Paper - The pharyngeal pouches and their derivatives in the mammalia
| Embryology - 27 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Fox H. The pharyngeal pouches and their derivatives in the mammalia. (1908) Amer. J Anat. 8(3): 187-250.
| Online Editor |
|---|
| Note this paper was published in 1908 and our understanding of pharyngeal arch pouch derivatives has improved since this historic study using cat, pig and rabbit embryos.
|
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
The Pharyngeal Pouches and their Derivatives in the Mammalia
By Henry Fox, Ph.D.
With 73 Figures.
Introduction
The present paper is an outgrowth of an earlier unpublished article submitted to the Faculty of the University of Pennsylvania in partial fulfilment of the requirements for the degree of Ph.D. The original article gave the results of a study of six or seven different stages in the development of the pig. Subsequently, through the kindness of Dr. C. S. Minot, I was enabled to study the extensive series of mammalian embryos in the collection of the Harvard Medical School. Of these I studied most thoroughly the series of pigs and cats, but also gave some attention to the later stages in the rabbit. The results of this additional study, along with those included in my former article, I now offer in the present paper. My aim is to give a complete history of the pharnygeal pouches and their derivatives as typically exemplified in the mammalia. The main facts of this history had been largely determined previous to my starting the investigation, but the interpretations attached to these facts by various authors differed considerably, and, moreover, there remained a number of details about which there was much confusion. These unsettled matters seemed to me to warrant a full investigation of the subject.
Soon after I had begun my observations an important article by Hammar appeared treating of the development of the fore-gut in man. Hammar had in his possession a large series of embryos, and from these he made out a full and consistent history of the middle ear and Eustachian tube. He also compared with his own results the statements made by earlier authors, and, through the more abundant material at his command, was enabled to show how their conclusions were in most cases the result of mistaken interpretation based on an insufficient body of facts.
While primarily concerned with the development of the pharynx in man, Hammar also examined a series of rabbit embryos, and while he does not in his article treat of them particularly, he yet mentions that he finds an essential agreement in the formation of homologous parts in both forms. Accordingly, he is inclined to assume that the essential features of the development in man will hold good in the case of other mammals.
Hammar’s first article was followed by a second on the fate of the second pharyngeal pouch. This is the last article of his I have seen, and, so far as I know, he has not published any articles on the fate or the last two pouches.
The appearance of Hammar’s paper seemed to me at first to do away with the necessity of further study of the first two pairs of pouches, but as Gaupp had already expressed the idea—based upon the conflicting statements of earlier investigators——that the formation of these parts probably differed considerably in different species of mammals, I concluded that a further contribution on the subject in the three species examined by me would not be without value. Moreover, as Kastschenko, the chief authority on the process in the pig, had declared that the middle ear tube did not arise in any way from the first pharyngeal pouch, I considered this an additional reason for continuing my investigation.
The results of this investigation, so far as the first and second pharyngeal pouches are concerned, are largely confirmatory of the conclusions reached by Hammar in man and the rabbit. The probability therefore is that the development of these parts is essentially similar in the majority of placental mammalia.
In the case of the third and fourth pharyngeal pouches I have obtained results which clear up certain details about which there has been much confiict of opinion. Among these may be mentioned the determination of the origin and structure of the carotid gland, a reconciliation of the confiicting statements regarding this structure made by Kastschenko and Prenant, a confirmation of the ectodermal origin of the so—called thymus superficialis of Kastschenko, and finally the origin of a second structure—beyond doubt the glandule thyroidienne of Prenant—from the fourth pouch.
My earlier studies were made by the aid of the wax reconstruction method. Later, owing to the lack of facilities for continuing the use of this method, I adopted the method of graphic projection, making dorsal, ventral and lateral views of each stage studied. The Pharyngeal Pouches in the Mamnialia 189 During the progress of this investigation I received much assistance from a number of investigators. To Dr. E. G. Conklin I am greatly indebted for his kind encouragement and helpful suggestions, and for these I desire to express my hearty thanks. To Dr. C. S. Minot I am under special obligations for his kindness in allowing me to examine the fine series of embryos in his charge. I also desire to thank Dr. C. B. Davenport for permission to continue part of this work in the laboratory at Cold Spring Harbor, Long Island.
I shall present the results of my studies under the following headings:
- The Formation and Structure of the Pharnygeal Pouches.
- The Later Modifications and Fate of the Pharnygeal Pouches.
In this study I was enabled to examine the following stages, which I here present in the order of progressive development:
(3) 6.5 mm., (M?) (1) 4.6 mm., N0. 398 (13) 14 days, 10.0 mm., No. 157 (6) 9.0 mm., (M5) (2) “ 413 (19) 16% “ 17.8 mm., “ 576 (7) 10.0 111111., No. 401 (4) 6.2 mm., “ 380 (20) 18 “ (8) 12.0 mm., “ 518 (5) 9.7 mm., “ 446 (25) 20 “ 29.0 mm., “ 172 (9) 13.5 mm., (14) 10.7 mm., “ 4:74 (26) 21 “ (10) 14.0 mm., N0. 65 (16) 15.0 mm., “ 436 (11) 17.0 mm., “ 51 (21) 23.0 mm., “ 466 (12) 18.0 mm., (M3) (24) 31.0 mm., “ 500 (15) 20.0 mm., No. 542 (17) 24.0 mm., “ 64 (18) 25.0 mm., (M4) (22) 32.0 mm., N0. 74 (23) 35.0 mm., (M)
1. The Formation and Structure of the Pharyngeal Pouches
Under this heading I shall describe all stages leading up to the complete formation of the four pairs of pharyngeal pouches characteristic of the mammalian embryo. The stages here considered include Nos. 1 to 4, inclusive.
The earliest stage of development of the pharynx and its appendages was shown in a cat embryo of 4.6 mm., No. 398 of the Harvard collection (figs. 55 and 56.) The embryo is approximately straight, the headfold is distinctly differentiated, but the posterior two-thirds of the enteric cavity opens widely into the yolk vesicle (at x in the figures). The neural tube is closed except anteriorly, where a narrow cleft still persists. The optic vesicles are present, but there is no sign of the optic cups. The pharynx anteriorly is in contact with the stomatodeal plate (St.). As a whole it is a relatively wide, dorso-ventrally flattened sac. Only two pairs of pharyngeal pouches are present as wide lateral diverticula of the pharynx. Of these the first (Ph. P. I) alone reaches the ectoderm and joins with it for a short distance. The second pair (P11. P. 2) are only barely indicated as faint outbulgings, the one on the left being the more distinct of the two.
The pericardium is of small size, in striking contrast to the enormous bulk it attains in later stages. It contains the inner portions of the great vitelline veins (V. v.), which are joined together only at their extreme anterior ends. Only one pair of fully developed aortic arches is present—the first or mandibular (ao. i). These extend dorsally in front of the first pouch and join the paired dorsal aortas. Two prominent out—bulgings from the sides of the vitelline veins are probably destined to form the common trunk from which the remaining aortic arches subsequently arise. ' A cat embryo, No. 413 of the Harvard collection, shows the next stage in advance (fig. 5'7). The posterior part of the body is still approximately straight, but the head portion is strongly flexed upon‘ it and is of relatively much greater extent than before.
Anteriorly the stomatodeal plate has disappeared. The pharynx is, as before, a wide flattened sac, but its width in its anterior portion is somewhat greater than in its posterior part. Its floor is somewhat deeper than before and close to the mouth is produced into a deep median groove— the median oral groove GR).
The hypophysis (HYP) appears as a blunt protuberance from the dorsal side of the pharynx close to its anterior extremity. Three pairs of pharyngeal pouches are now present. The first two pairs reach the ectoderm and join with it for a considerable extent (see light areas of Ph. P. 1 and 2, fig. 57). Of the third pair, the pouch on the right side reaches the octoderm, While that on the left is still removed by a slight interval from it. _ The first pharyngeal pouch forms a relatively large transverse fold. The greater part of its lateral margin is in contact with the ectoderm. The area of contact is widest dorsally and diminishes progressively in width toward the ventral side. The extreme ventral part of this margin extends a slight distance below the region of contact as a free edge, which then turns suddenly inwards as the ventral margin of the pouch. This part of the pouch projects slightly below the floor of the pharynx and thus forms a ventral diverticulum of the pouch. The second pharyngeal pouch, although considerably smaller than the first, is essentially similar to it. It has a ventral diverticulum, which is somewhat less prominent than the same part in the first pouch.
The third pharyngeal pouch is considerably smaller than its two predecessors. It forms a finger—like outgrowth, which extends outwards and downwards and, in the case of that on the right side, joins the ectoderm. The left pouch does not quite reach the latter.
The fourth pharyngeal pouch is only barely indicated by a slight bulging of the walls of the pharynx behind the base of the third pouch.
The first three pairs of aortic arches are now fully developed and a fourth is beginning to develop. _ In a pig embryo of 6.5 mm. (M2 of my collection) and a cat of 6.2 mm. (No. 380, Harvard collection) all the pharyngeal pouches and their associated parts are typically developed. The two embryos show almost the same relative stage of development, but that of the pig shows a slightly more primitive condition. It will, accordingly, be considered first.
The pharynx (figs. 1, 2 and 3) shows four complete pairs of pharyngeal pouches, all of which have a more or less extensive contact with the ectoderm of the corresponding grooves. Between the first two pairs of pouches the pharynx is considerably wider than in the region between the last two pairs. Anteriorly the hypophysis (HYP.) projects forward as a blunt protuberance, and immediately back of it arises a minute conical process, the representative of Seessel’s pocket.
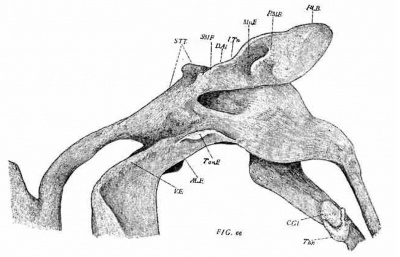
The pharyngeal pouches in general have the form of vertical winglike expansions projecting outwards and slightly backwards from the side walls of the pharynx. Typically, they are joined to the pharynx by a relatively narrow base and only laterally dilate into the wing-like expansions mentioned. Each pouch is attached laterally to the ectoderrn. The extent of this attachment is shown by the clear areas in fig. 1. As these show, it varies greatly, being most extensive in the second, where it includes almost the entire lateral margin. A similar relation is noted by I-Iammar in the corresponding stage in man, and, as the figures of the next stage show (see fig. 58), it holds in the cat.
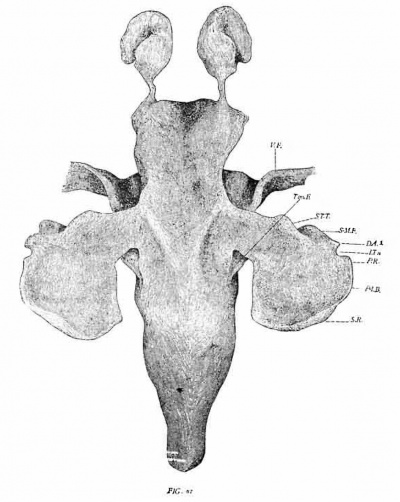
A conspicuous feature—shown best in the second and third pouches—is the presence of deep ventral projections to the pouches (V.D. 1-4). They reach to a greater or less extent below the floor of _the pharynx. Hammar calls them the ventral diverticula. They appear, from all published figures examined, to be constant at the corresponding stage in all mammals so far investigated. A ventral view (fig. 3) shows a number of important features. Projecting from each side of the pharynx are the four pharyngeal pouches, each with its ventral diverticulum. That of the first pouch (V. D. 1) fOI‘1II3S a low narrow ridge extending from the infero-lateral angle of the pouch inwards and slightly backwards quite to the median line, where it joins the corresponding ridge of the opposite side. There is thus formed a complete transverse V-shaped fold, the apex of the fold being the meeting point of the two opposite limbs. The ridge corresponding to the median oral groove begins immediately in front of this apex. The shallow impression between corresponds to the tuber~ culum impar of His. Just behind the apex is the median thyroid. The latter consists of two lobules joined to each other and to the pharynx by a slender epithelioid cord.
The ventral diverticula of the next two pouches are much deeper than that of the first, but are largely confined to the lateral half of the pharynx. A faint ridge, however, extends from the base of the second diverticulum to the median line, where it is joined by a similar ridge from the third pouch (see fig. 60 of the next series for this condition). The two sets of ridges thus converge to form a rather low protuberance immediately above the thyroid and in front of the tracheal ridge.
The presence of these inner low ridges connecting the opposite ventral diverticula of the second and third pouches shows their essential agreement in this respect with the first pouch. Only, in the case of the two former, the lateral half of each ridge is produced far below the level of the inner portion, while in the first pouch the depth (its height) of the ridge is throughout approximately uniform.
Owing to the form of the ventral diverticula of the second and third pouches there is left between their opposite lateral halves a considerable space, in which is lodged the apical portion of the heart along with the large arteries radiating from it (fig. 3). The prominent aortic arches at this time are the third (carotid), fourth (aorta typica) and the fifth (pulmonary). The latter has a small posteriorly directed braneh—the later pulmonary artery (fig. 3, Pul.), The first aortic arch is much reduced in size and has lost all connection with the dorsal aorta. The second is also extremely reduced and is only connected with the dorsal aorta by an extremely narrow (apparently functionless) vessel.
Immediately back of the common origin of the aortic arches begins a sharp median ridge, which deepens posteriorly. It represents the future larynx and trachea. The first pharyngeal pouch has a greater lateral extent than any of the succeeding. As the figures show, the lateral extension of the pouches decreases regularly from before backwards. The first pouch blends with the lateral portion of the pharynx by a broad base, so that it is impossible to draw any definite line between the two. The limits assigned by Hammar, whose usage in this matter I adopt, will be given presently. Laterally the outer extremity of the pouch is produced upwards as‘ a blunt prominence——the dorsal diverticulum of I-Iammar—which projects considerably above the roof of the pharynx. The dorsal-diverticulum terminates in a narrow apex—the dorsal apex (d. a. 1) (Recessus tympani anterior, Hammar).
Hammar includes in the first pouch the following parts: (1) The sulcus tubo-tympanicum. This is Mo1denhauer’s term for the prominent ridge (fig. 2, S. T. T.) representing the antero—lateral border of the pouch. It begins externally at the dorsal apex and extends downwards, inwards and forwards, terminating close to the base of the hypophysis. (2) The latero-ventral ridge and its continuation, the ventral diverticulum. This is a narrow ridge which in its dorso-lateral portion is joined to the ectoderm. The connection includes the dorsal two-thirds of its lateral extent. The lower third forms a free edge, and this, at its lower outer angle, turns sharply inwards and backwards to form the ventral diverticulum. (3) The sulcus tensoris tympani (S. T. Ty.). This is a term applied. by Hammar to the border extending from the dorsal apex backwards and inwards to join the next part along the inner border of the hyoid arch. (4) The sulcus tympanicus posterior (S. T. P.). This term, also given by Hammar, includes the longitudinal ridge forming the inner boundary of the hyoid arch arid connecting the sulcus tensoris tympani with the base of the second‘ pouch. (5) The impressio cochlearis. This I-Iarnmar defines as a conspicuous depression on the dorsal wall of the pouch close to its origin from the pharynx. The auditory sac lies immediately above this area.
The second pharyngeal pouch is characterized, as already mentioned, by the extensive contact of its lateral margin with the ectoderm. Only at its extreme lower end does this border have a free margin. So far as the present specimen is concerned, there is no communication between the lumen of the pouch and the exterior. The closing membrane is exceedingly thin, but examination shows no break in its continuity.
The ventral diverticulum of this pouch forms a. prominent quadrangular folcl. The mesial half forms only a faint ridge, but the lateral portion is very deep. The deepest part is represented by the blunt angle immediately below the lower end of the lateral border.
Posterior to the region of the second pouch the pharynx diminishes considerably in width. Its lateral margin forms a low ridge connecting the second pouch with the third. Between this ridge and the median dorsal ridge of the pharynx is a shallow longitudinal furrow, in which is lodged the dorsal aorta.
The third pharyngeal pouch is slightly smaller than the second. It is joined by a relatively narrow base with the pharynx, but distally expands into a broad wing—like fold with a prominent ventral divertic- \ ulum. A slight dorsal diverticulum is also present. The lateral margin is in contact with the ectoderm for almost its entire length.
As in the case of the second pouch, the deep portion of the ventral diverticulum is limited to the lateral half of the pharynx. Its mesial portion is represented by a low ridge, which extends from the root of the lateral half forwards and inwards close to the median line, where it joins with the same part of the second pouch. The extreme ventral tip of the ventral diverticulum is turned toward the mesial side.
The fourth pharyngeal pouch is the smallest of the series. It is divided by a shallow constriction into two parts, a dorso-posterior portion (Ph. P. IV), which projects laterally and at one point comes into contact with the ectoderm, and a ventro-anterior bulge, which terminates blindly and corresponds to a ventral diverticulum.
From the base of the ventral diverticulum a low ridge extends forwards to the base of the third pouch. It corresponds to the mesial extension of the ventral diverticulurn.
In the cat embryo of 6.22 mm. (No. 480, Harvard collection) the condition of the pharynx is essentially similar to that just described in the pig. As in the latter, four pairs of pharyngeal pouches are present.
