Paper - The origin and developmental mechanics of the avian sternum
| Embryology - 28 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Feli HB. The origin and developmental mechanics of the avian sternum. (1939) Phil. Trans. Roy. Soc. Lon. 229 pp.407-463.
| Online Editor |
|---|
| This historic 1939 paper by Feli describes early avian embryo sternum development. Philosophical Transactions of the Royal Society Of London Series B. Biological Sciences No. 563 Vol 229 pp. 407-463 20 March 1939
|
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
The Origin and Developmental Mechanics of the Avian Sternum
By
Honor B. Feli
Messel Research Fellow, Strangeways Research Laboratory, Cambridge
(Communicated by E. S. Goodrich, F.R.S.—Received 21 April 1938) Plates 33-41 Published 20 March 1939
General Introduction
Comparatively little work has been done on the development of the avian sternum. As previous publications on this subject are primarily concerned with phylogenetic problems, it occurred to the present writer to study the embryology of the avian sternum from the standpoint of developmental mechanics. This bone shows such remarkable adaptations to functional requirements in different species that the factors concerned in its development were likely to be of particular interest.
Moreover, from the technical point of view, the embryonic bird’s sternum seemed very promising material. In the first place it would be very suitable for tissue-culture experiments, as its thin, flat shape would facilitate nutrition through the external surface; in the second place it has a very definite and characteristic form, so that abnormalities produced experimentally could be readily detected; and in the third place it does not ossify during embryonic life, so that structural complications resulting from bone deposition would be eliminated.
It was decided first to study in detail the normal anatomical and _ histological development of the sternum and thus obtain as much information as possible about its normal morphogenesis. Points which could not be conclusively established by morphological evidence alone, or problems which could not be approached at all by ordinary morphological methods, would then be investigated experimentally by the tissue-culture technique.
The normal development of the bird’s sternum is briefly as follows. It appears as two mesodermal plates on either side of the antero-lateral body wall. ‘These plates, the origin of which is a matter of dispute, chondrify, move together and fuse in the mid- line. Fusion, which begins at the front end and passes backwards, is immediately followed by the development of the keel. In the present investigation, three main problems have been studied: (1) the origin of the sternum, (2) its differentiation, and (3) the mechanism of the closure of the sternal plates. Part I deals with the normal embryology of the sternum and Parts II, II] and IV with experiments in vitro. The results are described in their logical order, but it should be stated that this does not always correspond with the chronological order in which they were obtained.
I am greatly indebted to Dr A. F. W. Hughes and to Dr F. G. Spear for much invaluable constructive criticism. I also wish to express my sincere thanks to Dr W. Jacobson for his kind assistance with the German literature, to Mr V. C. Norfield, senior assistant at the $.R.L., who took all the photomicrographs, and to A. J. E. Freeman, Esq., for his help and advice in the breeding and management of the budgerigars from which most of the material was obtained. The expenses of the investigation were defrayed by the Medical Research Council.
Historical
Rathke (1838) was the first to observe the bilateral origin of the avian sternum. He states that the sternal rudiments are derived from the ribs and that the keel is formed from the sternal plates.
Bruch (1852) denies that the ribs give rise to the sternum, and maintains that their fusion with the sternal plates is secondary.
Parker (1868), in his large monograph on the shoulder girdle and breast bone, includes a section on the anatomy of the avian pectoral girdle and sternum in various types of birds. He refers mainly to adults, but a number of embryonic stages are also described. Like Rathke he believes that the sternum develops from the ribs: **'The sternum is nothing else than the lower part of certain of the costal arches—a part in which the segmentation. ..dies out more or less.”’
Goette (1877) also accepts the costal origin of the sternum, but differs from Rathke in maintaining that the keel has a separate origin from the rest of the sternum and 1s formed from the episternal process of the clavicle.
Hoffmann (1879) agrees with Goette that the paired sternal rudiment is derived from the distal ends of the sternal ribs and the keel from the fused clavicles.
Lindsay (1885) has produced the only paper in the literature which deals exclusively with the embryology of the avian sternum. Unfortunately her descriptions refer to dissections only and not to histological preparations. She accepts the theory of the costal origin of the sternum but rejects the view that the keel is formed by the backward growth of the clavicle. She concludes that “the keel is an outgrowth of the sternum of comparatively late phylogenetic date, and created by and for the attachment of the pectoral muscles”’.
