transition being clearly marked (Pl. 2, fig. 4). The shield ectoderm consists of high columnar cells which in places appear to be stratified, but this is due to the obliquity of the section; those of the central area are larger than those nearer the margin and are more vacuolated and granular. At the posterior end of the shield and reaching its margin there is a median linear strip of lighter more loosely arranged polyhedral cells (Pl. 2, fig. 5); the strip extends over six sections and as measured by reconstruction is 0-059 mm. long and 0-036 mm. broad, that is, it is a little more than one-fifth of the length of the shield (Text-fig. 2). The cells forming it are continuous in front and at the sides with the general shield ectoderm, and below they are in close contact with the gut endoderm but can be distinguished from it; there can be little doubt that it is the primitive node and streak.
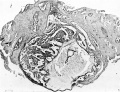
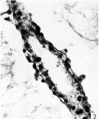
The main interest of the specimen lies in the condition of the yolk-sac. The form of its three parts is shown in Text-fig. 1. The wall of the whole of the proximal part consists of a single layer of round or oval cells much smaller than the overlying ectodermal cells and easily distinguishable from them. Cells of the same kind bound a small wide diverticulum which passes backwards, below the level of the connecting stalk, under the posterior end of the shield, which may represent the allantoic diverticulum or may be a part of the expanding yolk-sac which is later incorporated in it as described by Florian (1930). The cells lining the duct part of the sac are cuboidal in form, have large round nuclei and are arranged in a single continuous layer (Pl. 1, fig. 2); on the whole they are slightly larger than the cells of the proximal part. The cells lining the distal expansion (Pl. 1, fig. 3) gradually change from cuboidal above to thin flattened squamous—like cells below, and over the greater part are easily distinguishable from the covering layer of mesoderm; but the central part of the ab-embryonic wall is very thin and consists of a single layer of very flattened cells the nature of which, endoderm or mesoderm, I am unable to decide.
The mesoderm covers the duct and the proximal part of the yolk-sac and is a continuous layer over the amnion; it forms the broad irregular connecting stalk. There is a small amount of intra-embryonic mesoderm in the peripheral rim of the embryonic shield which is directly continuous with the extra-embryonic mesoderm; and in the angle between the anterior end of the shield and the yolk-sac there is a more loosely arranged mass of mesoderm which may well be the protocardiac area. There are a few projections, with indications of cavity formation, in the mesoderm of the proximal yolk-sac and cavities in the chorionic mesoderm, especially in the region of the connecting stalk (Pl. 2, figs. 4, 5); these have been taken to be the first evidences of angiogenesis.
The next menstruation, from the history given, should have begun on 14 July, and, taking ovulation to have occurred 14 ;I-_ 2 days before this, the single coitus probably on 2 July, falls within the probable period of ovulation 28 June to 2 July; the probable coital age is thus 13% days. The general dimensions, structure and differentiation of the embryo, and the appearances of the decidua agree with this estimate and place the ovum in Streeter’s (1942) Group VII; and in comparison with known ova it appears to be more developed than embryo No. 7801 (13; days) of Heuser, Rock and Hertig (1945), of about the same stage as the Yale embryo (14 days) (Ramsey, 1938), and younger than the Falkiner ovum (15-17 days) (Martin & Falkiner, 1938). The interest lies in the hitherto undescribed form of the yolk-sac.