The characteristic features of this stage are shown in figs. 58, 59 and 60. fig. 58 shows the lateral aspect. The clear areas on the lateral margins of the pouches show the extent to which these are attached to the ectoderm. It will be noticed that they are essentially similar to the same parts in the preceding specimen. The dorsal diverticulum of the first pouch is somewhat more elevated. The ventral diverticulum of the third pouch extends to a slightly lower. level. The fourth pouch shows more clearly its division into two portions. The dorso-posterior portion is somewhat bulbous. Its more dorsal part is flattened and is produced outwards as a thin process which reaches the ectoderm. The ventral diverticulum projects almost directly forwards.
The median thyroid is of a relatively large size. It has lost all connection with the pharynx and lies at a lower level than in the preceding specimen.
Owing to the more rapid growth of the mandibular and hyoid arches as compared with that of the arches posterior to them, the originally almost transverse plane of the second and third pouches becomes postero-lateral. Their originally anterior and posterior surfaces thus become antero—lateral and postero-internal, respectively. Their lateral margins thus come to project backwards.
The increased antero-posterior growth of the mandibular arch leads to a change in the direction of the tubo—tympanal border of the first pouch. The latter is at first almost transverse, but later assumes a more anterior direction. The more antero-mesial direction of this border in the present stage as compared with that in the preceding shows the beginning of the change. As growth continues the border progressively lengthens, thus giving an increased width to the basal portion of the pouch. These relations are clearly indicated in the dorsal view (fig. 59).
figs. 59 and 60 show the relations of the more posterior pouches to the now fully formed sinus praecervicalis—relations which are of considerable importance in view of later developments. Owing to the great increase in bulk of the hyoid arch the posterior border of the latter projects backwards. The third and fourth arches increase but slightly in bulk and thus remain at a considerably lower level than the arches in front. The sinus is thereby formed as a deep recess, the bottom being formed by the arches mentioned. Just within the anterior margin of the sinus opens the second pharyngeal groove. The third groove occupies the middle of the inner walls. Dorsally it meets the upper extremity of the fourth groove. From this point the latter turns strongly downwards, backwards and inwards to where it meets the fourth pouch. As the latter lies at a considerably lower level than the other pouches, this part of the sinus projects inwards as a prominent, pointed process.
The ventral view (fig. 60) shows some additional features. On the right side the section is taken at a slightly lower level than on the opposite side, and accordingly it shows the entire exterior of the first two arches, together with the ventral extension of the first pharyngeal groove. It also shows how the antero-internal angle of the sinus praecer— vicalis is continued ventrally into the ventral extension of the second groove. The latter has a decided anterior course, and at its mesial end meets the first groove.
On the left side the ventral wall of the sinus praecervicalis is represented as having been removed, so that its interior is clearly shown. The internal process of the sinus is less deep than the same part on the right.
The continuous transverse fold formed by the ventral diverticula of the first pouch is clearly shown in this view. As in the case of the pig, there is no contact between this fold and the corresponding ventral extension of the groove, the two being separated by a considerable thickness of mesenchyrne.
II. The Later Modifications and Fate of the Pharyngeal Pouches
Owing to the more or less independent course which the different pouches take in their later history, I think it will conduce to greater clearness if I consider them separately, and accordingly I subdivide the above topic as follows:
A. The Modifications of the first Pharyngeal Pouch.
- (a’) The Formation of the Primary Tympanic Pouch.
- (a”) The Differentiation of the Tympanic Cavity and Eustachian Tube.
B. The Modifications and Fate of the Second Pharyngeal Pouch.
- (b’) The Retrogressive Modifications of the Pouch.
- (b”) The Formation of the Tonsillar Fold.
C. The Metamorphoses of the Third Pharyngeal Pouch and its Derivatives.
- (c') The Elongation of the Ventral Diverticulum and the Formation of the Thymus.
- (c”) The Origin and Structure of the Carotid Gland.
- (c’”) The Sinus Praecervicalis and its Relation to the Thymus.
D. The Fourth Pharyngeal Pouch and its Transformation into the Lateral Thyroid and Glandule Thyroidienne.
A. The Modifications of the First Pharyngeal Pouch
(a’) The Formation of the Primary Tympanic Pouch
The pharynx is essentially alike in a pig of 9 mm. (Series M5, my collection) and in a eat of 9.’? mm. (No. 446, Harvard series). fig. 61 gives a ventral View in the latter. The first pharyngeal pouch is wider in the antero—poste1'ior direction than before——a change connected witlr the anterior growth and elongation of the oral cavity and the consequent prolongation in the same direction of the attached tubo-tympanal rim. The ventral diverticula are slightly less prominent. T0gether they form a low V—shaped elevation on the floor of the pharynx. Just external to the median apex formed by the convergence of the arms of the V each is joined by one of the pair of folds forming the outer line of the tuberculum impar (Tub.) . Close to the lateral margin each arm is crossed by the broad alveolo-lingual fold (AL.F.). A slight distance in front the latter meets the vestibular fold (V.F.). Immediately back of the point of convergence a lateral fold (SM is given off, which extends obliquely outwards and backwards over the lateral ridge of the latter. The formation of this fold marks the initial step in the development of the later important submeckelian fold.
The tuberculum impar arises as a result of the bipartition of the median oral ridge. The crest of the latter widens and its middle part then becomes depressed to form a shallow concavity—the ventral counterpart of the tuberculum.
In the pig of 10 mm. (No. 401, Harvard series, figs. 4-6) the pouch is joined to the ectoderm by only the dorsal third of its lateral ridge. The remainder of this border is now free and forms a low fold separating the antero-lateral and postero-lateral surfaces of the pouch. Ventrally it is continuous with the ventral diverticulum. Where the transition takes place the alveolo-lingual swelling cuts across it at right angles, forming here the line of demarcation between the pouch and the pharynx.
The ventral cliverticula present no new points of interest. The swellings which marked the lateral boundaries of the tuberculum impar are now relatively inconspicuous, having been absorbed along with the adjacent parts of the pharyngeal floor in the broad depression (representing the anlage of the tongue) lying between the alveola—lingual ridges.
Dorsally the pouch projects relatively higher than hitherto and terminates in a more acute apex. This condition is not due to the growth dorsalwards of the pouch, but is a result of a ventral displacement of the pharynx. As a comparison of the figures shows, the formation of the neck of the animal is attended with a ventral (caudal) fiexure of the posterior half of the pharynx. The flexure also afiects to a minor degree the remainder of the pharynx, tending to displace it to a lower level. This tendency, however, is checked by the fact that the first pair of pouches is still attached to the ectoderm by their lateral extremities. These points are accordingly relatively fixed in position, and, as the basal portion of the pouch sinks in response to the general lowering of the pharynx, the structure attains the pronounced ascending course characteristic of it at this stage.
In consequence of this change the basal portion of the pouch has assumed an almost horizontal plane, while its peripheral part ascends almost vertically. Where the two parts meet there is on the lateral surface a slight ridge extending from the lateral ridge to the base of the vestibular fold. It corresponds with the fold mentioned in the preceding stage as forming the beginning of the submeckelian fold (fig. 6, S.M.F.).
This fold subdivides the antero-lateral wall into two surfaces, an external, dorso—lateral and a mesial ventro—lateral surface. The former forms an elongated triangular area limited dorsally by the tubetympanal crest and posteriorly by the lateral ridge. The latter forms a smaller triangle bounded internally by the alveolo-lingual fold and posteriorly by the lateral ridge.
The anterior prolongation of the tubo-tyrnpanal ridge is more pronounced than in the preceding stage. The difference is due to a continuance of the process, already mentioned, of anterior elongation of the oral cavity.
A pig of 12 mm. (No. 518, Harvard series, figs. 9-11) shows the pharynx only slightly larger than in the stage just descr_ibed. The continued anterior elongation of the oral cavity has given the tubetympanal crest a decided antero-posterior course. The pouch retains its connection with the ectoderm only at its dorsal apex. The lateral ridge forms only a low prominence extending from the dorsal apex to the ventral diverticulum.
The dorsal apex appears broader and more depressed than in the preceding stage. This condition, I think, results from the lateral flex ure of the apex in consequence of the general growth in width of the head.
At this stage the pouch has the essential features of the primary tympanic pouch of Kastschenko. This investigator considered the pouch as merely a widened diverticulum of the lateral wall of the pharynx and regarded the lateral ridge as alone representing the first pharyngeal pouch. As Hammar shows and my observations confirm, Kastschenko’s conception of the pouch was entirely too limited and was doubtless due to his not examining earlier stages in which it is more typically developed.
The primary tympanic pouch at this stage is a dorso—ventrally flattened triangular fold which arises by a broad base from the pharynx and terminates peripherally in the dorsal apex. The pouch as a whole lies almost horizontally, but towards the lateral edge it turns sharply upwards. Its walls are medic-dorsal and lateral. The former is limited laterally by the tubo-tympanal crest, dorsal apex and posterior tympanal borders. All below these limits is embraced in the lateral wall. This is divided by the lateral ridge into two surfaces, antero-lateral and latero—posterior. The antero-lateral surface is further subdivided into two areas—dorso—lateral and ventro—lateral—by the submeckelian fold.
The ventral diverticula now form a pair of low swellings. Mesially they are interrupted by a shallow longitudinal groove connecting the tongue concavity with the deep hollow in front of the larynx.
In a pig of 13.5 mm. (Series M1 of my collection) the condition of the pouch is intermediate between that last described and the next. The only feature that calls for remark is the presence at the dorsal apex of a short narrow process by which the pouch retains its last connection with the ectoderm (fig. 3'7).
In the pig of 14 mm. (No. 65, Harvard series, figs. 14-16) the primary tympanic pouch has separated entirely from the ectoderm and now lies some distance below it-—a condition due to the greater lateral growth of the head compared with that of the pouch.
The pharynx at thistime begins to show modifications due to its own differential growth. The increase in width of the anterior half is considerably greater than in the posterior portion. Thus, while the distance between the apices of the first pair of pouches has increased appreciably since the last stage, that between the same parts in the second pair remains approximately the same. In consequence of this the pouch now shows a more pronounced lateral projection. The tensortympani crest turns sharply inwards and joins the posterior tympanal border at an obtuse angle. The latter border also shows a tendency to assume a more transverse trend. The second pouch appears as a rounded prominence at the postero-internal angle of the tympanic pouch.
The ventral half of the lateral ridge and its continuation, the ventral diverticulum, have disappeared. Their former position is only indistinctly indicated by low swellings on the under side of both pouch and pharynx. The dorsal half of the lateral ridge, however, is continued into the submeckelian fold, and these are now slightly more prominent, Together they now form a continuous crescent—shaped fold extending from the dorsal apex to the base of the vestibular fold. It underlies, for the greater part of its length, l\Ieckel’s cartilage. For this reason I have called it the submeckelian fold. The shallow depression in the lateral wall which it subtends I call the Meckelian fossa.
The paired ridges which formerly limited the tuberculum impar laterally have now become blended with the epithelium covering the tongue anlage. The formation of the latter has been accompanied by the progressive downgrowth of the surrounding alveolo-lingual crests, particularly in their anterior portion. The deep space thus enclosed is filled with the tissues of the organ. Posteriorly this space is now connected by a deep groove with the space in front of the larynx.
An early stage in the formation of the external auditory meatus is shown by the conical indentation projecting under the pouch. Its inner angle terminates a short distance below the latero—posterior surface. The two structures are nowhere in contact, a moderately thick layer of mesenchyme intervening between them.
In the pig of 1*?’ mm. (No. 51, Harvard series, figs. 19-21) the primary tympanic pouch is slightly more expanded and depressed. The dorsal apex has become flattened out to a low rounded prominence and has sunken to a lower level, so that it scarcely projects above the level of the pharyngeal roof. The tubo-tympanal crest in consequence is almost horizontal. Anteriorly it turns sharply inwards to form the relatively short tubal portion, the remainder forming the tympanic part (see fig. 20).
On the lateral wall the submeckelian fold forms a prominent, projecting ridge. It extends from the dorsal apex downwards and forwards to the latero—inferior edge, when it projects as a convex ventral pocket. In front of this region it suddenly dies out, forming only a low fold (fig. 19, 37.), continued to the base of the vestibular fold. The interval outside of this part is occupied by Meckel’s cartilage. The latter ascends from the mandibular arch in the angle between the submeckelian and the vestibular folds and thereby comes to lie in front of and above the former. The presence of the cartilage in the angle mentioned has probably some close connection with the separation of the two folds. Its presence would inhibit continued lateral extension of the connecting portion, while it would not interfere with such growth in the remainder of the fold. The latter would then continue to expand laterally and would thus give rise to the prominent projection which it forms at this stage.
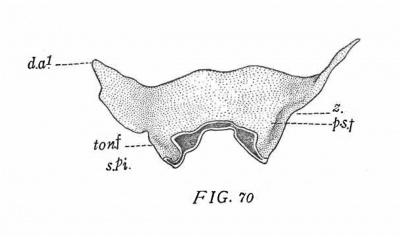
The form of the pharynx is essentially the same in a pig of 18 mm. (Series M3, my collection) and a rabbit of 14 days (No. 157, Harvard series, fig. 70). It differs but slightly from that last described. The greater part of the tympanic pouch lies in an almost horizontal plane, only its extreme lateral portion being slightly upturned (figs. 41-46). The dorsal apex forms only a low eminence, the meeting point of tubetympanal border, submeckelian fold and tensor tympani border.
The most important feature of this stage consists in the definite segregation of the neighboring skeletal structures, particulary l\Z[eckel’s cartilage and the auditory capsule. Their formation is so intimately associated with certain later modifications of the pouch that a short description of their essential characteristics is necessary. Meckel’s cartilage is a stout rod, which, as already mentioned, rises from the mandibular arch in the angle between the submeckelian and vestibular folds and then turns obliquely backwards above the former fold (figs. 41-43). Close to the posterior margin of the fold it sends down the stout manubrium which curves around the back of the fold and terminates in a slight depression——the manubrial fossa—immediately beneath (figs. 43-44). The submeckelian fold is thus wedged in the angle between Meckel’s cartilage and the manubrium and is thus relatively fixed in position (fig. 43). This relative fixity of the fold is an important factor in the final transformation of the pouch into the definitive tympanic pouch and Eustachian tube.
The auditory capsule occupies the depression (Impressio cochlearis) between the dorso-internal surface of the pouch and the roof of the pharynx.
(a”) The Differentiation of the Tympanic Pouch and Eustachian Tube
In a pig of 20 mm. (No. 542, Harvard series, figs. 23-27) we observe the beginning of the changes leading to the final transformation of the primary tympanic pouch into the definitive pouch and Eustachian tube. The transformation appears to be closely connected with a continuance of the processes already indicated. Of these We may recall (1) the ventral (caudal) flexure and elongation of the posterior half of the pharynx in connection with the formation of the neck, (2) the anterior extension and fiexure of the mouth, and (3) the relative fixation of the primary tympanic pouch by the differentiation of the surrounding cartilages.
As a result of the iiexures of the pharynx and mouth the common structure now has the form of an arch (fig. 23), the apex of the arch being that part lying between the primary tympanic pouches. From the side of this part each pouch projects as a broad, flattened fold, which towards its periphery turns strongly upwards so that the apex again extends some distance above the roof of the pharynx. Together the tub0—tympanal and tensor tympani borders form an arched curve, the apex being formed by the dorsal apex (fig. 23). The submeckelian fold (S-ELF.) has much the same appearance as before. It is completely separated from the vestibular fold. It, however, no longer projects below the ventral line of the pouch, but lies a slight distance above it on the lateral surface. This change has been eifected by a process which only becomes noticeable in the region of the pouch at this time. This is the downward growth and posterior extension of the alveolo-lingual folds As these grow down they carry with them the adjacent ventro-lateral wall of the pouch, and thus the latter loses its original horizontality and assumes an inclined position. Its surface thus comes to be more nearly continuous with the plane of the dorso-lateral portion. Since the submeckelian fold forms the dividing line between the two portions, it comes, in consequence of this change, to occupy its present relatively higher level on the side of the pouch.
An important, but at this stage inconspicuous, feature is a shallow indentation on the posterior tympanal rim between it and the second pouch (see figs. 2-6-27, z.). The latter pouch is now so small that the exact line of demarcation is not easily recognizable. A slight ridge (fig. 26, p-m.f.), however, which extends from the indentation to the submeckelian fold, enables one to fix upon this point as being between the two structures. The same ridge, showing the same relations, is present in the immediately «preceding stage when the second pouch was still clearly recognizable. This ridge later becomes the prominent elevation limiting the manubrial fossa posteriorly.