Paterson (1903) gives a brief account of the development of the sternum in fowl embryos of 6—7 days’ incubation. His observations on the origin of the sternal plates agree with those of Bruch. According to Paterson, when the sternum “‘first appears the ribs are unconnected with it, and it is only secondarily and after the sternum becomes cartilaginous that fusion occurs’’. He states that the keel is formed “‘ out of the median border of each half sternum”’, thus confirming Rathke’s and Lindsay’s accounts of keel formation.
Kopfli (1918) * reverts to the theory of the costal origin of the sternum. He records the appearance of two sternal bands, formed independently of the ribs, into which the costal tissue spreads.
Lillie (1919) believes that the sternum of the fowl develops “‘from a pair of mem- branous expansions formed by the fusion of the distal ends of the first four true thoracic ribs’’, The halves unite and “‘the carina arises as a median projection very soon after concrescence in any region, and progresses backwards, rapidly following the con- crescence”’.
Quoted from Gladstone and Wakeley (1932).
Hommes (1921) describes the first sternal rudiment as a paired mesodermal thickening which arises independently of the coracoid, the clavicle and the ribs. The rest of his account is substantially the same as Lillie’s.
Gladstone and Wakeley (1932) also maintain that the ribs play no part in the formation of the chicken sternum, but “in the earliest stages of mesodermal con- densation”’ the sternal rudiments “‘are very closely associated with the inner ends of the coracoids”’.
Part I. The Normal Embryonic Development Of The Budgerigar Sternum
Introduction
Previous work on the embryology of the avian sternum has dealt mainly and some- times exclusively with its gross anatomical development. In the hope that a combined histological and anatomical investigation might shed some light on the developmental mechanics of this complex bone, an attempt has been made in the present study to correlate the fine histological changes which occur in the sternal rudiment and its adjacent tissues with the gross anatomical changes.
The budgerigar is particularly favourable material for such an investigation, as the small size of the embryo makes accurate fixation possible without a preliminary dissection and also minimizes the time and labour involved in making wax reconstruc- tions for a study of the gross anatomy.
Material and methods
The artificial incubation of budgerigar eggs for biological purposes requires special precautions. The method as elaborated by Mr Harold Hignell (senior mechanic to the Strangeways Laboratory) is as follows. The eggs must be collected within a few hours of being laid and placed immediately in a very damp incubator kept at a temperature of 107° F. To provide a sufficiently moist atmosphere in the immediate neighbourhood of the eggs a shallow dish of saturated cotton-wool is laid on the tray beside them. It is most important that the eggs should be turned at least six times a day, preferably after moistening the fingers on the wet cotton-wool. If these conditions are observed, 80-90°%, of the eggs should develop, provided the birds have not been allowed to lay for more than a few months without a rest. When the percentage of development begins to decline, it is usually a sign that the birds are becoming exhausted. The sexes should then be separated and rested for 2-3 months. It is best to have two sets of birds available, so that one set can be rested while the other is laying. Eggs can then be obtained throughout the year.
Complete serial sections were made of the sternal regions of thirty-five embryos ranging in age from 3 days’ incubation to 1 day after hatching. Most of the material was either fixed in 3°, acetic Zenker’s solution and stained with safranin and picro-indigo-carmine or fixed in Carnoy’s solution and stained with haematoxylin and erythrosin. Some of the younger specimens were fixed with Flemming’s solution less acetic acid and stained for mitochondria with iron haematoxylin. One of the oldest sterna was fixed in Bouin’s fluid and stained with safranin and picro-indigo-carmine, and nine 6—9-day embryos were fixed in Zenker’s solution, treated by Wilder’s silver method for the demonstration of intercellular fibres and stained with carmalum and light green.*
The sternal regions of seventeen embryos were mounted whole. Four specimens from embryos of 5 and 6 days’ incubation were fixed in 5°% acetic alcohol, stained with dilute haematoxylin and then dehydrated, cleared in xylol and mounted whole in Canada balsam. The sternal rudiments from embryos of not less than 8 days’ incubation were first dissected from the rest of the body with or without the ribs and pectoral girdle, after which they were fixed in acetic alcohol, mordanted for 24 hr. in 4% iron alum, washed for 24 hr. in distilled water and stained for 24 hr. in very dilute thionin. After differentiation in 70° alcohol the stain was fixed by immersing the tissue for 24 hr. in 5°/, ammonium molybdate. This method renders the cartilage a deep violet and the surrounding tissue pale green. The rudiments were dehydrated, cleared and mounted in balsam in the usual way.