The presence of a duct-like process leading distally from the yolk-sac and connected to the chorionic mesoderm was described by Bryce (1924) in the ovum T.B. 2 and he noted that similar formations were present in other ova. (Schlagenhaufer and Verocay, Fetzer, Strahl-Benecke) and that in the Frassi ovum there is a detached vesicle lined with endoderm which is connected to the yolk-sac by mesoderm. Heuser et al. (1945) describe in embryo No. 7801 several large vesicles in the peripheral part of the chorionic cavity, the walls of which are for the most part composed of a single layer of flat squamous-like cells, but occasionally containing patches of cuboidal cells; the largest vesicle is attached by a faint strand of cytoplasm, which itself contains a vacuolated group of cells, to the yolk-sac. The same authors also describe in embryo N o. 7802 (16% days) a tapering funnel-shaped ventral process of the yolk-sac the mesodermal layer of which can be traced, as a fine filament, across the chorionic cavity to the mesoderm of the opposite side. Heuser & Wislocki (1935) have shown in the sloth (Brad;/pus griseus) that in early stages there is a chorionic attachment of the ab-embryonic wall of the yolk-sac and the formation of two yolk-sac vesicles connected, for a time, by a narrow duct. Later the connecting duct disappears, leaving a definitive yolk-sac under the shield and an endoderm-lined cyst-like cavity attached to the ab-embryonic wall of the chorion. Martin & Falkiner (1938) state that the yolk-sac of the Falkiner ovum is partially divided into two by a septum; and that the endoderm lining the cavity of the yolk-sac dorsal to the septum is cuboidal, and the endoderm of the part ventral to it is thin, flattened and smooth, except at the extreme ventral pole where it also is cuboidal. They also give details of a similar partial subdivision of the yolk-sac in a 15-day-old macaque embryo (No. C 571) and state that the differences of "the two types of endoderm are more evident than in the human. They offer two explanations for the occurrence of the divisions of the yolk-sac. They state first (p. 257) that the grooves (external to parts of the septum) probably represent the earliest sign of the folding-oil’ of the embryo, and that they separate the gut endoderm dorsally from the yolk-sac vesicle endoderm ventrally, as suggested by Streeter. On p. 266 they state that if the process of taking up evaginations of the yolk-sac ‘had gone a little further the septum partially dividing this region of the yolk-sac would have disappeared and the part of the allantois situated in the caudal wall of the yolk~sac ’ (i.e. below the septum) ‘would have been incorporated in the yolk-sac cavity’. This implies that some of the thin flattened endoderm assigned earlier to the yolk-sac vesicle becomes transformed into cuboidal gut endoderm. It is apparent, however, from a study of the Falkiner ovum, that the partial division of the yolk-sac seen in it is of a different nature from that seen in the present specimen. The endoderm cells lining the duct part of the yolk-sac of the Biggart ovum are slightly larger than the cells lying below the shield ectoderm and lining the proximal part of the yolk-sac cavity. It is only in the distal expansion of the yolk-sac that the lining cells become thin, flat and smooth, and in part of the ab-embryonic wall they are even more flattened and are either endoderm alone or mesoderm (Pl. 1, fig. 3). The appearances in the Biggart ovum are much more suggestive of the condition found in the 12-somite sloth. This stage could easily lead to the condition found in the 13%-day human (No. 7801) by Heuser et al. or to that in the 38-somite sloth embryo. The appearance of the endoderm cells of the ab-embryonic parts of the vesicle possibly is due to a slow rate or absence of division of the cells, or perhaps to a falling off of the activity gradient and to relatively poorer nutrition at the ab-embryonic region. The ‘ stretching-out’ of the yolk-sac by the expansion of the whole chorionic vesicle, to which the yolk-sac is attached, probably is the direct‘ cause of the thinning of the distal yolk- sac cells, the duct cells remaining, or becoming, cuboidal because of the small diameter of the duct relative to the rest of the yolk-sac.
The origin of the endoderm cells lining the yolk-sac still remains uncertain. The spread round of the endoderm cells from the periphery of the embryonic endoderm as seen in the macaque yolk-sac, must, in the human, be either very rapid or altogether omitted. If the spreading of endoderm cells round the interior of the exo-coelomic membrane is absent or only partial, then the yolk-sac cells, as distinct from the endoderm cells below the shield ectoderm, must arise by delamination from the precocious mesoderm forming the exo-coelomic membrane as is stated to be the case by Streeter (1937). The absence of a definite double layer of cells over the ab-embryonic pole of the yolk-sac may suggest a migration of endoderm cells from the original embryonic endoderm cells, the migration not having been completed; the single layer present would then be described as mesoderm. But, alternatively, the delamination process may have been delayed over the less well-nourished ab-embryonic region or the second layer of cells may have already disappeared. It is unfortunate that the histological appearances are such that one cannot be certain whether this layer belongs to endoderm or mesoderm.