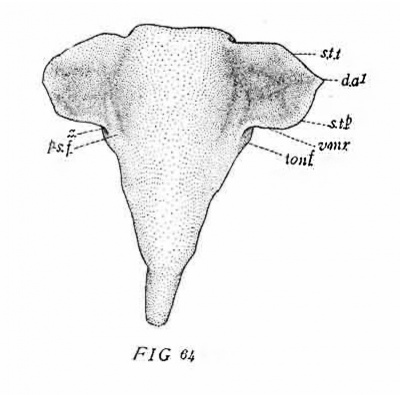
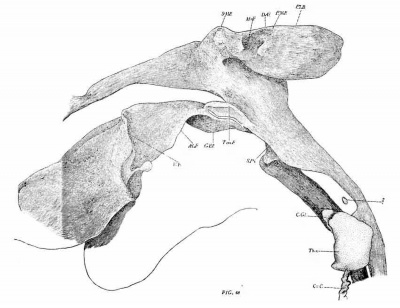
In the cat of 10.7 mm. (No. 474, Harvard series) the tympanic pouch shows a slight advance. The indentation between the pouch and the second pouch is slightly deeper and consequently the posterior tympanal crest now forms a rounded lobe projecting dorsally. In all other respects this stage is so similar to the preceding that further description is unnecessary. The l?haryngeal Pouches in the Mammalia 203 The eat of 15 mm. (No. 436, Harvard series, fig. 63, 64) presents the next stage in the modification of the tympanic pouch. The incision, which in the preceding stages had just begun to form between the tympanic pouch and the dorsum of the second pouch, is now much deeper. The postero-lateral lobe of the pouch in consequence protrudes more\ strongly in the dorso-posterior direction. As a result of the incision a new ventro-mesial border (V.M.R.) has begun to form between the base of the pouch and the pharynx. Posteriorly this border connects by a rounded angle with the posterior tympanal border (fig. 64, s. t. p.). 6 The connection of the pouch with the pharynx is both relatively and actually of lesser extent than in the preceding stages. This condition represents the commencement of the gradual constriction of the connecting part as a result of the anterior extension of the incision.
Owing to the increased depth of the intervening incision the tympanic pouch is now completely separated from the second pouch. This condition is apparently produced in the following manner: It will be recalled that the pouch has now become relatively fixed in position by being included between Mecke1’s cartilage with its manubrial process and the auditory capsule. The neighboring lateral walls of the pharynx, on the other hand, are continuously being displaced to a. lower level by the downgrowth of the alveolo-lingual margins. Among the parts thus carried down is the concavo-convex fold representing the dorsal remnant of the second pouch (Ton.F.). In consequence of this displacement of the second pouch and the relative flxity of the tympanic pouch, the incision (z) between the two spreads dorsally over the second pouch and reaches the longitudinal ridge (P—S.F.) lying immediately internal to the base of the pouch (cf. figs. 26, '70 and 64). Thus the base of the tympanic pouch is placed in connection with this ridge, which is the external expression of the groove extending backward from the Eustachian opening between the levator cushion and the salpingo-pharyngeal fold. For convenience in description I shall speak of it as the post-salpingeal groove.
As a result of the process. just described the dorsum of the second pouch comes to lie on the lateral surface of the pharynx below the posterior margin of the tympanic pouch. With the subsequent downgrowth of the a.lveolo-lingual folds it is carried farther ventralwards, and, as will be described later, is finally transformed into the tonsillar recess. On the expanded lateral wall of the tympanic pouch two prominent outstanding folds are now present. The anterior is the submeckelian fold; it shows the now deep Meckelian fossa on its dorsal side. The posterior fold is less prominent; it corresponds to the ridge formerly mentioned as forming the posterior limit of the manubrial fossa. The latter now forms a depression of considerable depth.
A rabbit of 161/2 days, 17.8 mm. (No. 576, Harvard series, fig. '71) shows the constriction of the tympanic pouch still further progressed. The post-salpingeal fold (p. s. f.) is more convex. The dorsum of the second pouch (ton. f.) is separated by a short interval from the base of the tympanic pouch. The remaining features of the pouch are essentially like those in the following stage.
This stage is represented by a pig of 24 mm. (No. 64, Harvard series, figs. 29, 30). The constriction of the tympanic pouch has now reached a stage where its connection with the pharynx embraces about twothirds of its former extent. The ventro-mesial margin is accordingly of considerable length. The posterior half of the pouch projects strongly backwards as a wide, cup-shaped fold.
The submeckelian fold forms a wide, almost horizontal shelf (figs. 47-50, s. In. f.). Laterally it reaches considerably beyond the dorsal apex, so that it is clearly visible from above (fig. 30). On lateral View it appears at a considerably higher level than before. This position it has obtained partly as a result of its lateral extension and the consequent flattening of its dorsal surface and partly from the ventral dovmgrowth of that portion of the pouch lying immediately below it (cf. figs. 48-50, with fig. 43).
The manubrial fcssa forms a shallow impression between the submeckelian (s. m. f.) and post-manubrial folds (p. m. f.). The latter is much less prominent in the pig than in the equivalent stage of the cat.
The pig of 25 mm. (Series M3, my collection), while slightly more advanced than the preceding, is essentially similar so far as the tympanic pouch is concerned. figs. 4-7-51 give views of several transverse sections of the structure.
The next step in advance is shown by a eat of 23.1 mm. (No. 466, Harvard collection, figs. 66-67). In this case the tympanic pouch and Eustachian tube are first clearly diiferentiated from each other. The former is a wide, cup—shaped expanse, concave dorsally. As a whole, it has a decided ascending direction. The postero-lateral border (P-L.B.) forms a highly elevated ridge. Immediately back of the dorsal apex it is interrupted by a deep incision—the incissura tensoris (I.Tn.). Anterior to the apex is the submeckelian fold (SM.F.) facing at this stage in the antero—dorsal direction. Laterally its margin is so far upturned as to hide from view the adjacent part of the tubetympanal border.
The msanubrial fossa (i\In.F.) is Very deep. It lies immediately below the tensor incision, bounded anteriorly by the submeckelian fold and posteriorly by the post~manubrial fold (P—M.F.). The external auditory tube lies a short space below the fossa, but is still separated from it by a considerable thickness of connective tissue.
The most noteworthy feature of this stage is the initial division of the pharynx into its oral and nasal portions by the backward extension of the palatine incisions. The oral cavity has been entirely separated from the nasal tube, but the constriction of the pharynx has only begun in its more anterior part. The constriction, as the figures show, takes place in the part lying below the post—salpingeal fold, between it and the tonsillar fold.
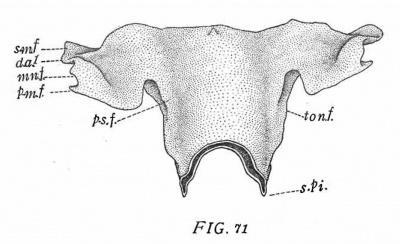
The rabbit of 18 days (Harvard series), while showing a slight difference from the preceding, is yet so closely similar that a full description is unnecessary. Its general features can be seen by consulting fig. '72. The most marked feature is the greater posterior extension of the palatal constriction. The broad grooves continued back from the latter over the sides of the pharynx represent the posterior palatine grooves (Arcus pharyngo-palatinus), p. pl.
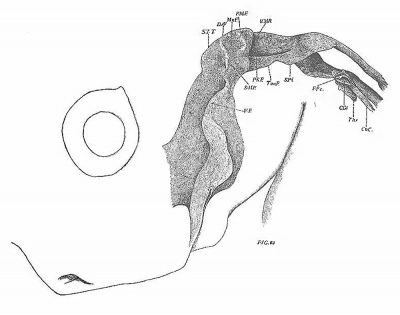
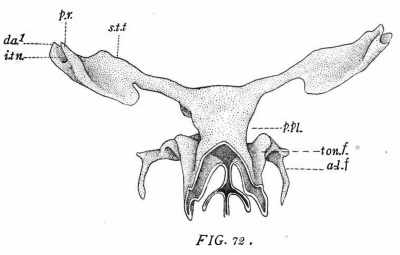
In the pig of 32 mm. (No. 74, Harvard series, figs. 32, 33, 52) the Eustachian tube is more constricted than in the preceding stage. The tympanic pouch projects sharply outwards and backwards. Close to where it joins the tube it gives off the still prominent submeckelian fold. Where the latter and the tubo—tympanal borders meet is the dorsal apex (Recessus anterior, D.A.1). Below the latter on the lateral wall is the now crescent-shaped post—nianubrial fold. The latter arches around underneath the manubrial fossa (Mn.F.) and becomes continuous with the portion extending to the dorso-lateral margin of the pouch. Immediately below the fossa the ventro—lateral surface of the pouch is flattened and is adpressed against the inner part of the external auditory tube. Only an exceedingly thin layer of connective tissue intervenes between the two structures (fig. 52).
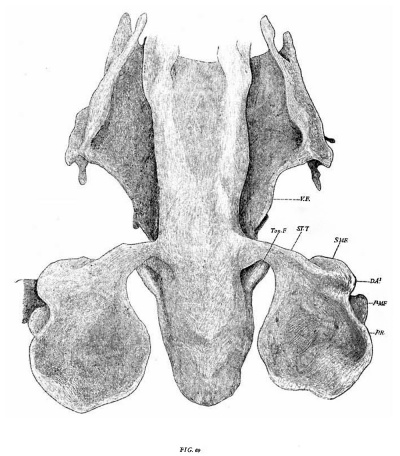
The eat of 31 mm. (No. 500, Harvard series, figs. 68-69) gives the final stage in its species. The Eustachian tube is very narrow, while the tympanic pouch is widely expanded, particularly in its posterior part. It still retains its cup—like form, the concave surface fitting closely against the ventro—lateral wall of the auditory capsule. The' submeckelian fold (S—M.F.) is relatively not so prominent as earlier. The dorsal apex or anterior recess (D.A.1) projects strongly outwards. The manubrial fossa (Mn.F.) forms a deep hollow on the more dorsal portion of the lateral surface. It is largely surrounded by the new high and conspicuous post-manubrial fold (P.M.F.). Below the fossa is the surface already mentioned as being in close relation with the external auditory tube. The remaining posterior extension of the pouch is simply applied to the neighboring part of the auditory capsule.
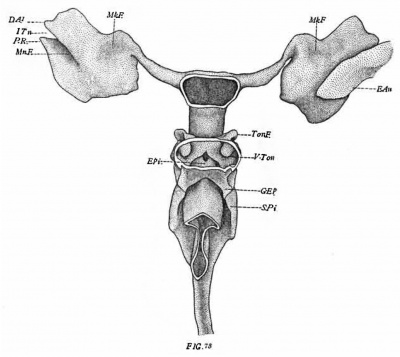
The latest stage studied is shown by a rabbit of twenty-one days. fig. '73 shows the more important points. The manubrial fossa (Mn.F.) is still rather deep in its dorsal half, but vertrally becomes very shallow and there tapers out to a point. The area entering into the constitution of the tympanic membrane is more extensive than in the preceding stage. It now includes a considerable part of the surface in which the manubrial fossa is located.
The Meckelian fossa (Mk.F.) is almost obliterated; it persists as a very shallow impression on the antero-dorsal margin close to the union of the pouch with the Eustachian tube.
Review and Comparisons
The foregoing results render it highly probable that the developmental history of the first pharyngeal pouch is essentially the same in the three species of mammals studied.
This history I have subdivided into three periods, as follows:
- The period of formation of the typical pouch.
- The period of transformation of the pouch into the primary tympanic pouch.
- The period of differentiation of the tympanic pouch and Eustachian tube and their subsequent modifications.
Period I.
The formation of the pharnygeal pouches takes place in the usual manner~—beginning with the most anterior and ending with the most posterior.
When typically developed the first pharyngeal pouch has the form of an approximately transverse vertical fold. At its dorsal lateral angle it projects dorsalwards as a narrow prominence, the dorsal apex (recessus anterior). From this apex three prominent ridges diverge, i. e., antero—lateral, lateral and postero-lateral. - The first extends diagonally inwards and slightly forwards. It forms the sulcus tubetympanicus of Moldenhauer. The lateral. ridge is that by which attachment to the ectoderm is effected. The area of attachment includes nearly its entire extent. Ventrally this ridge is continued into the ventral diverticulum. The postero—lateral ridge extends obliquely inwards and backwards from the dorsal apex to the dorsum of the second pouch.
The ventral diverticula of the first pair of pouches are at first more prominent than those of the succeeding, but they are soon outstripped by the latter. Typically they form a pair of low, but sharp folds, which at first are continuous across the median line of the pharynx.
Period II.
The most important changes leading to the transformation of the first pouch into the primary tympanic pouch are the following: The gradual separation of the pouch from the ectoderm. This process begins on the Ventral side and progresses dorsalwards until complete separation has been effected. In consequence of this separation the lateral ridge becomes greatly reduced and partly absorbed into the neighboring walls of the pouch.
The tubo-tympanal border becomes prolonged in the anterior direction. This change is produced as a result of the elongation in the same direction of the adjacent part of the oral cavity.
The ventral diverticula first become interrupted in the mid-line, and later gradually disappear as a result of absorption into the floor of the pharynx.
The basal or mesial portion of the pouch is displaced ventralwards in consequence of a corresponding displacement in the adjacent part of the pharynx itself. This portion of the pouch thus assumes an almost horizontal position. At first the peripheral part, owing to its continued attachment to the ectoderm, retains its ascending course, joining the mesial portion at a sharp angle. Later, after complete separation from the ectoderm, the peripheral portion also sinks, and thereby assumes a plane more nearly like that of the mesial portion.
The submeckelian fold is formed by the union of the dorsal remnant of the lateral ridge with the diagonal fold separating the basal and peripheral portions of the pouch. At first the fold is continuous anteriorly with the lateral margin (vestibular fold) of the oral cavity. Subsequently this connectionis interrupted and the submeckelian fold then grows out as a prominent shelf—like protuberance underlying Meckel’s cartilage.
Period III.
The transformation of the primary tympanic pouch into the definitive tympanic pouch and Eustachian tube is marked by the following features: The peripheral portion of the pouch becomes relatively fixed in position by the segregation of Meckel’s cartilage with its manubrial process and the auditory capsule.
The basal portion, on the other hand, continues to be carried down by the downgrowth of the alveolo-lingual fold.
The combined effect of these two processes is to give the pouch a peripherally ascending course.
An incision forms at the postero—internal angle of the pouch between it and the dorsum of the second pouch. This incision rapidly extends forwards as an ever—widening cleft between the base of the pouch and the wall of the pharynx.
In consequence of this process the connecting part of the pouch is progressively constricted until it forms a narrow tube, the Eustachian tube.
The remainder of the pouch retains its original wide extent and forms the tympanic pouch.
The later changes relate mainly to modifications in the detailed structure of the tympanic pouch. Among them are the formation of the manubrial fossa, the reduction of the submeckelian fold and the formation of the tympanic membrane.
The manubrial fossa lodges the ventral extremity of the manubrium. At first it is a shallow impression on the lateral surface immediately underlying the posterior part of the submeckelian fold. With the formation of the definitive tympanic pouch it rapidly deepens to form a cup—like depression. Subsequently this elongates at its ventral extremity to form the fissure-like groove characteristic of its final stage.
The submeckelian fold is at first very prominent and partly encloses a Meckelian fossa. The latter later assumes a more flattened form and the fold at the same time broadens until it is absorbed into the wall of the pouch. In the latest stage the submeckelian fold forms only ‘an inconspicuous swelling on the outside of the tubo-tympanal border.
The tympanic membrane is formed by the progressive approximation of the ventro-lateral portion of the tympanic pouch and the neighboring dorso-internal surface of the external auditory tube. At first the two surfaces are separated by a considerable interval filled with connective tissue. This interval later becomes narrower until it is reduced to an exceedingly thin layer—the membrana propria of the definitive membrane. The formation of the tympanic membrane begins on the ventrolateral surface of the pouch, but subsequently it extends dorsalwards so as to include the portion containing the manubrial fossa.
After its differentiation the pouch as a whole increases in width both laterally and longitudinally. Its posterior portion extends backwards as a prominent projection (posterior recess). The margins become upraised and thus the pouch as a whole assumes a cup—like form. The concavity on the dorsal side, corresponding to the promontory, lies close to the latero-Ventral surface of the auditory capsule.
As already mentioned, my results make it highly probable that the developmental history of the first pharyngeal pouch is in all important respects similar in the three types studied. This probability is still further heightened when the results are compared with those obtained by other investigators. Thus Piersol has described and figured the earlier stages in the rabbit. They agree in every important particular with the corresponding stages in the cat and pig.
The most complete comparison can, thanks to the work of Hammar, be made with the human species. Hammar figures nearly every stage from the typical first pharyngeal pouch to the end of foetal life. I have carefully compared Hammar’s descriptions and figures with mine and find that in every important particular they are applicable to the types examined by me. It is in fact diflicult to recognize any important differences, at least as late as the stage when the tympanic pouch and Eustachian tube have been fully differentiated. In the case of the human species the tympanic pouch during the later foetal life gives rise to several outgrowths from its dorso-lateral margin. From one of these the mastoidal cells arise as a complex series of buds. In the rabbit these outgrowths had not formed at as late a stage as that of an animal of 21 days. Whether they are present at the same stage of development in the other two forms I am, at present, unable to say. The latest stages of each, Which I was enabled to examine, showed no trace of them.
Hammar does not lay as much stress as I on the submeckelian fold. He describes its formation correctly, but apparently fails to note its separation from the vestibular folds and its later lateral expansion. His figures, however, leave no doubt that in these particulars the human species agrees with the other types. In some of his figures I am 1101‘. certain whether Hammar means to include the subrneckelian fold as a part of the recessus anterior or to limit the latter to the dorsal apex. His descriptions seem to me to favor the latter alternative. He applies, at any rate, no distinctive term to the fold, and accordingly I have felt free to call it the submeckelian fold.
The foregoing remarks make it apparent that the same essential type of development of the tympanic pouch and Eustachian tube holds in species belonging to four different orders of mammals, *5. e., Rodentia (Lepus), Ungulata (Sus), Carnivora (Felis), and Primates (Homo). So far as known, other species, which have been much less thoroughly investigated, agree with this type. Accordingly, it seems reasonable to suppose that the same type prevails in the majority of ordinary placental mammals and that it represents the typical development of the structures in the class. In forms which are adapted to a special environment (Cetacea, for example) or which are farther removed from the main phylogenetic series (Edentata) it may show important modifications. So far as I am aware, these forms have not yet been investigated in regard to this point. The Marsupials and Monotremes have also not been sufficiently investigated to allow of any assertions being made concerning them. It may be added that a figure by Maurer, showing an early stage of the pharynx in Echidna, bears a striking likeness to that of my 6.5 mm. pig and 6.2 mm. cat.