Five sterna, in embryos of 74, 8, 84, 94 and 114 days’ incubation respectively, were reconstructed from serial sections by means of waxed blotting paper.
The origin of the sternum
As stated in the General Introduction, the sternum is usually thought to arise from the ribs, and particular attention has therefore been paid to the early development of the sternal rudiment in relation to that of the costal processes.
There is a rather wide individual and seasonal variation in the degree of differentia- tion reached by embryos of the same age, so that the age given for each stage of development is approximate only.
The first sign of the sternal rudiment usually appears about the sixth or seventh day of incubation. At this stage the humerus has just begun to chondrify, but the coracoid is still procartilaginous with a somewhat indefinite outline. The sternal tissue lies immediately behind and below the ventral end of the coracoid with which it is con- tinuous, and forms a narrow, very diffuse condensation in the mesoderm of the dorso-lateral wall of the thorax. Dorsally this condensation merges with a mass of myoblasts and externally is partly covered by the oval rudiment of the pectoral muscles. It extends backwards as far as the second sternal rib process.
The costal processes are at a very early mesenchymatous stage. The first process in the sternal region terminates a long way dorsal to the sternal rudiment. The next, that of the first sternal rib, is very diffuse distally, and passes into the large mass of myoblasts dorsal to the sternum; no connexion between the rib rudiment and the sternum could be traced. The second sternal rib process already shows division into vertebral and sternal parts, the latter being represented by a small, oval mass of cells surrounded by myoblasts and connected with the vertebral part by an extremely diffuse strand of tissue. As already stated, the sternal plates do not extend beyond this point. The vertebral and sternal divisions could also be seen in the third, fourth and fifth sternal ribs, although the outline of the rib processes becomes less distinct towards the posterior end of the thorax. The sixth sternal rib has a very indefinite outline and no sternal division could be identified with certainty. No evidence of proliferation into the sternal rudiments from the sternal divisions of the costal processes was found.
I am indebted to Mr George Lenney, assistant at the Strangeways Research Laboratory, formaking the Wilder preparations.
A few hours later (figs. 1, 5, Plate 33) the sternal rudiment, though still in a fairly early procartilaginous state, has a more distinct outline, extends backwards as far as the third thoracic rib, and has broadened considerably. The articulation between the chondrifying coracoid and the sternum has become distinguishable as a zone of flattened cells, but the two structures are still continuous. The pectoral muscles cover the dorsal two-thirds of each sternal plate as far as the level of the first thoracic rib. The two halves of the sternum are very widely separated throughout their length; their ventral margins are farther apart than their dorsal margins (fig. 5, Plate 33).
The anterior sternal ribs are very distinct at this stage and consist of late pro- cartilage; the histological development of the ribs is always in advance of that of the sternum. The sternal division of the first sternal rib is now clearly seen and is connected with the vertebral division by the usual diffuse band of cells (fig. 1, Plate 33) ; it ends well above the sternal plate. The sternal divisions of the second and third sternal ribs have made contact with, but are sharply demarcated from, the sternum. Those of the fourth, fifth and sixth sternal ribs are also clearly outlined from the surrounding mesoderm and do not show the least sign of proliferating to form the posterior part of the sternum.
The above results afford no evidence in support of the view that the sternum is formed from the distal ends of the ribs. In the first place, in the budgerigar the sternal divisions of the ribs are not at first in contact with the sternal rudiments, an observation which confirms those of Bruch (1852), Paterson (1903) and Gladstone and Wakeley (1932). Moreover, the comparatively sharp outline of the sternal ends of the rib rudiments gives no indication of any downward emigration of cells in the sternal region and the histological appearance differs markedly from that of other structures in the embryo, e.g. the myotomes, where proliferation and emigration of cells are known to be taking place.
The fact that the first mesodermal condensation of each sternal plate is continuous with that of the corresponding coracoid, suggests that in its origin the sternum is more closely related to the pectoral girdle than to the ribs. This does not imply, however, that the sternum is actually derived from the coracoid.
The development of the sternum
Owing to the variation in the degree of development of embryos of the same age, the development of the sternum is described as a series of numbered stages, the approximate age at which each stage is reached being given.