Whatever the exact mode of origin of the endoderm cells may be, it is evident that this ovum illustrates a stage in the formation of the human definitive yolk-sac intermediate between that described by Hertig & Rock (1941) for ovum No. 7700 (12% days) and that by Brewer (1938) for the 15-day Edwards-Jones-Brewer embryo. Also that this stage, which has not previously been described for man, resembles the similar stage in the 12-somite sloth, and closely resembles the condition postulated by Hamilton, Boyd & Mossman (1945) in their scheme for the development of the yolk-sac.
The Macafee Ovum
The Macafee ovum was recovered from a woman aged 26 years; her last menstruation was from 20 to 26 January; coitus occurred on 22, 25 and 30 January; she was admitted to hospital on 3 February and was curetted on 5 February; the expected date of her next menstruation was 11 February.
The material obtained from the curetting was cut into a diagnostic section and fifty-one serial sections separated from the former by a small interval due to loss of sections. The diagnostic section, of which Pl. 2, fig. 6 is a photograph, is nearest the centre of the ovum, and showsian endometrium well prepared for implantation. The endometrium is not grossly oedematous and there is no marked leucocytosis and no large haemorrhagic areas. Some capillary sinusoids were found to communicate with the spaces in the outer part of the trophoblast. The glands are dilated, but do not contain blood or pent-up secretion; some of them are being engulfed by the syncytiotrophoblast and their cells are disintegrating in the trophoblast spaces. There is no necrotic zone, and no syncytial masses of peripheral trophoblast were seen.
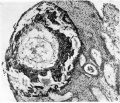
The ovum, which from the wax-plate reconstruction is seen to be ovoid in form, is very superficially embedded so that a large part of it is exposed and uncovered by uterine tissue. The measurements of the largest section are: trophoblast, maximum external 1-13 x 0-63 mm. and trophoblast cavity 0-58 x0-33 mm. The trophoblast wall consists of two layers, an inner of large cuboidal cells lining the cavity, and projecting outwards as cell columns, and an outer thin covering layer of syncytium. This syncytium bounds lacunar-like spaces and peripherally forms a continuous layer which constitutes the junctional zone with the maternal stroma. There is a general absence of activity penetrating syncytium from this layer and the line of demarcation between it and the uterine stroma is quite definite. The trophoblast spaces contain a coagulum and only a small amount of maternal blood cells. The trophoblast is lined internally with a layer of extra-embryonic mesoderm which does not indent the trophoblast columns. In the section nearest the centre of the ovum (Pl. 2, fig. 6) the mesoderm forms what appears to be an exo-coelomic membrane, but this cannot be traced in the more peripheral sections; there the mesoderm is smaller in amount and forms a much thinner layer. There is no embryonic rudiment.
Discussion
The superficial implantation and the general histological appearance of the specimen indicate that the ovum was probably dying and being cast off by the maternal tissues. The coital and menstrual histories are believed to be reliable, and assuming that the coitus on 25 January was the fertile one, give to the ovum a maximum coital age of 104} days. The measurements of this ovum, as compared with other pre-villous ova, notably the Hertig & Rock ova (Carnegie Nos. 7699, 7700, 7950, and 8020), the Davies-Harding, Barnes, Miller, Dible and West, Kleinhans, Werner, Scipiades and Marchetti ova, also place it between 9 and 13 days of age. In appearance it closely resembles that of Carnegie No. 7950 (Rock & Hertig, 1942). The engulfment and disintegration of the uterine glands by the syncytium are unusual in an ovum of this early stage of development and indicate that the invasive power of the syncytium must, at one time, have been considerable.