B. The Modifications and Fate of the Second Pharyngeal Pouch
(b’) The Retrogressive Modifications of the Pouch
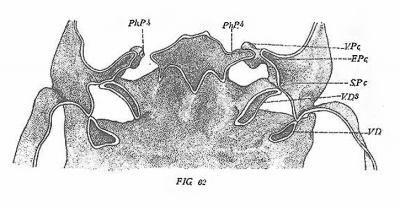
We left the second pharyngeal pouch fully and typically developed in a eat of 6.2 mm. (figs. 58-60). Its form at that stage is that of a postero-laterally projecting, vertical fold, which is connected by its entire peripheral margin with the ectoderm of the corresponding groove. At its dorso-lateral angle it is produced into a slight elevation forming a dorsal apex (D.A.2) similar to that of the preceding pouch, but considerably less prominent. On its ventral side the pouch is continued as a prominently projecting ventral diverticulum (V.D.2). The deep portion of the latter is limited to the lateral half of the pharynx, its internal border forming a free edge (fig. 60). At the base of this edge the diverticulum is continued mesially as a low fold similar to the same part in the first pouch. Like the first pouch, the second has four borders and three surfaces. The borders are antero-lateral, posterointernal, lateral and ventral. The surfaces are antero-lateral, medioThe Pharyngeal Pouches in the Mainmalia 211 posterior and dorsal. The antero-lateral border (Ton.F.) extends from the dorsal apex diagonally forwards and inwards to the postero—internal angle of the first pharyngeal pouch. It forms the dividing line between the ‘dorsal and antero—lateral surfaces. The postero—internal border is approximately crescentiform. Laterally, owing to the posterior flexure of the pouch, its course is almost longitudinal, but basally it bends first inwards and then posteriorly and connects with the lateral ridge extending to the third pouch. It separates the dorsal and postero-internal surfaces. The lateral margin forms the part connected with the ectoderm. It separates the antero—lateral and postero—internal surfaces. Ventrally it is continued into the ventral margin, which forms the free edge of the ventral diverticulum.
In a cat of 9.7 mm. (No. 446, Harvard series, fig. 61) the second pouch, beyond a slight increase in size, shows but few new features. For a short distance below the dorsal apex it has separated from the ectoderm—the initial step in the process which in this case begins at the dorsal end and progresses towards the ventral. The separation is accompanied by the ingrowth of mesenchyme into the intervening space.
The latero-ventral angle of the ventral diverticulum is produced into a slight projection. As a result the inner border of the diverticuluin ascends more diagonally to the floor of the pharynx.
In the pig of 10 mm. (No. 401, Harvard series) a departure from the preceding stage is shown by the slightly lower level of the second pouch. In the preceding examples the dorsal line of the pouch lay a short distance above the same line of the pharynx, while in the present stage it lies below it. This condition is probably produced by the changes taking place in the neighboring parts. The hyoid region increases in thickness more rapidly in its dorsal portion than in its relatively passive ventral part. The dorsal portion thus projects outwards over the lower and consequently the second ectodermal groove assumes a more inclined direction than before. With the latero-ventral rotation which the dorsal half of the groove undergoes it naturally results that the attached dorsal portion of the internal pouch accompanies it, at least in part, in the same direction, and thus assumes a more lateral, as well as lower, position.
In the 12 mm. pig (No. 518, Harvard series, figs. 9-12) the second pouch comes to a standstill so far as any lateral growth is concerned. Thus the distance between the lateral margins of the two opposite pouches remains the same as in the preceding stage. The first pharyn» geal pouch, on the contrary, continues to extend rapidly in that direction, and at the same time carries with it the attached adjacent parts. As already mentioned, the antero-lateral margin of the second pouch is continuous anteriorly with the latero-posterior border (S.T.T.) of the first pouch. At first the two join at a considerable angle, but as this border of the first pouch is carried outwards by the growth of the pouch, the attached antero-lateral border of the second pouch (Ton.F.) follows it and thus the angle tends to become drawn out and the borders to form a continuum. Consequently at this stage the antero—lateral border extends diagonally outwards instead of inwards, as in preceding stages.
The lateral fiexure of the antero-lateral margin causes it to arch outwards above the underlying antero-lateral wall. The latter thus forms a well—marked concavity, which is limited internally by the low fold (later part of the alveolo—lingual sinus) connecting the ventral diverticula of the first and second pouches. Owing to its inclined position, this surface will henceforth be called ventro-lateral (fig. 12, c. v.).
Corresponding to the depressed condition of the vcntro-lateral surface, the dorsal surface, which is everywhere closely adpressed against the underlying wall, is raised into a low dome-shaped convexity. The latter I shall call the dorsal prominence (fig. 10, D.Pr.).
The ventral diverticulum (v. d. 2) is reduced to about three-fourths of its former vertical extent. This change I am inclined to attribute, in part at least, to the outward extension of the antero—lateral portion of the pouch. The latter would set up a tension in the remainder of the pouch which would lead to a partial absorption of the diverticulum into the adjacent portion of the pouch. A fact favoring the existence of such a tension is the presence upon the ventro-lateral wall of a narrow fold extending obliquely upwards from the base of the diver-ticulum (fig. 12).
The lateral margin of the second pouch is now largely free from the ectoderm, the connection with the latter persisting only in its more ventral portion, where the corresponding ectodermal groove forms»a deep, vertical pit (fig. 11).
The pig of 14 mm. (No. 65, Harvard series, figs. 14-17) shows the second pouch slightly reduced in vertical extent, but produced at its ventro-lateral angle into a long, fine process (Fl.P.), the distal end of which is attached to the ectoderm. Elsewhere the pouch is free and is removed by a wide interval from the ectoderm. This condition is the result of the rapid growth in thickness of the hyoid region. As the figures (12 of last stage and 17 of this) show, this growth has not been accompanied by a corresponding increase in the pouch, which at this stage remains of the same width as in the preceding stage. Consequently, as the second pharyngeal groove is displaced more and more lateralwards, the attached ventro—lateral angle becomes drawn out into the process here shown. Owing to its form, I designate the latter the filiform process (fl. p.).
The lateral margin of the pouch is considerably less prominent than hitherto. This change appears to be produced by an actual regression of the margin. This is indicated by the fact that the distance between the lateral margins of the two opposite pouches is slightly less than in the preceding stage. The regression is probably attributable to the tension exerted upon this margin by the continued lateral extension of the adjoining antero-lateral margin with which it now joins at a. wide angle.
As just mentioned, the antero—lateral margin (ton. f.) has continued to extend in the lateral direction. It thus has a decided antero—latera1 course. For this reason it is inappropriate to call it by the term hitherto used, and accordingly I shall hereafter speak of it as the dorsolateral margin.
The dorsal apex (D.A.2) of the pouch now forms only a slight protuberance at the posterior extremity of this margin (fig. 15).
In consequence of the extension laterally of the dorso-lateral margin the underlying ventro—lateral surface has acquired the form of a deep concavity (fig. 17, c. v.). The overlying dorsal wall is correspondingly raised as a broad dome—shaped prominence (fig. 15, D.Pr.). I The ventral diverticulum (v. d. 2)- has almost ceased to exist as a distinct feature. Only in its more peripheral part does it project to a fair degree below the ventro-lateral line of the pharynx. Its middle part has largely disappeared owing" to the downgrowth of the alveololingual ridge (fig. 17, al. f.) and the union of the latter with the sinus piriformis (fig. 17, s. pi.). The place of the original diverticulum is indicated by a widening of the continuous ventro-lateral fold thus formed.
The more internal part of the diverticulum persists as a slight ridge on the inner side of the ventro-lateral fold (fig. 17).
In the 17 mm. pig (figs. 19-22) the second pouch has entirely severed its connection with the ectoderm, leaving the filiform process terminating blindly in the mesenchyme. Owing to the continued lateral extension of the dorso—lateral margin (Ton.F.), the original lateral border now forms a continuum with it. This leaves the dorsal apex as a minute protuberance at its posterior extremity.
The dorso-lateral margin shows no increase in length, but anteriorly it has been carried farther outwards in consequence of the extension of the tympanic pouch in that direction. It thus acquires a course almost in line with the postero—lateral margin (s. t. p.) of the latter. Only a slight notch remains to indicate the dividing line between them.
The ventral diverticulum (v. d. 2) has now nearly disappeared, having been absorbed by the downgrowth of the ventro-lateral margins of the pharynx.
The features of the second pouch in the pig of 17 mm. are essentially duplicated in cats of 10.7 mm. and 12 mm.
A rabbit of 14 days (No. 157, Harvard series, fig. '70) shows a stage somewhat intermediate between that just described and the next.
The same remark is also applicable to an 18 mm. pig (Series M3, of my collection).
In a pig of 20 mm. (No. 542, Harvard series, figs. 23-27) the second pharyngeal pouch is chiefly modified as regards length and direction. As indicated by the figures, these modifications are related to the ventral (caudal) flexure of the posterior half of the pharynx. As already mentioned (see account of tympanic pouch), the tympanic pouch has at this time become relatively fixed in position by the formation about it of the related cartilages. Consequently, as the pharynx continues to bend toward the ventral side, the attached second pouch tends to be drawn out and flattened (fig. 23). This process is also accelerated by the continued deepening of the ventro-lateral margin of the pharynx.
The hitherto prominent dorsal prominence is now reduced to a low swelling located at the postero-internal angle of the tympanic pouch. No lateral extension of this part of the pouch has taken place since the 17 mm. stage. Its middle and posterior portions, on the contrary, have shrunken slightly, due probably to the tension produced by its elongation in the ventral direction and the continued downgrowth of the adjacent ventro-lateral margin (A-L.F.) of the pharynx.
Posteriorly the second pouch thus forms a low ridge situated on the outer side of the ventro-lateral fold (= conjoined alveolo-lingual and piriform sinuses).
The shrunken filiform process is still recognizable. The Pharyngeal Pouches in the Mamrnalia 215 The dorsal apex has been absorbed into the neighboring surface of the dorsal prominence.
The ventral diverticulum forms only an inconspicuous fold in the same situation as hitherto.
(b") The Formation of the Tonsillar Fold
The eat of 15 mm. (No. 436, Harvard series, figs. 63-64) gives us the initial step in the transformation of the remnant of the second pouch into the tonsillar fold.
In this stage the dorso—lateral fold, representing the second pouch, no longer forms a continuum with the adjacent border of the tympanic pouch, but is separated from the latter by an indentation which extends quite across its dorsal side to the longitudinal ridge (P.S.F.), forming its mesial boundary. .
The second pouch thus forms an arched lateral fold. The lateral margin (Ton.F.) of the fold lies on a level with the ventro—lateral line of the pharynx. The ventral side is concave; the dorsal correspondingly convex. Anteriorly the fold is continued immediately under the ventr0internal angle of the tympanic pouch and extends to the base of the vestibular fold. Internally the fold is limited on the ventral side by the alveolo—lingual fold, on the dorsal by the adjacent surface of the pharynx. The structure thus defined is the tonsillar fold (tonsillar sinus). The concavity on its ventral side corresponds to the tonsillar prominence (tonsillenhocker).
There is no trace at this stage of the filiform process.
The rabbit of 161/2 days (No. 5'76, Harvard series, fig. '71) shows an essentially similar condition. The fold (ton. f.) is more strongly arched and its lateral edge lies some distance above the lower edge of the alveolo—lingual groove. The fold is widest immediately under the post-salpingeal ridge (p. s. f.). Its anterior continuation forms a low ridge, which probably represents an extension of the fold over the adjacent surface of the pharynx.
In the 24 mm. pig (No. 64, Harvard series, figs. 29-30) the tonsillar fold is removed by a considerable interval from the base of the tympanic pouch (fig. 30"). Between them the surface of the pharynx is depressed, forming the palatal constriction. The present position of the fold is due to the formation of this constriction and to the continued downgrowth of the alveolo—lingual margin (A-L.F.) with which it is closely associated. In form the tonsillar fold (Ton.F.) of the pig of this stage bears a greater resemblance to that of the cat than to the same structure in the rabbit. The tonsillar fold of the latter has a more decided ascending plane than the others.
In the eat of 23.1 mm. (No. 466, Harvard series, figs. 66-67) the tonsillar fold (Ton.F.) has attained its definite position. The palatal constriction has now separated the nasal cavity from the mouth and has begun to encroach upon the pharynx. The tonsillar fold forms a wide, diagonally ascending arched fold on the side of the oral portion. Its ventro—lateral surface is, as usual, deeply concave.
The pig of 32 mm. (No. 74, Harvard series, fig. 32) shows the tonsillar fold (Ton.F.) more nearly erect than in the cat. In outline it is approximately quadrangular and its outer (: ventral) surface is less concave than in the cat. Ventrally it is limited by the alveolo— lingual ridge (A-L.F.), which at this stage no longer forms the lower line of the pharynx, but lies on the outer side of the glosso-epiglottic fold (vallecula glosso-epiglottica).
In the 31 mm. cat (No. 500, Harvard series, figs. 68-69) the tonsillar fold (Ton.F.) has essentially the same form as in the 23 mm. cat. As in the pig last described, its lower boundary——the alveolo—lingual ridge——now lies on the outer side of the glosso—epiglottic fold The palatal constriction has now completed the division of the pharynx into nasal and oral portions.
The narrow cord shown in the figure parallel with the tonsillar fold is an epithelial structure which lies free in the connective tissue to the outer side of the fold. Its significance I have not been able to solve.
In none of the stages so far studied did I observe any clear indications of the formation of lymphoidal tissue in connection with the tonsillar fold.
In the rabbit of 21 days (fig. '73) the tonsillar fold (Ton.F.) is approximately vertical. Its lateral surface is deeply concave and lies between a dorsal and a ventral fold. The former corresponds to the supra-tonsillar recess and evidently represents the derivative of the second pouch. The ventral fold I am inclined to homologize with the infra-tonsillar recess (V.T.) which is a derivative of the pharynx. I-Iammar, however, who describes a similar stage in the rabbit as well as in man and several other mammals, fails to mention this fold as the part in question. I regret that with the relatively few later stages at my disposal I have not been able to solve this problem satisfactorily.
Review and Comparisons
My investigations make it probable that the history of the second pouch, so far, at least, as its earlier stages are concerned, is similar in the forms studied. Unfortunately, my rabbit and cat material was not sufficiently abundant to enable me to make this statement without qualification. However, the specimens I did examine agreed very closely with corresponding stages in the pig series. The later stages were not sufficiently numerous to enable me to make comparisons. In its general features the development of the tonsillar fold seems to agree in all forms; in details, there are undoubtedly considerable differences in tlie different species.
The history of the pouch, as mainly determined in the pig, divides itself in two periods—-the first characterized by a series of retrogressive changes in the pouch, the second by a series of progressive changes converting the remains of the pouch into the tonsillar fold.
When typically developed the second pouch has the form of a poster0laterally projecting vertical fold. Dorsally the dorso-lateral angle is produced as a dorsal apex. Ventrally it shows a prominent ventral diverticulum. Connection with the ectoderm is more extensive than in any other pouch, the entire lateral margin taking part in the formation of the verschlussmembmn.
The earlier modifications of the pouch are connected with the rapid lateral growth of the hyoid region. The pouch, on the other hand, remains stationary. Parts of it are, however, connected with adjoining structures, and, as these undergo displacement connected with subsequent growth, the pouch becomes profoundly modified.
Separation of the pouch from the ectoderm begins on the dorsal side and extends progressively toward the ventral. The last point to remain attached is the ventro-lateral angle of the ventral diverticulum, which becomes drawn out into a thin cord, the filiform process. The latter subsequently separates and then shrinks in length and disappears.
Largely as a result of the lateral extension of the adjoining tympanic pouch the dorso-anterior portion of the second pouch is drawn farther outwards. Its margin, which originally extended forwards and inwards, acquires an antero-lateral course and thus comes to form a continuum with the posterior border of the tympanic pouch. The underlying antero-lateral surface becomes ventro-lateral and its wall becomes depressed to form a deep concavity, which corresponds to the later tonsillar projection. The closely adpressed dorsal Wall is correspondingly raised into a dome—shaped swelling, the dorsal prominence.
After its separation from the ectoderm the original lateral margin of the pouch recedes towards the median line. At first it forms a slight projection at the postero-internal angle of the pouch, but later this is absorbed and then forms a continuum with the dorso—lateral fold.
The ventral diverticulum early diminishes in size and later is absorbed by the downgrowth of the alveolo—lingual fold.
At the termination of the first period the remains of the second pouch form a laterally ascending arched fold, which lies at the postero—internal angle of the tympanic pouch. On its ventral side it is concave and on the dorsal correspondingly convex. Its inner boundary is formed by the alveolo-lingual ridge.
The second or progressive stage is marked (1) by the separation of the second pouch from the tympanic pouch, (2) its ventral displacement to its definitive position, and (3) its progressive modification to form the tonsillar fold.
The separation from the tympanic pouch takes place by the extension of the indentation between the two structures over the dorso-lateral surface. The second pouch thus comes to lie at a slightly lower level than the base of the tympanic pouch.
The ventral displacement takes place in connection with the continued downgrowth of the alveolo—lingua1 ridge and the accompanying formation of the palatal constriction. The latter forms between the base of the tympanic pouch and the dorsum of the tonsillar fold and as it enlarges the fold is pushed farther ventralwards, where it attains its final definitive position.
In its principal features the tonsillar fold in the later embryonic stages is similar in the species studied. It then forms a prominent arched fold on the lateral surface of the oral portion of the pharynx parallel to the alveolo-lingual sinus. Its ventro-lateral surface is concave, its dorsal convex.
The later modifications are concerned with the assumption of its definitive form. Owing to lack of materials, these modifications were not traced. The rabbit of 21 days shows that they may be considerable. I shall consider them further in the comparative part.
Contributions to the developmental history of the second pouch have been made by a number of investigators, chief among whom are Born, His, Rabl, Piersol, Kastschenko and Hammer. The results of these authors, while agreeing in certain respects, are hopelessly discordant in others. The most satisfactory account is that given by Hammar of the development in man. Hammar also describes a few stages in the formation of the tonsil in several other species of mammals. In the main my results are in harmony with his. The only statement of his to which I cannot subscribe is that the "Iciemengzmg” (: my filiform process?) is an ectodermal derivative. As his figures show, this struc— ture occupies the same relative position as my filiform process. In Hammar’s view this is formed by the passive deepening of the ectodermal groove produced by the growth of the hyoid region. He also pictures it as protruding above the margin of the pouch as a dorsal organ. In the pig, on the other hand, this structure is perfectly continuous with the ventro—lateral angle of the pouch and appears as a prolongation of the latter. In none of the specimens examined by me did I notice any communication between the lumen of the filiform process and the exterior. As for a dorsal organ projecting above the ventro—inferior edge of the pouch, I find no evidence of it. Accordingly, I am disposed to think that the filiform process and the “kiemengang” are independent structures. The latter would then be absent in the pig, while the former would be lacking in the human species. This view is supported by the observations of Piersol on the rabbit. He speaks of the ventro-lateral angle as continued in a blind tube. This evidently corresponds to the filiform process. He later speaks of the latter as cutting off from the pouch and undergoing changes reminiscent of the thymus. I find nothing of this in the pig. In the latter the process simply disappears——at least I have not seen any trace of it later than a 17 mm. animal. On the other hand, Piersol describes a long epithelial tube, which according to him arises from an insinking of the ectodermal groove. This corresponds to the “kiemengcmg” as described by Hammar. Piersol states that it originates while the filiform process is still present (twelfth day), but later (fourteenth day) it disappears without leaving a trace. If the facts as described are correct, the rabbit shows the filiform process and "kiemengang” as independent formations. The latter is evidently an extremely temporary structure. As already mentioned, I saw no trace of it in the pig, and in this I am in agreement with Rabl and Kastschenko, both ofi whom studied the same animal. The short duration of the “kieme.ngang” may perhaps have led to its being overlooked in this animal. I may add here that the term kiemengang was first applied by Rabl to the endodermal structure here called filiform process. Hammar considers Rabl’s account as contradicted by his results as determined in the human species, and accordingly applies the same term to the ectodermal structure. In case two independent structures, one endodermal and the other ectodermal, are found to exist in mammals, it will be necessary to return to Rabl’s original use of the term.
My rabbit and cat series throw no light on this puzzling matter. In the former I did not examine sufficiently early stages, while in the latter the stages which would show the structures under consideration were lacking in the collection.
The remaining results agree fairly closely with those of Hammar. The form of the early tonsil in the forms studied by me difiers somewhat from that in man, though the difference is a minor one. In regard to the rabbit of 21 days, Hammar does not speak of it as having an infratonsillar sinus. My specimen, on the other hand, shows a fold which, in my opinion, corresponds to this sinus. As, however, I was unable to examine forms in which the latter is undoubtedly present, I will not urge this homology.
The above comparisons would indicate that, although the chief features in the history of the second pouch agree in all species of mammals studied, there are considerable differences in detail. The matter of the filiform process and kiemengang would illustrate this. In later stages, as Hammar shows and as my specimens indicate, the tonsillar fold differs considerably in its structural details in various species of mammals.
C. The Metamorphoses of the Third Pharyngeal Pouch and its Derivatives
(c’) The Elongation of the Ventral Diverticulum and the Formation of the Thymus
When typically developed, as in the eat of 6.2 mm. (figs. 58-60), the third pharyngeal pouch bears a considerable resemblance to the second. Like the latter, it bears a deep ventral diverticulum (V.D.3), which likewise is limited to the lateral half of the pharynx, its inner half forming only an inconspicuous ridge. The deep part of the diverticulum is only slightly prolonged in a ventro-mesial direction.
The lateral margin is joined to the ectoderm for almost its entire extent, in this respect also resembling the preceding pouch (see clear area in fig. '7). In the 9.7 mm. cat (No. 466, Harvard series, figs, 61, 62) the ventral diverticulum (V.D.3) of the third pharyngeal pouch is slightly more elongated "at its ventro-internal angle. In other respects it is essentially similar to the stage last described.
The pig of 10 mm. (No. 401, Harvard series, figs. 4-8) shows clearly the initial steps in the formation of the thymus duct. The ventrointernal angle of the pouch is now clearly elongated in a ventro-mesial direction and ends in an acute angle which is wedged in the angle between the roots of the carotid and aortic arches (fig. 7). The downgrowths of the two sides are not quite symmetrical, the right being slightly larger and ending in a more acute angle than that on the opposite side. Laterally the lower part of the pouch has separated from the ectoderm, leaving only its dorsal half in contact with the latter. Below the point of contact the lateral border turns obliquely inwards and downwards and is continued into the ventro-lateral edge of the thymus downgrowth.
On the dorsal side the peripheral portion of the pouch projects slightly above the upper end of the verschlussmembmn as a dorsal diverticulum (figs. 4-8. D.A.3).
The carotid gland (figs. '7-8, C.G‘rl.) lies closely adpressed against the anterior wall of the pouch and on its dorsal side projects slightly above the upper margin of the latter (fig. 8). We shall defer further consideration of the gland until later.
In the 12 mm. pig (No. 518, Harvard series, figs. 9-11, 13) the ventral diverticulum (V.D.3) is further elongated. In addition to the Ventro-internal direction which it took in the earlier stage, it now shows a pronounced anterior trend, an effect of the ventral rotation of the adjacent part of the pharynx. At this time it has acquired a distinct tubular form.
The union of the pouch to the ectoderm is limited to a short stretch immediately below its dorsal apex. The rest of its lateral border is free and is continued ventrally into the outer edge of the tubular downgrowth.
The part connecting the pouch with the pharynx is considerably more constricted than in the preceding stage.
The tubular downgrowth is still further elongated in a 14 mm. pig (No. 65, Harvard collection, figs. 14-16, Thy.). Its blind ventral extremity lies a slight distance below the level of the pericardio—cervical groove and thus occupies the upper part of the pericardial region. It retains its tubular form, but shows a differentiation into two portions— a terminal, swollen portion and an intermediate, relatively narrow canal, which connects the former with the remaining dorsal body of the pouch. The latter now forms a compressed epithelial plate, bearing upon its anterior surface the voluminous carotid gland. It is still connected with the pharynx by a narrow connective.
The dorsal apex of the pouch has disappeared. The peripheral portion of the pouch is attached for a short distance to the anterior wall of the fundus praecervicalis (F.Pc.).
A lumen is present in the tubular downgrowth and in the pharyngeal connective, but has disappeared into the dorso—peripheral part by approximation of its anterior and posterior walls. The latter thus assumes the form of a vertical plate, connected with the fundus praecervicalis at its dorso—lateral angle and continuous with the intermediate cervical canal of the thymus at its ventro-internal angle.
In the 17 mm. pig (No. 51, Harvard collection, figs. 19-21) the thymus downgrowth (Thy.) has still further elongated and now shows clearly its segmentation into three portions. These include (1) the considerably swollen thoracic vesicle, (2) the intermediate cervical connective (CV.C.), and (3) the dorsal plate (D.Pl.), with which is closely associated the carotid gland (O.Gl.) and fundus praecervicalis (F.Pc.).
Only a few fragments of the original lumen now remain, mostly confined to the thoracic vesicle.
Connection with the pharynx is still maintained by an extremely thin, solid connective (fig. 45, of an 18 mm. pig).
The dorsal body of the pouch, as already noticed, has become reduced to a flattened plate and now constitutes a part of thymus. It has separated from the ectoderm of the surface of the body, but remains attached to that of the fundus praecervicalis, which constricts from the superficial ectoderm and accompanies the thymus as the latter passively recedes from the exterior. The detailed account of this process will be reserved for later treatment. It sutfices at this time to state that this ectodermal structure remains in close connection with the dorsal extremity of the thymus for a considerable period.
In an 18 mm. pig the thymus shows no essential deviations from that in the preceding stage. It is slightly longer and has a more vertical course than in the latter. I shall at this stage treat of the factors which appear to be operative in producing the present condition of the thymus. So far as I have been able to determine, the modifications which the third pouch has undergone are referable, in large part at least, to the operation of purely mechanical factors. The pouch itself shows but little power of active growth. Compared with the surrounding parts, it is relatively passive. The first important factor is found in the unequal rate of growth of the neck and of the ventral tubular process. The former carries the roots of the large arteries from their original position immediately under the pharyngeal pouch region to their definitive position in the upper part of the thorax. The ventral extremity of the thymus, it will be recalled, is from the start in close relation with the bases of the carotid and aortic arteries, and as the latter become displaced to successively lower levels, this portion of the thymus is carried down with them. The elongation of the neck takes place, however, at a more rapid rate than that of the thymus, and thus produces on the latter a tension tending to carry it downwards. This, however, is prevented by the fact that the dorsal extremity of the thymus is, during the same period, relatively fixed in position by its attachment to the skin and pharynx. The result of such conditions would be to produce a strong tension in the intermediate connecting portion, which in consequence would become drawn out in the form of a thin cord, similar to that shown in the present stage (fig. 21, Cv.C.). This view is supported by the fact that the diameter of the cord is actually, and not merely relatively, much less than in preceding stages.
As already mentioned, the dorsal extremity of the thymus is relatively fixed in position. This condition is readily explained when it is recalled that this portion is attached to both the ectoderm and the pharynx and also bears the voluminous carotid gland, the mere bull": of which alone would hinder any ready displacement, even through such a plastic medium as the surrounding mesenchyme.
An additional factor, first pointed out by Kastschenko, is found in the behavior of the hypoglossal nerve. The latter underlies the fundus praecervicalis. As this structure in harmony with the attached thymus becomes displaced in the ventral direction, it comes in contact with the nerve, which is also carried downwards with it until it forms a strong angular flexure, over which the outer part of the fundus curves like a hook over a cord. The elastic reaction of the nerve would naturally form a strong hindrance to any ready ventral movement of the head of the thymus. That a decided tension of the kind indicated is actually produced is evidenced by the later behavior of the nerve—a subject which I shall consider more fully when considering the modifications of the fundus praecervicalis.
In a cat of 10.7 mm. the thymus has approximately the same characteristics as in the pig just considered. It shows, however, no clear lumen in any part. A In a pig of 20 mm. (No. 542, Harvard collection, figs. 23-25) the thymus (Thy.) is considerably longer than hitherto and its ventral extremity is slightly lobed. Its lumen has entirely disappeared, owing to the thickening of its walls. The whole organ is thus composed of small-celled epithelioid tissue, which in every respect bears a close resemblance to ordinary lymphoid tissue. The dorsal plate is closely wedged in between the carotid gland and the fundus praeeervicalis (fig. 23).
The thymus on the left side has completely separated from the pharynx, while that on the right is still connected with it by an extremely thin cord (fig. 28).
The separation from the pharynx is probably connected with the inward flexure of the sinus piriformis and the consequent decrease in the lateral diameter of the pharynx. The dorsal extremity of the thymus and the attached carotid gland, however, retain their original position, with the result that the connecting..cord is drawn out to an exceedingly thin strand, which subsequently constricts.
In the eat of 15 mm. (fig. 63) the thymus shows no special features. It closely resembles the same structure in the pig just considered, but is located at a relatively lower level. In the pig its dorsal extremity is opposite the sinus piriformis, while in the cat it lies some distance below it.
In the 24 mm. pig (No. 64, Harvard collection, figs. 29-31) the thymus on each side is completely separated from the pharynx. Its terminal thoracic portion has grown considerably below the level of the thyroid. It is more swollen than hitherto and its distal portion is subdivided into a considerable number of convolutions (fig. 31, Thy.).
The intermediate cervical connective persists as an exceedingly thin solid cord (Cv.C.).
The dorsal plate is so intimately fused with the carotid gland and fundus praecervicalis (F.Pc.V.) as to be distinguishable from them only with difficulty.
The cat of 23.1 mm. (No. 466, Harvard collection) shows the thymus entirely free from the carotid gland, that on the left side being separated from the latter by a considerable interval. At its thoracic portion the organ is subdivided into numerous lobules.
In the 32 mm. pig (No. "/4, Harvard collection) dnly the dorsal end of the thymus was specially examined. It still forms a flattened plate associated with the carotid gland and the lobules of the fundus praecervicalis. It is connected by the cervical connective with the now large and much lobed thoracic thymus.
In the 32 mm. cat (No. 500, Harvard collection, fig. 68) the thymus is entirely unconnected with the carotid gland. The dorsal extremity of the cervical cord (Cv.C.) is placed immediately outside of the ventral edge of the lateral wing of the thyroid. The cord is somewhat convoluted. It is continuous at its ventral extremity with the thoracic thymus, which is much enlarged and subdivided into numerous lobules. The latter are solid and are composed of small—celled epithelial tissue. I could observe no indications of the formation of true lymphoid tissue in it.
In a rabbit of 20 days the thoracic thymus is very large and on each side it is subdivided into numerous lobules similar to those seen in the cat last described. It is connected by the cervical cord with the carotid gland, which is located close to the dorsal edge of the lateral wing of the thyroid.
(c") The Origin and Structure of the Carotid Gland
The carotid gland first appears in a 9 mm. pig (M5 of my series, fig. 36) as a mass of intertwined solid vesicles (C.Gl.), representing a series of folds of the anterior Wall of the third pharyngeal pouch. The gland is therefore a purely epithelial structure of endodermal origin. The carotid artery (Car.) lies immediately in front of it and shows a small branch extending back toward the gland. In the 10 mm. pig (figs. 7-8, C.Gl.) the carotid gland is somewhat larger. As in the preceding stage, it is continuous with the peripheral half of the anterior wall of the pouch and even projects a slight distance beyond its lateral margin (fig. 8). It here meets the ectoderifi of the anterior wall of the third pharyngeal groove. Dorsally it projects a short distance above the upper margin of the pouch and partly curves backwards over it. The carotid artery (Car.) gives off a small branch, which, on reaching the gland, divides into numerous capillaries which form a rich network interpenetrating it in all directions. Ventrally they unite into a common vessel which opens into the inferior jugular vein.
In the 12 mm. and 13 mm. pigs the carotid gland forms a more regularly circumscribed, voluminous mass (figs. 9-10, C.Gl.). It retains the same topographic relation to the third pouch as before, but has increased considerably in size (fig. 13). Its follicular structure is clearly shown, and reminds one of that of the liver. Like the latter, it consists of a reticulum of numerous, closely intertwined follicles interpenetrated by a rich system of capillaries, the latter derived from the carotid artery (fig. 53).
The gland and the associated solid wall of the pouch (= dorsal plate of the thymus) are intimately connected with each other (figs. 38-40). In many specimens, owing to unsuitable staining, the two parts cannot be clearly distinguished from each other. In those appropriately double stained with haematoxylin and Bordeaux red or with alum-cochineal and orange G the definitive reticular structure of the gland is clearly shown.
In the 14 mm. pig (figs. 14-15) the carotid gland shows no specially noteworthy features. On the dorsal side it projects considerably above the upper edge of the thymus and there comes in contact with the inner part of the fundus praecervicalis (F.Pc.).
In the 17 mm. pig (figs. 19-20) the carotid gland appears to differ only in size from that just described. The same remark applies also to an 18 mm. animal (figs. 45-46).
In the 20 mm. pig (figs. 23-24) the carotid gland forms a moderately large ovoid organ lying to the outside of the sinus piriformis (S.Pi.). It is distinctly follicular in structure, but its individual follicles show no lumen.
In cats of 10.7 and 15 mm. the carotid gland is similar in essential respects to that in the pig last described. It appears to be less compact than the latter, the interspaces between the follicles being relatively larger (fig. 63, C.Grl.).
In the pig of 24 mm. (figs. 29-31) the carotid gland (C.Gl.) shows no peculiar characteristics.
In the eat of 23 mm. (fig. 66) the carotid gland shows no special features. It resembles essentially that in a 15 mm. example.
In the pig of 32 mm. (figs. 34-35, C.Gl.) the carotid gland has the same characteristics as hitherto, but is closely invested by the lobules of the proliferated fundus praecervicalis.
The cat of 31 mm. (fig. 68, C.Gl.) shows the carotid gland as an ovoid body, located near the antero-dorsal angle of the lateral wing of the thyroid. It shows its follicular structure very clearly. It has lost its connection with the cervical cord of the thymus, the latter terminating opposite the ventral border of the thyroid.