Summary
Two human ova recovered from uterine scrapings are described. The older specimen (14 days) is normal, but is rather superficially embedded. It shows a large yolk-sac with proximal and distal dilatations connected by a narrow tubular duct. The proximal and duct’ parts are lined with cuboidal cells. The distal expansion is partially lined with flattened endoderm cells and also shows an area one cell in thickness over the ab-embryonic pole. The significance of the appearance of the yolk-sac and the general position of the ovum with regard to other early human ova are discussed.
The younger ovum (10 days) is incomplete. It is superficially embedded, and
probably pathological. Erosion and engulfment of the uterine glands have occurred
at an earlier stage than is usually described in the human. Its probable age and its
general chronological position are discussed.
References
Brewer JI. A human embryo in the bilaminar blastodisc stage (The Edwards-Jones-Brewer ovum). (1938) Contrib. Embryol., Carnegie Inst. Wash. Publ. 496, 27: 85-93.
Bryce TH. Observations on the early development of the human embryo. (1924) Trans. Roy. Soc. Edinburgh, 53: 533-567.
Florian J. The formation of the connecting stalk and the extension of the amniotic cavity towards the tissue of the connecting stalk in young human embryos. (1930) J. Anat., 64: 454-476.
Florian, J. (1930). The formation of the connecting stalk and the extension of the amniotic cavity towards the tissue of the connecting stalk in young human embryos. J. Anat., Lond.,64,454-476.
Hamilton WJ. Boyd JD. and Mossman HW. Human Embryology. (1945) Cambridge: Heffers.
Hertig AT. and Rock J. Two human ova of the pre-villous stage, having an ovulation age of about eleven and twelve days respectively. (1941) Carnegie Instn. Wash. Publ. 525, Contrib. Embryol., 29: 127-156.
Heuser CH. Rock J. and Hertig AT. Two human embryos showing early stages of the definitive yolk sac. (1945) Contrib. Embryol., Carnegie Inst. Wash. Publ. 557, 31: 85-99.
Heuser, C. H. & Wislocki, G. B. (1935). Early development of the sloth (Bradypus griseus) and its similarity to that of man. Contr. Embryol. Carneg. Instn, 25,1-13.
Martin CP. and Falkiner N. Mcl. The Falkiner ovum. (1938) Amer. J Anat., 63: 251-271.
Ramsey EM. The Yale embryo. (1938) Contrib. Embryol., Carnegie Inst. Wash. Publ. 496, 27: 67-84.
Rock J. and Hertig AT. Some aspects of early human development. (1942) Amer. f. Obstet. Gynecol, 44: 973-983.
Streeter GL. Origin of the yolk sac in primates. (1937) Anat. Rec, 70 suppl, 53-54.
Streeter GL. Developmental horizons in human embryos. Description of age group XI, 13 to 20 somites, and age group XII, 21 to 29 somites. (1942) Contrib. Embryol., Carnegie Inst. Wash. Publ. 541, 30: 211-245.
Explanation Of Plates
Figs. 1-5 are of the Biggart ovum, fig. 6 of the Macafee ovum.
Plate 1
Fig. 1. The ovum in situ. Sect. 81. x 38.
Fig. 2. The duct part of the yolk-sac. The distal end is inferior. Sect. 77. x 375.
Fig. 3. The distal expansion of the yolk-sac. The end of the duct just appears above, on the left. Sect.77. x375.
Plate 2
Fig.4. The embryonic rudiment. The distal expansion of the yolk-sac is not figured. Sect.81. x140.
Fig. 5. The opening of the duct part into the distal expansion of the yolk-sac which shows its thin ab-embryonic wall below. Sect.75. x95.
Fig. 6. The Macafee ovum in situ. Diagnostic section. x 65.
Cite this page: Hill, M.A. (2024, April 27) Embryology Paper - Two Early Human Embryos. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Paper_-_Two_Early_Human_Embryos
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G