In a 20-day rabbit (No. 172, Harvard series) the carotid gland shows its typical form and structure. It lies outside of the antero-dorsal angle of the lateral wing of the thyroid and is connected by the cervical cord with the thoracic thymus.
In the 21-day rabbit the gland occupies a depression in the outer surface of the lateral wing of the thyroid. It is now entirely unconnected with the cervical cord, the latter lying at a much lower level.
(c”’) The Sinus Praecervicalis and its Relation to the Thymus
It will be recalled that in a 6.2 mm. cat (figs. 59-60) the sinus praecervicalis (S.Pc.) forms an approximately funnel-shaped depression. The inner posterior portion corresponding to the stem of the funnel forms a deep pit, which extends diagonally inwards and backwards and terminates in a sharp edge, which at its ventral extremity is in contact with the lateral process of the fourth pouch. The outer relatively wide vestibule forms an approximately triangular depression, surrounded on all sides by the overhanging prominences of the adjacent parts—anteriorly by the hyoid arch, posteriorly by the anterior cervical region and ventrally by the pericardial prominence. Dorsally it becomes shallow and there blends with the side of the head without any perceptible break. At its ventro-anterior angle it is continued into the pericardio-cervical groove (fig. 60). The inner wall of the sinus is formed by two low prominences representing the third and fourth pharyngeal arches.
In the 9.7 mm. cat the sinus praecervicalis (fig. 61, S.Pc.) is deeper and narrower than in the stage just described—a diiference due to the continued outgrowth and approximation of the adjacent hyoid and anterior cervical regions. That part of the third arch which immediately adjoins the hyoid region is rotated outwards with the latter and thus comes to face obliquely backwards. In this way the outer opening of the deeper part comes to lie on a level with the third pharyngeal groove (Ph.G.3). We shall henceforth designate this deeper part of the sinus the fundus praecervicalis (F.Pc.), a term‘ applied to it by Kastschenko.
The opening of the fundus is triangular, narrow above, wider below (see right side of figure). At its dorsal apex the third pharyngeal arch and anterior cervical prominence converge. The fourth pharyngeal arch, which is located at the inner extremity of the fundus, is thus partly hidden in lateral view.
The fundus has much the same features as hitherto. Its inner extremity, while still connected at one point with the fourth pouch (fig. 62, P11. P. 4), is prolonged above the latter and forms a slight dilatation situated a short distance back of the dorsal edge of the third pouch (fig. 62, V.Pc.). This free, dilated portion is evidently to be compared with the vesicula praecervicalis (: vesicula thymicus of Kastschenko), which will be more fully considered in the descriptions of the P1g~ The 10 mm. pig (figs. 5-8) shows the sinus in a condition somewhat intermediate between the two last described. On the right side the fundus and the fourth pharyngeal pouch are still connected with each other, but on the opposite side they are entirely separate and are removed from each other by a considerable interval, which is largely occupied by the aortic arch proper (Ao.). The large size of the latter suggests that it may have been an active agent in effecting the separation of the pouch from the fundus. Thus, on the side where the two structures are still connected, the ventral side of the artery is closely pressed against the connecting part and is evidently exerting a pressure upon it which would tend to efiect its separation. As we shall see, this soon takes place.
The inner extremity of the fundus is formed by a narrow, obliquely vertical groove—the fourth pharyngeal groove (fig. 8, Ph. G4). Where the fourth pouch retains its connection, it is confined to the ventrointernal angle of the fundus. From this point the remainder of the groove ascends diagonally forwards and at its dorsal extremity meets the corresponding part of the third groove (fig. '7, Ph.G.3). There is thus included between the two a triangular convexity which represents the fourth pharyngeal arch. The third groove in its entire extent is connected with the underlying pouch. The latter is not joined to the bottom of the groove, but to its anterior wall, the lateral margin of the pouch reaching some distance beyond the deep part of the groove (figs. 7-8). ' On the side where it is no longer joined to the fourth pouch the blind, inner end of the fundus is turned obliquely backwards and comes into close relation with the inferior ganglion of the vagus. This part we shall hereafter designate the vesicula praecervicalis. In my estimation, this is a more appropriate name for it than the term vesicula thymica, applied by Kastschenko.
In a 12 mm. pig, in consequence of the passive behavior of the sinus and the continued outgrowth of the surrounding parts, the sinus is still deeper and its margins are so near each other that, with the exception of its most external part, the entire sinus may be considered as included in the fundus (fig. 13). The external opening of the latter is now relatively small. On its dorsal side the adjacent borders of the third pharyngeal arch and anterior cervical region have fused, leaving only a faint groove to mark the earlier extension of the sinus in that direction. On its ventral side the part of the head underlying the sinus has grown out and has united with the ventral extremity of the third arch, at the same time obliterating the groove earlier connecting the sinus with the pericardio—cervical fissure.
The outer limit of the fundus is approximately formed by the middle of the third pharyngeal arch. From this point it extends inwards and slightly backwards as a deep pocket, the blind inner extremity of which —-the vesicula praecervicalis—terminates close to the inferior ganglion of the vagus. This part is formed by the third and fourth pharyngeal grooves and the intermediate fourth arch. In consequence of the diminished depth of the fourth groove, the fourth arch does not form as prominent a convexity as in the preceding stage.
In the 14 mm. pig (figs. 14-16 and 18) all that remains of the sinus prwcervicalis has become, by virture of its passive deepening and constriction, included in the fundus praecervicalis (F.Pc.), which now forms a deep, blind pocket opening to the exterior by a much reduced opening placed immediately under the posterior rim of the hyoid arch. The outer half of the fundus forms a relatively narrow duct—the ductus praecervicalis of Kastschenko—leading to the external opening (D.Pc.). The inner half is relatively wider and at its mesial end is continued into the vesicula (V.Pc.). In this part the earlier prominence of the fourth arch has flattened out and thus the third and fourth grooves cease to be longer distinguishable. In this way the inner end of the fundus assumes the form of a bulb.
In the next stage, i.e.,_ a 17 mm. pig, the narrow ductus forms a solid cord, which has just severed its connection with the external ectoderm (figs. 20-21). The inner portion of the fundus now forms a relatively broad, flattened band, which is slightly concave on its posterior side (fig. 21, F.Pc.). By its anterior wall it is closely connected with the carotid gland and the epithelial plate now forming the dorsal end of the thymus, but representing originally the peripheral body of the third pouch (D.Pl.). With the exception of its vesicula, the fundus is without a distinct lumen. The vesicula originates at its ventro-internal angle, then bends backwards and terminates, as before, in the anterior part of the ganglion of the vagus.
In the 20 mm. pig the ductus has shrunken to a mere remnant (fig. 28, f. pc.). The remainder of the fundus praecervicalis, excepting the vesicula (v. pc.), forms a flattened band, which is closely wedged in between the lateral surface of the carotid gland (c. gl.) and the hypoglossal nerve (xii), over which its free end curves after the manner of a hook. At the lower posterior side of the carotid gland it expands to form the vesicula, which retains the same relation to the ganglion of the vagus as hitherto. The dorsal extremity of the thymus is wedged in between this part of the fundus and the carotid gland (fig. 45).
It will be noticed that the fundus now has an ascending course. Beginning at its vesicular extremity, it extends diagonally upwards and outwards over the lateral surface of the carotid gland to the upper side of the hypoglossal nerve, over which it curves like a hook. This condition, as I shall attempt to show presently, is to be correlated with the changes in position of the nerve mentioned.
The condition of the fundus praecervicalis is essentially similar in a. eat of 10.’? mm. The left ductus, however, is considerably longer than that on the opposite side.
In a cat of 15 mm. the ductus has disappeared. The rest of the fundus (fig. 63, F.Pc.) is coiled over the top and back of the carotid gland. It shows a distinct vesicle, located on the posterior surface of the gland.
The pig of 24 mm. shows the fundus as a much coiled, mostly solid, band. A slight lumen persists in the vesicula praecervicalis, which is now located on the postero-inferior surface of the carotid gland, some distance in front of the ganglion of the vagus. From this part the fundus curves upwards over the outer surface of the gland as an exceedingly thin, flattened ribbon, which at its dorsal extremity expands slightly into the hook—shaped process, which, as before, is curved outwards over the hypoglossal nerve (fig. 31, F.Pc. and F.Pc.V.).
In a pig of 25 mm. the condition of the fundus is very similar to that just described. The part of it adjoining the vesicula is somewhat broader and is sub-divided into a number of small lobules.
In the 32 mm. pig (figs. 34-35) the fundus shows a remarkable increase in size and now forms a prominent, irregularly lobed mass appendaged to the carotid gland (F. Pc.). That portion (F. Pc. V.) adjoining the vesicula has subdivided into several lobules, from which the original vesicula itself is not clearly distinguishable. From this part the thin band curves dorsally around the outer side of the carotid gland, and at its upper extremity is continued into the hook-shaped process, which has undergone a remarkable proliferation into a relatively immense, much convoluted mass (F. Pc.). The hypoglossal nerve (XII) partly divides it on the left side (fig. 34) into two unequal lobes———a large outer and a smaller internal. On the right side (fig. 35) the division is complete, the outer being separated from the inner by a thin plate of connective tissue. The outer, free portion represents the so-called thymus superficialis of Kastchenko. (fig. 35; Th.S.).
This division of fundus is evidently—as indeed Kastchenko first pointed out-——a result of the pressure produced by the hypoglossal nerve. On the left side (fig. 34), where the two divisions of the praecervical mass are united, this nerve lies in the constriction between them immediately under the connecting cord. Where, as on the right side (fig. 35), the division is complete, the nerve lies entirely above the praecervical mass. These relations naturally suggest that the displacement of the nerve itself has been the active cause in producing the present condition of the structure. This view is further supported by the relations between the two structures as observed in earlier stages. In pigs of 10, 12 and 14 mm, the nerve, after descending behind the sinus praecervicalis, curves forward some distance below its ventral margin (fig. 40). In the 1’? mm. pig it lies immediately under the fundus close to where the latter meets the carotid gland (figs. 45-46). It has thus assumed, relative to the fundus, a more dorsal position——a change associated with the ‘ventral displacement of the thymus owing to the elongation of the neck. Later, as these alterations in position continue, the nerve produces an upward pressure on the fundus, and thus causes it to assume an ascending course with its peripheral free extremity hanging loosely, like a hook, over the nerve. This is the condition observed in a 25 mm. pig (fig. 31). This portion of the fundus then undergoes a rapid proliferation, perhaps an indirect result of the mechanical irritation produced by the nerve, and thus attains the form characteristic of the present stage, when the praecervical mass is being finally constricted into two separate parts.
As this is the latest stage in the series of pig embryos which I have examined, I can state nothing as to the future history or fate of the praecervical body in this animal. It seems probable from its large size that it would be present at birth. Kastschenko observed it in an 80 mm. pig. Prenant claims that it is present at birth in the sheep.
In cats of 23.1 mm. and 31 mm. I was unable to find any certain traces of a fundus praecervioalis. This fact indicates that in this animal the later behavior of the organ must be less complicated than in the pig. It is probable that it rapidly disappears} In two late stages of the rabbit I could find only extremely uncertain traces of the fundus. In a twenty days’ foetus a slight process is present at the dorsal apex of the left carotid gland. In a twenty-'—one days’ example this has apparently disappeared, but some distance above the gland and entirely disconnected from it is a minute lymphoid body. This may possibly represent the transformed process seen in the twenty days’ individual. However, this point cannot be settled until additional material is examined.
Review and Comparisons
The third pharyngeal pouch when typically developed closely resembles the second. Like the latter, it has a prominent ventral diverticulum.
The pouch becomes transformed into the thymus. The greater part of the latter, i. (-1., its thoracic portion and cervical cord, are formed by the downgrowth of the ventral diverticulum.
The carotid gland is a derivative of the dorsal portion of the pouch. It arises as a series of follicular outgrowths from the anterior wall of the latter.
The pouch does not separate entirely from the ectoderm, but remains attached to that of the sinus praecervicalis. Separation from the superficial ectoderm takes place by the deepening and subsequent constriction of the sinus, which accompanies the pouch in its passive withdrawal from the surface of the body.
The connection of the pouch with the pharynx is at first formed by a wide opening. Later this is reduced to a solid cord, which subsequently constricts, thus leaving the pouch as an entirely independent body.
‘Verdun asserts that it had entirely disappeared in an embryo of 16 mm. The dorsal body of the pouch after the separation of the attached fundus praecervicalis from the skin forms the relatively inconspicuous dorsal extremity of the thymus. It loses all trace of a lumen and thus forms a solid epithelial plate wedged in between the carotid gland and fundus praecervicalis.
The fully formed thymus is diiferentiated into three parts—a ventral, thoracic thymus, an intermediate cervical cord and a dorsal plate to which the carotid gland is attached. In the cat the connection of the thymus with the carotid gland is interrupted in the later stages of development. In the rabbit the two structures become disconnected by the twenty-first day of development.
The carotid gland is typically an ovoidal body located in the neck close to the outer side of the lateral wing of the thyroid. Structurally it is a reticulum of solid follicles, interpenetrated by a system of capillaries derived from the carotid artery.
The sinus praecervicalis, as a result of its passive deepening by the outgrowth of surrounding parts, is transformed into a deep recess, the fundus praecervicalis. The latter is finally cut off from the ectoderm, and, by retaining its connection with the dorsal plate of the thymus, comes to lie at a considerable distance below the surface. Its inner extremity forms for some time a vesicle, which enters into close relation with the inferior ganglion of the vagus.
In the cat the fundus apparently is early atrophied. In the pig, however, it undergoes a strong proliferation, giving rise to a prominent, irregularly convoluted mass closely associated with the carotid gland. The peripheral lobe is separated from the remainder by the constriction effected by the hypoglossal nerve. This portion represents the so-called thymus superficialis of Kastschenko. ‘ In late stages of the rabbit all remains of the fundus przecervicalis have largely, if not entirely, disappeared. Only very doubtful traces of it remain. .
The formation of the thymus as described in this paper is in harmony with all the more recent observations. These prove that the organ is of purely endodermal origin and that all but an insignificant portion arises from the ventral diverticulum of the third pouch.
With regard to the final lymphatic transformation of the thymus, I can say little, owing to the fact that I did not have at my disposal a sufiicient number of older stages to enable me to form any decided opinion as to the process by which the change took place. In the rabbit in which I examined a large series of relatively late foetal stages the follicles as late as the twenty—first day maintained the same histological character, that is, they were composed of small—celled epithelial tissue. The latter, however, bears a strong resemblance to ordinary lymphoid tissue, and its persistence at this late period in this animal suggests that the fundamental tissue of the definitive thymus may be really epithelial and not lymphoid tissue. Such a view has recently been supported by Stohr. On page 9 of his article “Ueber die Thymus” (Sitiungs—Berichte der physikalisch-medicinischen Gesellschaft zu Wiirzburg) he writes, “Die Thymus ist ein epitheliales Orgcm mm Anfang bis zu Ende, so gut wie etwa. cine ;S’peicheldriise.” My own observations do not cover sufficiently late stages to enable me to give any strong support to this view.
Regarding the origin and structure of the carotid gland there has been considerable diversity of opinion. Steida first described the organ and postulated its origin from the endoderm. This view was later supported by fischelis. Kastschenko, on the other hand, makes no distinction between the carotid gland and the associated dorsal extremity of the thymus, both of which are included in his nodulus thymicus. The latter he regards as simply the much swollen dorsal end of the thymus. He apparently overlooks the vesicular structure of the gland—an oversight which is not surprising when one bears in mind that this structure is only shown in sections suitably double-stained.
Steida, while correct in his derivation of the gland from the endoderm, errs when he states that it later separates from the thymus anlage and comes into contact with the carotid artery. Kastschenko maintains that the nodulus thymicus, with which he considers the carotid gland of Steida to correspond, never separates from the thymus. He shows that the body which Steida took for the gland in later stages is of an entirely different character. It forms ein verliingerter ellipsoider Ifnofen, which surrounds the internal carotid at the bifurcation of the common artery. This he maintains is merely a local thickening of the adventitia of the artery.
My observations show that the contention of Kastschenko is correct. The carotid gland does not separate from the thymus, at least not in any stages examined by the two investigators mentioned. I find the same peculiar thickening of the adventitia of the internal carotid artery as described by Kastschenko in both the pig and cat. It is particularly large and prominent in pigs of 17-24 mm. and cats of 23-31 mm. In both it is located at a considerably higher level than the true carotid gland.
Piersol in his study of the rabbit does not, like Kastschenko, distinguish between the carotid gland and the dorsal extremity of the thymus. It, however, is probably present, as it is clearly distinguishable in the later stages of the same animal. Its presence in the earlier stages has been shown by Verdun.
According to Prenant, the merit of having determined that the carotid gland is a proliferation from the epithelium of the third pouch belongs to de Meuron. Prenant describes and figures correctly the histological structure of the organ. His work is based upon the sheep, but his results are in all respects in harmony with what I have observed in the pig.
In Verdun’s work, “Dérivés branchiaux chez les vertébrés supérieurs,” the term “la glandule bmnchiale II ” is applied to this organ. The term “carotid gland” he applies to the conjunctival proliferation surrounding the carotid artery at its bifurcation. There has been much confusion in the use of this term. As already mentioned, Steida applied it to both structures, though in the first part of his description, 1'. e., of the earlier stages, he applies it to the endodermal derivative. I have, therefore, retained it for the latter. The conjunctival swelling itself is no gland and consequently does not deserve to be called one.
The exact share taken by the ectoderm in the formation of the thymus has been a puzzling problem. His early advanced the view that the entire thymus was an ectodermal structure, but soon abandoned it. fischelis would apparently consider it as half endodermal, half ectodermal. Kastschenko derives the bulk of the organ from the endoderm, but considers that its dorso-peripheral portion, which he designates by the term, thymus superficialis, is of ectodermal origin.
Since the last investigator the part taken by the ectoderm in the formation of the thymus has been largely ignored. The prevailing opinion regards the thymus as of purely endodermal origin. My own observations, however, corroborate the statements of Kastschenko so far as his facts are concerned, but, unlike the latter, I do not consider the so-called thymus superficialis as of sufficient constancy or importance to warrant the application to it of this rather pretentious term or to be considered as an actual constituent of the thymus.
Kastschenko describes correctly the origin of his thymus superficialis by the constriction of the fundus prmcervicalisz His observations were ‘This is “Le fond du troisiéme sillon eotodermique” of Verdun made upon pigs, and the large size which the outer free lobe of the fundus attains in these creatures probably led him to consider it as an important part of the dorsal extremity of the thymus. Its similarity in histological structure to the thoracic thymus was another fact upon which he based his view as to its importance.
To me these facts do not warrant the ascription of the ectodermal structure under consideration to the thymus. first, as regards the prominence of the outer lobe of the fundus, it is evident from my observations on the cat that this condition is not general among mammals. Even in the pig it does not seem to be absolutely constant, since Kastschenko himself speaks of an animal of 80 mm., in which he could find no trace of a thymus superficialis. In the sheep, according to Prenant’s account, it is evidently similar to that in the pig. It might be inferred from these facts that a prominent fundus praecervicalis is limited in late foetal life to the ungulatesf’ but is probably more or less early atrophied in other forms.
With regard to the histological structure of the so—called thymus superficialis, it will sufllce to state that its similarity in this regard to the thymus is no greater than that which any branching epithelial mass shows. In specimens which I examined the lobuli of the fundus praecervicalis bore as strong a resemblance to those of the salivary glands as to the same parts in the thymus. The resemblance is therefore unimportant.
I would therefore conclude that the fundus praecervicalis is to be looked upon as an associate of the thymus, but not as an integral part of it.
Prenant evidently considers the vesicula thymica of Kastschenko as including all the derivatives of the fundus praecervicalis. He regards it apparently as of endodermal origin and hence as a part of the thymus. He says, “La téte du thymus se developpe aux dépens de la 3d poche entodermique et d’un diverticule de cette poche; celui—ci, qui est sans doute identique a la vesieule thymique Kastschenko, s’enforce dans le ganglion du vague.” This statement is erroneous. The vesicula thymica of Kastschenko is not a diverticulum of the third pouch, but represents the inner blind recess of the fundus praecervicalis, which I prefer to call the vesicula praecervicalis.
Verdum correctly considers his “la fond du t1*0ist'e‘me sillon ectodermique” as corresponding to the “vesicule thymique” of Kastschenko.
‘Verdun, however, states that it disappears entirely in an 18 mm. calf. He, however, does not trace its transformations in the pig, merely stating that he had found it and its outer connective in a 19 mm. embryo.
D. The Fourth Pharyngeal and its Transformation into the Lateral Thyroid and Glandule Thyroidienne
In a 6.2 mm. cat we noticed the division of the fourth pouch into two segments by a lateral constriction (figs. 58-59). The more dorsal of these constitutes the body of the pouch; it projects strongly backwards and on its outer side gives off a slender process, which connects with the ectoderm of the inner extremity of the sinus praecervicalis (fig. 60). The remaining segment forms a ventral diverticulum (V.D.4), which at this period extends for a short distance downwards, forwards and inwards.
The 9.7 mm. cat shows nearly similar conditions. The ventral diverticulum (fig. 61, V.D.4) has lengthened slightly and begins to assume a more tubular aspect. The dorsal extremity forms a more distinct posterior process. The lateral process still persists, and, as before, connects with the ectoderm of the fundus praecervicalis.
In a 10 mm. pig (Student collection, Harvard) the fourth pharyngeal pouch of one side has entirely separated from the ectoderm, while on the opposite side the slender connecting process still persists (figs. 7-8). The dorsal process (Gl.T.) projects strongly backwards. The ventral diverticulum forms a relatively short, rounded protuberance. Its ventral extremity lies immediately under the aortic arch. It bears the same relation to the latter that the corresponding part of the preceding pouch does to the carotid arch. _ In a second 10 mm. pig (No. 401, Harvard series) the fourth pouch of each side has severed its connection with the ectoderm (figs. 4-6). The ventral diverticulum (V.D.4) forms a compressed, antero-ventrally projecting sac. The lateral process has disappeared. The dorsal process (Gl.T.) retains the same characteristics as hitherto.
A 12 mm. pig is apparently exceptional in that the fourth pouch of one side retains its connection with the fundus praecervicalis (fig. 10). On the opposite it has essentially the same features as in the preceding stage.
In two pigs of 13 and 14: mm., respectively, the fourth pouch has been transferred to a considerably lower level by the downward flexure of the sinus piriformis. The ventral diverticulum (figs. 1-1 and 20, La.T.) shows increased length and forms a tubular process, whose axis is more nearly vertical than in the preceding stages. Its distal extremity is somewhat bulb—like in form (fig. 39, la. t.), but its basal portion has become constricted to a narrow duct, opening into the sinus piriformis (fig. 40). The dorsal portion is much reduced; it is represented only by the dorsal process, which forms a spheroidal body attached by a narrow stalk to the duct (figs. 14, 16, Gl.T.). It contains only a slight lumen. Otherwise it forms a solid mass, whose walls are apparently thrown into series of tight folds. These produce an appearance sirnu~ lating that of the carotid gland—an organ produced from the ho1nologous part of the preceding pouch.
At this period the distal extremity of the ventral diverticulum is without any connection with the median thyroid. The latter at this time is rather small and lies in the mesial plane above and between the ventral extremities of the thymus downgrowths.
In both the 17 and 18 mm. pigs the ventral diverticulum has still further elongated, largely as a result of the lengthening of its duct, which now appears as a slender, solid cord (figs. 19, 21). A lumen is present only in the terminal vesicular part. The dorsal process (Gl.T.) presents the same appearance as before; its lumen, however, has disappeared and its structure more clearly resembles that of the carotid gland (fig. 45, c.gl.). We shall henceforth designate it the “glandule thyroidienne”—a term applied to it by Prenant.
The median thyroid now occupies a position considerably posterior to that occupied by it fin the preceding stage. It has grown considerably and has assumed a horseshoe shape, owing to the outgrowth of its lateral wings, which at their outer extremities almost touch the vesicles of the ventral diverticulum.
In a eat of 10.7 mm. the ventral diverticulum has separated from the pharynx and forms a pear-shaped vesicle — the lateral thyroid vesicle — lying free in the mesenchyme by the side of the trachea. Its lumen is reduced to a mere slit—a result of the internal proliferation of its walls. It bears the same relation to the thyroid as in the pig of the stage last described.
I was unable to distinguish in this individual any clear evidence of the presence of a “glandule thyroidienne.” In a 20 mm. pig the lateral thyroid vesicle shows only fragments of a lumen. Otherwise it is a solid structure and is composed of several layers of small cells of epithelial nature, but closely resembling lymphoid tissue. They have essentially the same character as the elements forming the lobules of the thymus. The median thyroid has moved backwards to a position immediately in front of the trachea (figs. 23, 25). Its lateral wings have grown back over the outer side of the lateral thyroids, the latter thus being partly embedded on their inner sides.
An exceedingly fine duct still connects the lateral thyroid with the pharynx.
The dorsal process (Gl.'I‘.) forms a small spheroid attached to the remains of the duct. It is clearly composed like the carotid gland of a close network of solid follicles, the interstices of which are traversed by a system of capillaries (fig. 54).
A eat of 15 mm. (fig. 65) shows each lateral thyroid embedded in a depression on the inner side of the corresponding lateral wing of the median thyroid. Its minute structure is easily distinguishable from that of the latter organ by its solid lymphoid character.
A “glandule thyroidienne” does not appear to be present.
In a pig of 24 mm. the lateral thyroid is also largely surrounded by and embedded in the lateral wing of the thyroid. Only its more dorsal portion projects above the latter (fig. 29, La.T.).
The “glandule thyroidienne” is present, at least on the right side. I found no clear trace of it on the opposite side.
In a 25 mm. pig conditions are similar to those just described, but both glands are present. That on the left, however, is much reduced, being only about a fifth the size of thatféon the right. I may add that the same difference in size between the glandules of the two sides was noticed in an 18 mm. pig.
In a 23.1 mm. cat the lateral thyroid bears the same relation to the lateral Wings of the thyroid as in the pig last described. I could, however, find no readily distinguishable traces of the “glandule thyroidienne.” In a 31 mm. cat such lateral thyroid is deeply embedded in a concavity on the inner surface of the corresponding wing of the thyroid. No part of it projects beyond the periphery of the latter at any point.
Two minute bodies (fig. 68) were observed, one on each side, close to the lateral border of the oesophagus. They apparently correspond in position with the glandules, but, as I could not readily determine their minute structure in the specimen examined, I think it improbable that they represent these. They are probably independent structures, lym ' phatic in origin.
In a 21-day rabbit the lateral thyroid forms a solid, ovoidal mass deeply embedded in a flask-shaped depression on the inner side of the lateral wing of the thyroid. As in earlier stages, it is readily/distim guishable from the latter by its difierent histological structure.
Review and Comparisons
The fourth pharyngeal pouch resembles the third in producing two distinct structures, the lateral thyroid and the “glandule thyroidienne.” The lateral thyroid is formed by the elongation of the ventral diverticulum. This at first is perfectly continuous with the side of the pharynx, but the connecting part early becomes constricted, assumes the form of a solid cord and subsequently separates from the pharynx. The remaining ventral portion forms a piriforrn vesicle, which soon becomes solid and later by backward growth of the median thyroid becomes embedded in the lateral wings of the latter.
The dorsal body of the pouch early loses its connection with the ectoderm and undergoes partial atrophy. A considerable portion, how-‘ ever, is transformed into the "glcmdule thyr0idienne” of Prenant. In the cat I have not been able to trace the history of this structure, but Verdun, who examined a large series in this type, asserts that along with the lateral thyroid it becomes embedded in the lateral lobe of the median thyroid. In the pig it persists for a considerable period, but never forms any connection with the median thyroid.
The results of my study of the fourth pouch and its derivatives are, as a whole, corroborative of previous investigations. The main facts in its-development had been ably presented by Kastschenko, although he overlooked the “glandule thyroidienne” which was described by Prenant. The latter gives a full account of the microscopic structure of the organ and “distinctly asserts its homology with the carotid gland of the preceding pouch.
Verdun derives from the fourth pouch another structure which he terms “thymus IV” on account of its supposed homology with the thymus of the preceding pouch. This, he states, arises as a “diverticule externe et ventral.” In all the examples examined by me I have noticed nothing to suggest this structure. Verdun describes it most fully in the case of the eat, but states that it is only exceptionally, and then only sli.ghtly, developed in the rabbit. In the camel and ox he finds it doubtfully represented by certain lobules associated with the "glcmdule thyroidt'enne.” In the other forms—man, mole, opussum, dog, pig, sheep—he gives no very convincing evidence of its presence. In view of these facts, 1). e., its exceptional presence in one form and its doubtful presence in certain others, it seems to be that this thymus IV cannot in the mammals have the significance Verdun attributes to it. At least, to my mind, the actual facts do not bear it out, whatever its theoretical support may be.
The lateral thyroid Verdun regards as an autonomous structure, representing the post-branchial bodies of lower vertebrates. To me it appears that this is done for the most part on purely theoretical grounds. So far as actual facts are concerned, the lateral thyroid in the mammals closely follows the thymus in its behavior, and I can see no clear and convincing reason for regarding it as other than the ventral diverticulum of the fourth pouch and therefore homodynamous with the thymus of the preceding pouch. I know of no decisive evidence against the view advocated by Verdun, but the facts adduced by him are not sufficient to establish his point. I therefore follow the usage of most writers in regarding the lateral thyroid as a part of the fourth pouch.
References
1. Bell ET. The development of the thymus. (1905) Amer. J Anat. 5: 29-62.
2. BORN. Ueber die Derivate der embryonalen Schlundbogen und Sch1undspalten bei Saugethieren. Archiv f. mikr. Anat., Bd. 22.
3. FISCHELIS, P. Beitrage zur Kenntniss der Entwicklungsgeschichte der G1. Thyroldea und GI. Thymus. Archiv f. mikr. Anat., Bd. 25, 1885.
4. FRORIEP. Ueber Anlagen von Sinnesorganen am Facialis, G1ossopharyn geus und Vagus u. s. w., Archiv f. Anat. u. Physio1., Anat. I-Iefte, 1885.
5. Flint JM. On the Framework of the Glandula parathyroidea. (1904) Amer. J Anat. 4. FLINT, J. M. On the Framework of the Glandula parathyroidea. Amer. Jour. of Anat, Vol. IV, 1904.
6. GRADENIGO, G. Die embryonale Anlage der Gehtirkntichelchen und des tubo-tympanalen Raumes. Centralbl. f. d. med. W1ss., 1886.
7. HAMMAR. Studien fiber die Entwicklung des Vorderdarms und einiger angrenzenden Organe:—Die Entwicklung des Mittelohres und des ‘ziusseren Gehiirganges. Archiv f. mikr. Anat., Bd. 59, 1902. Das Schicksal der zweiten Schlundspalte. Zur vergleichenden Embryologie und Morphologie der Tonsille. Ibid., Bd. 61, 1903.
8. Hrs, WILHELM. Ueber die Sinus praecervicalis und fiber die Thymusanlage. Archiv f. Anat. u. Phys1o1., Anat. Abth., 1886.
9. KASTSCHENKO, N. Das Schicksal der embryonalen Schlundspalten bel Séiugethieren. Archiv f. mikr. Anat., Bd. 30, 1887. 242 10.
21 MALL, F. P. The Branchial Clefts of the Dog. Studies from the Biolog ical Laboratory of Johns Hopkins University, Baltimore. Vol. IV, 1888.
22. Minot CS. A Laboratory Text-Book Of Embryology. (1903) Philadelphia:P. Blakiston's Son & Co.
23. MOLDENHAUER, W. Die Entwicklung des mittleren und des éiusseren Ohres. Morph. J ahrbuch, Bd. 3, 1877.
24. PIERSON, G. Ueber die Entwicklung der embryonalen Schlundspalten und ihre Derivate bei Siiugethieren. Zeitschr. f. wiss. Zoologie. Bd. 47, 1888,
25. PRENANT. Développement organique et histologique du thymus, de la glande thyroide et de la glande carotidienne. La Cellule, T. 10, 1894. Sriirm, P. Ueber die Thymus. Gese1l schaft zu Wiirzburg, 1905. Sitzungsberichte der phys. med.
26. Sudler MT. The development of the nose and of the pharynx and its derivatives in man. (1902) Amer. J Anat. 1:391–416.
27. URBANTSCHIRTSCH. Ueber die erste Anlage des Mittelohres und des Trommelfelles. Mittheil. aus Clem embryologischen Institut der k. k. Univ. in “Men, 1877.
28. VERDUN, M, P. Sur les derives de la quatrieme poche branehiale chez le chat. Compt. rend. Soc. Biol., 1897.
29. VERDUN ET TOURNEIZX. Sur les premiers developpements de la thyroide du thymus et des glandules parathyroidiennes chez 1’homme. Jour. de l’Anat. et de la Physiol., 1897.
30. VERDUN ET SOULE. Sur les premiers developpements de la glande thyroide, du thymus et des glandules satellites de la thyroide chez le lapin et ehez la taupe. Journ. de. 1’Anat. et de la Physiol., 1897.
31. VERDUN. Dérives branchiaux chez les vertébres supérieurs. 1898. Toulouse,
Explanation of Figures
Fig. 1. Lateral view of the pharynx in a 6.5 mm. pig, M’, for parts consult Fig. 58. X 60, reduced 143.
Fig. 2.Dorsal View of the pharynx in the same embryo. D. A. 1, dorsal apex of first pharyngeal pouch; HYP., hypophysis; P11. P. 1-4, pharyngeal pouches; S. P., Seessel’s pocket; S.’1.‘. P., sulcus tympanicus posterior; S. T. '1‘. sulcus tubo-tympanicus; S. T. '1‘y., suleus tensoris tympani; V. D. 1-3, ventral (liverticula of the pharyngeal pouches; RF. 4, posterior process of the fourth pouch. X 50, reduced 34;.
Fig. 3. Ventral view of pharynx and larger blood-vessels in the same embryo. I-IYP., hypophysis; Ph. P. 1-4, pharyngeal pouches; V. D. 1-4, ventral diverticula; M., mouth; Tr., trachea; Ao. 2-5, aortic arches; D. Ao., dorsal aorta; Pul., pulmonary artery; T. Ao., truncus arteriosus; Tyr., thyroid; Ch. Ty., chorda tympani. X 50, reduced %.
Fig. 4. Lateral view of the pharynx in a 10 mm. pig, No. 401, Harvard medical collection. D. A. 1-3, dorsal apices of first three pouches; G]. T., dorsal process of the fourth pouch, the primordium of the glomdule thyroidienne; S. M. F., submeckelian fold; S. T. T., sulcus tubo-tympanicus; S. T. Ty., sulcus tensoris tympani; Ton. F., dorso-lateral region of the second pouch, later transformed into the tonsillar recess; V. D. 1-4, ventral diverticula; V. F., vestibular fold. X 60, reduced 3/5.
Fig. 5. Dorsal view of the pharyngeal region in the same specimen. Symbols as before. X 60, reduced 3/5.
Fig. 6. Ventral view of the same region in the same embryo. Symbols as before. X 60, reduced 3/5.
Fig. 7. Ventral view of the region of the third and fourth pouches in a 10 mm. pig, students’ collection, Harvard medical collection. F. ]?c., fundus praecervicalis; Ph. G. 3, third pharyngeal groove; 10, vagus; other symbols as in Fig. 8. X 80, reduced 1/2. figure inverted.
Fig. 8. Dorsal view of the same region shown in Fig. 7. Ao., aorta; Car., carotid; 0. GL, carotid gland; D. A. 3, dorsal apex of third pouch; Gl. T., dorsal process of fourth pouch, later the glamlule thyroidicnne; Ph. G. 4, fourth pharyngeal groove, forming the inner edge of the fundus praecervicalis; S. Pc., sinus praecervicalis; V. D. 2-3, ventral diverticula of second and third pouches. X 80, reduced 1/2.
Fig. 9. Lateral view of the pharynx in a 12 mm. pig, No. 518, Harvard medical collection. 0. Gl., carotid gland; remaining symbols as in figs. 4-6. X 60, reduced 3/5.
Fig. 10. Dorsal view of the pharyngeal region in the same specimen. D. Pr., dorsal prominence of the tonsillar fold; F. Pc., fundus praecervicalis; V. Pc., vesicula praecervicalis; other symbols as in preceding figures. X 60, reduced 3/...
Fig. 11. Ventral View of same parts as in Fig. 10. Symbols as in preceding figures. X 60, reduced 3/5.
Fig. 12. Region of the second pharyngeal pouch in a 12 mm. pig, No. 518, Harvard medical collection, viewed from below. cv., concavity on the lower surface of tonsillar fold (ton. f.); 0., ridge connecting second and third pouches; v. (1. 1-2, ventral diverticula of first and second pouches. X 30.
Fig. 13. Region of sinus praecervicalis in a 12 mm. pig, students’ collection, Harvard medical collection, ventral view. G. Nod., ganglion nodosum. Remaining symbols as before. X 80, reduced 1A.
Fig. 14. Lateral view of the pharynx in a 14 mm. pig, No. 65, Harvard medical collection. A-L. F., alveolo-lingual ridge; 0. Gl., carotid gland; C. V., concavity on ventro-lateral wall of tonsillar fold; D. A. 1-2, dorsal apex of first and second pouches; F. Pc., fundus praecervicalis; Fl. P., filiform process of second pouch; Gl. T., glandule thyroidienne; La. T., vesicle of lateral thyroid; P. T. R., posterior tympanal margin; S. M. F., submeckelian fold; S. T. T., sulcus tubo-tympanicus; S. T. Ty., sulcus tensoristympani; Thy., thymus; Ton. F., tonsillar fold (= sinus tonsillaris) ;. Tyr., median thyroid; V. D. 2, ventral diverticuluin of second pouch; V. F., vestibular fold; y., interval between vestibular and submeckelian fold. X 60, reduced 3/5.
Fig. 15. Dorsal view of pharyngeal region in the same embryo, ectoderm shown only on one side. D. Pr., dorsal prominence of tonsillar fold; F. Pc., fundus praecervicalis; V. Pc., vesicula praecervicalis; other symbols as before. X 60, reduced 3/5.
Fig. 16. Ventral view of the parts shown in Fig. 15. X 60, reduced 3/5.
Fig. 17. The region of the second pharyngeal pouch, ventral view, in a 14 mm. pig, No. 65, Harvard collection. a—l. f., alveolo~lingual ridge; fl. p., filiform process of second pouch; s. pi., sinus piriformis; s t p., sulcus tympanicus posterior. Other symbols as in Fig. 12. X 30.
Fig. 18. The region of the sinus praecervicalis in a 14 mm. pig, same specimen, Ventral view. D. Pc., ductus praecervicalis; Fl. P., ventral end of filiform process of second pouch; F. Pc., fundus praecervicalis ; G. Nod.,' ganglion nodosum; Ph. P. 3, third pharyngeal pouch; V. Pc., vesicula praecervicalis. X 80, reduced %,.
Fig. 19. Lateral View of pharynx in a 17 mm. pig, No. 51, Harvard medical collection. A-L. F., alveolo-lingual fold; C. V., concavity on ventro—lateral wall of tonsillar fold; CV. 0., cervical cord of the thymus; D. A. 1-2, dorsal apex of first and second pouches; D. Pl., dorsal plate of thymus (the carotid gland is closely associated with this part, but in this specimen it was not sufficiently differentiated by the stain to enable its outline to be accurately traced); Fl. P., filiform appendix of second pouch; F. Pc., fundus praecervicalis; La. T., lateral thyroid vesicle, the glcmdule thyroidienne projects backwards from its dorsal end; S. M. F., submeckelian fold; S. Pi., sinus piriformis; S. T. T., sulcus tubo-tympanicus; Ton. F., tonsillai: fold; Thy., thymus; Tyr., thyroid; V. F., vestibular fold; y., space between vestibular and submeckelian folds. X 60, reduced 3/5.
Fig. 20. Dorsal view of same parts shown in Fig. 19. S. T. Ty., sulcus tensoris tympani; S. T. P., sulcus tynipanicus posterior; D. Pr., dorsal prominence of tonsillar ‘fold; 0. GI., carotid gland. X 60, reduced 3/5.
Fig. 21. Ventral view of same parts shown in figs. 18-19. E. Au., external auditory tube; Gl. T., glamlule tlzyroidienne. Other symbols as in Fig. 19. X 60, reduced 3/5.
Fig. 22. Region of the second pharyngeal pouch in a 17 mm. pig, No. 51, seen from below. Symbols as in figs. 12 and 17. X 30.
Fig. 23. Lateral View of pharynx in a 20 min. pig, No. 542, Harvard medical collection. D. A. 1, dorsal apex (recessus anterior) of tympanic pouch; Mn. F., manubrial fossa; S. Pi., sinus piriformis; z., indentation between tympanic pouch and tonsillar fold. X 60, reduced 3/5.
Fig. 24. Dorsal view of pharynx in same specimen. X 60, reduced 3/5.
Fig. 25. Ventral view in same specimen. X 60, reduced 3/5.
Fig. 26. A portion of the pharynx, including tyinpanic pouch and tonsillar fold in a 20 mm. pig, viewed from the posterior side. pm. f., post—manubrial ridge; ps. f., post—salpingea1 fold. Other symbols as before. X 30, reduced 1/3.
Fig. 27. The same structure as in Fig. 26, viewed from anterior side. Symbols as in preceding figure. X 30, reduced 1/3.
Fig. 28. Thymus and associated parts in the same animal, front view, 12, hypoglossal nerve; other symbols as before. X 30.
Fig. 29. Lateral view of pharynx in a 24 mm. pig, Harvard medical collection. X 60, reduced 3/5.
Fig. 30. Dorsal view of the same pharynx as in Fig. 29. X 60, reduced ‘/5.
Fig. 31. Left thymus of same animal with carotid gland and praecervical appendix. Cv. C., cervical cord; F. Pc., hook-shaped portion of fundus precervicalis; F. Pc. V., ventral segment of the fundus-this is joined to F. Pc. by a narrow connective extending over the outer surface of the carotid gland. but hidden from view in figure by the oblique position of the latter. X 230, reduced %.
Fig. 32. Lateral view of pharynx in a 32 mm. pig, Harvard medical collection. G. Ep., glosso-epiglottic fold; Mk. F., Meckelian fossa; Mn. F., manubrial fossa; other symbols as before. X 60, reduced 3/5.
Fig. 33. Dorsal view of same structure as in Fig. 32. The naso—pharynX is represented as cut across, thereby showing its interior. X 30, reduced 15.
Fig. 34. Left carotid gland and associated przecervical mass and hypoglossal nerve in 32 mm. pig, anterior view. For symbols, see Fig. 31. X 80, reduced 1/2.
Fig. 35. Right carotid gland and associated parts in the same animal, posterior view. The carotid gland is represented as sectioned in the plane of the paper. The praecervical mass has divided into two parts, internal, F. Pc., and external, Th. S. The latter is the thymus superficialis of Kastschenko. X 80, reduced V2.
Fig. 36. -Part of a sagittal section of the head of a 9 mm. pig, showing origin of carotid gland. 0. GL, carotid gland; Per., pericardial cavity. Other symbols as in preceding figures. X 50, reduced 1/3. '
Fig. 37. Part of a transverse section of a 13 mm. pig (M1) showing the final separation of the first pouch from the skin. Coch., cochlea; Ch. Ty., chorda tympani; D. A. 1, dorsal apex with the part by which the pouch is last connected with the skin; Impi-., impressio cochlearis; Ph. G. 1, the first pharyngeal groove, its undulating course causes it to be sectioned twice in the section; V. D. 1, ventral diverticulum of first pouch. X 50, reduced %.
Fig. 38. Part of a transverse section of the region of the third pouch in a 13 mm. pig (M1). (1. pl., dorsal lamina of the thymus. Other symbols as in preceding figures. X 50, reduced 1/2.
Fig. 39. Similar section slightly posterior to last. ph. g. 2, second pharyngeal groove. X 50, reduced 1/3.
Fig. 40. Section posterior to last, passing through fundus praecervicalis, f. pc.; or 3, ectodermal organ of third pouch; pul., pulmonary artery. X 50, reduced 1/2.
Fig. 41. Part of a transverse section through the head of an 18 mm. pig, M3. The section passes close to the oral extremity of the tympanic pouch. al. f., alveolo-lingual fold; G. Gas, gasserian ganglion; s. p. c., superior petrosal nerve; 53, inferior maxillary division of trigeminal. X 50, reduced 1/2.
Fig. 42. Part of a similar section, a few sections behind the preceding. X 50, reduced Va.
Fig. 43. Similar section, posterior to preceding. d. 1. s., dorso-lateral wall of tympanic pouch; G. Gn., geniculate ganglion; p. g. 1, flrst pharyngeal groove. X 50, reduced 1/3.
Fm. 44. Similar section in region of the dorsal apex of the tympanic pouch. G. Au., auditory ganglion; hy., hyoid; thr., thyroid cartilage; 7, main trunk of facial, showing its two divisions, the outer being the basal portion of the chorda tympani. X 50, reduced %.
Fig. 45. Section near hind margin of tympanic pouch. The latter lies in the upper right-hand corner. dc. t., duct of lateral thyroid; 1’. pc., fundus praecervicalis-the dorsal plate of the thymus lies between this and the carotid gland, c. gl. X 50, reduced 1/2.
Fig. 46. Section slightly posterior to preceding. gl. t., glandule thyroidienne; ps. f., post-salpingeal fold; v. pc., vesicula praecervicalis. X 50, reduced 1A.
Fig. 47. Part of a transverse section of the head of a 25 mm. pig, M‘. The section passes near the front end of the tympanic pouch. v. f., vestibular groove. X 50, reduced 1/2.
Fig. 48. Similar section near anterior edge of submeckelian fold in same animal. Mk. 1., Meckelian fossa; s-m. f., submeckelian fold; vls., ventrolateral wall of tympanic pouch. X 50, reduced 1/;
Fig. 49. Section through middle part of the submeckelian fold in same animal. e. au., external auditory meatus. X 50, reduced 15.
Fig. 50. Section slightly posterior to the point where tympanic pouch and pharynx are connected, pig, 25 mm. The post-salpingeal fold projects from the sides of the pharynx immediately internal to the tympanic pouch. mn., manubrium of the malleus. X 50, reduced 1A.
Fig. 51. Section through the posterior segment of the tympanic pouch. p. m. f., post-manubrial ridge; s. t. ty., sulcus tensoris tympani; t. s., tensor muscle; v. m. r., ventro-mesial border. X 50, reduced 1/3.
Fig. 52. Part of a transverse section of the head of a 36 mm. pig, showing relation of the external auditory Ineatus. E. Au., to the tympanic pouch. prom, promontory of the tympanic pouch; the projection below the manubrium is the post-manubrial ridge. X 50, reduced %.
Fig. 53. Transverse section through carotid gland and adjacent part of third pouch. fig, 13 mm., M‘. X 300 circa, reduced 1A»,
Fig. 54. Cross section of a glandule thyroidiemie in a 20 mm. pig, Harvard medical collection. X 300 circa, reduced 1/2.
Fig. 55. Ventral view of the pharyngeal region of cat of 4.6 mm., No. 398, Harvard medical collection, showing associated blood-vessels. st., stomato deuni; V. V., vitelline veins; X., opening of enteron into yolk sac. X 50, reduced 1/3.
Fig. 56. Lateral view of the same region. X 50, reduced %,.
Fig. 57. Lateral view of the pharyngeal region in a cat, No. 413, Harvard medical collection, showing pharynx and associated aortic arches. M. Gr., median oral groove; Ph. P. 1-3, first to third pharyngeal pouches. X 80, reduced
Fig. 58. Lateral View of the pharynx in a 6.2 mm. cat, No. 380, Harvard medical collection. Ton. F.. antero-lateral border of the second pouch, in later stages the tonsillar sinus. X 60, reduced %.
Fig. 59. Dorsa1 View of the pharynx in the same cat, showing also the related ectodermal parts. S. Pc., sinus praecervicalis; F. Pc., fundus praecervicalis. X 60, reduced 15.
‘Fig. 60. Ventral View of the pharyngeal region in the same cat. X 60, reduced 1/2.
Fig. 61. Ventral View of the pharyngeal region in a 9.7 mm. cat, No. 446, Harvard medical collection. On the right the plane of section is highei than on the left side. SM. F., submeckelian fold, barely visible at this stage; on the right the interior of the sinus praecervicalis is shown; Tub., tuberculum impar; V. F., vestibular fold. X 60, reduced 1/3.
Fig. 62. Region of the sinus praecervicalis in the same cat. F. Pc., fundus praecervicalis; Ph. P. 4, fourth pharyngeal pouch, only its outer tip showing on the left side; V’. D. 2-3, ventral diverticula of second and third pouches, their inferior parts cut off; V. Pc., vesicula prsecervicalis. X 80, reduced 1/9.
Fig. 63. Lateral view of the pharynx in a 15 mm. cat, No. 436, Harvard medical collection. Mn. f., manubrial fossa; P. S. F., postsalpingeal ridge; S. Pi., sinus piriformis; V. M. R., ventro-mesial border. X 60, reduced 3/5.
Fig. 64. Dorso-posterior view of pharynx in the same animal. The figure shows the initial separation of the tympanic pouch and tonsillar fold. p f., postsalpingeal ridge; s. t. p., sulcus tympanicus posterior; ton. f., tonsillar fold; v. m. r., ventro-mesial border; z., incision between tympanic pouch and tonsillar fold. X 30, reduced lg.
Fig. 65. Median thyroid, lateral thyroid, la. t., carotid gland, c. gl., and praecervical body, 15. pc., in a 15 mm. cat. X 30.
Fig. 66. Lateral view of pharynx in a 23 mm. cat, No. 466, Harvard medical collection. I. '.[‘n., incissura tensoris; Mn. F., manubrlal fossa; Pl. B., postero-lateral border of the tympanic pouch; P. M. F., post-manubrial ridge: S. M. F., submeckelian fold; Ton. F., tonsillar fold. X 60, reduced 3/.,.
Fig. 67. Dorsal view of the pharynx in a 23 mm. cat. P. B., recessus posterior; S. B., recessus superior. X 60, reduced 3/5.
Fig. 68. Lateral view of pharynx and mouth in a 32 mm. cat, Harvard medical collection; a doubtful body of lymphoid nature. X 60, reduced 3/5.
Fig. 69. Dorsal view of pharynx in the same cat. X 60, reduced 3/.,.
Fig. 70. A part of the pharynx, including tympanlc pouch and tonsillar fold in a 14 days rabbit, viewed from the posterior side. Symbols as in Fig. 26. X 30.
Fig. 71. Part of the pharynx in the region of the tympanic pouch and tonsillar fold, rabbit, 16% days. X 30.
Fig. 72. Part of the pharynx in an 18 days rabbit, Harvard medical collection. i. tn., incissura teusoris; p. pl., palatlne constriction; D. r., recessus posterior; ton. f., tonsillar fold. X 30.
Fig. 73. The posterior portion of the pharynx of a rabbit of 21 days, Harvard medical collection, viewed from the front. The nasopharynx is represented as sectioned close to the base of the Eustachian tubes and the oral pharynx immediately in front of the tonsils. E. Au., external auditory meatus; Epi., epiglottls; G. Ep., glossi-eplglottic fold; I. Tn., incissura tensoris; Mk. I4‘., Meckelian fossa; Mn. F., manubrial fossa; S. Pi., sinus piriformis; Ton., F., tonsillar fold; V. '1‘on., inferior tonsillar recess. X 30, reduced 14.
Abbreviations
|
|
Cite this page: Hill, M.A. (2024, April 27) Embryology Paper - The pharyngeal pouches and their derivatives in the mammalia. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Paper_-_The_pharyngeal_pouches_and_their_derivatives_in_the_mammalia
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G