The development of the corpus sterni. As described in the preceding section, the primary sternal plates first appear at the sixth or seventh day of incubation (stage 1) as a pair of mesenchymatous structures in the dorsal wall of the thoracic cavity (fig. 5, Plate 33). At this stage the plates slope away from each other, so that the ventral edges are farther apart than the dorsal edges.
In 7—8-day embryos (stage 2) the general orientation of the plates has changed. ‘They now occupy a vertical position on either side of the body cavity and so appear parallel in transverse section, while the anterior ends have begun to converge so that they are now closer together than the posterior ends. ‘The sternum extends slightly beyond the fifth sternal rib and has fused with the sternal divisions of all but the sixth sternal rib.
The plates have begun to chondrify. Chondrification begins and is always most advanced in a relatively large, antero-lateral region, whence it gradually spreads backwards, downwards and forwards. The ventral edges of the plates consist of a curved fringe of undifferentiated cells of irregular form with their long axes directed towards the mid-ventral line (fig. 4, Plate 33).
By about the end of the eighth day of incubation (stage 3) the sternal plates, which have considerably enlarged, have the following anatomical structure (fig. 36).
Seen in lateral view the plates, which are still vertical, have a pear-shaped outline, the narrow end being anterior. In transverse section (fig. 6, Plate 33) they appear almost flat except in the anterior third where they have a slight inward curvature. A small, triangular structure, the antero-lateral process, projects from the dorsal edge of each plate at a point about one-third the way along its length from the anterior end. The articular surface for the coracoid forms a fairly deep groove running backwards along the narrow, anterior part of the plates to the level of the antero-lateral process. Its outer edge is continued for some distance along the side of the sternum as an increasingly shallow ridge which disappears posteriorly. The anterior ends of the plates are closer together than at the previous stage.
Between the eighth and ninth day (stage 4) the sternal plates begin to fuse (fig. 37).
The extreme anterior ends unite, so that, seen from above, the sternum appears as a V with the apex anterior. The plates are no longer vertical, but incline at an angle (fig. 7, Plate 33), because the ventral margins have begun to draw together. In side view the outline of the plates has changed. The anterior end has elongated considerably so that it now extends beyond the sternal ends of the coracoids; it has also become much deeper and at the same time projects sharply upward to form the rudiment of the spina sterni. The antero-lateral process is relatively longer and the sixth pair of sternal ribs are now in contact with the sternum. The sternum is chondrified with the exception of the extreme anterior and posterior ends and the ventral margins which remain membranous,
Fic. 36. Sternal rudiment at stage 3, drawn from a wax reconstruction. The anterior ends of the sternal plates have begun to approach each other; the plates are still vertical. a./.p. anterior lateral process; ¢c.a. coracoid articulation; r. ribs.
A few hours later (stage 5) the keel begins to develop. At the level of the coracoid articulation the sternal plates are bent sharply, almost at right angles, the upper, horizontal parts forming the corpus sterni and the ventral, vertical parts the dorsal portion of the keel. ‘The development of the keel will be described separately. Behind the level of the fourth sternal rib the keel ends and the sternal plates are separated by a gap which widens gradually towards the hinder end.
Between the ninth and tenth days of incubation (stage 6) the corpus sterni has the following gross anatomical structure (fig. 38).
It is markedly concave and is closed in front by a transverse wall, on the inner surface of which is a heart-shaped lump with the apex downwards. This lump is formed by a median backward folding of the anterior part of the primary sternal plates. The articular grooves for the coracoids, which laterally are very deep, meet anteriorly in the mid-line to form a shallow depression separating the corpus sterni from the spina. The spina is now a relatively large, transversely flattened structure projecting sharply upwards. Behind the level of the sixth pair of sternal ribs, the sternal plates have not yet fused and are separated by a triangular gap. Two broad, flat, inward-curving projections, the posterior lateral processes, have now appeared at the hind-end of the sternum but are still unchondrified.
Fic. 37. Sternal rudiment at stage 4, from a wax reconstruction. The anterior ends of the sternal plates have fused and the plates now incline at an angle to each other. ‘The spina sterni has begun to develop in front. a./.p. anterior lateral process; ¢c.a. coracoid articulation; r. ribs; s. spina sterni.
A few hours later (stage 7) the antero-lateral processes have lengthened relative to the body of the sternum, and the posterior lateral processes have also elongated and curved backwards to fuse with the corpus sterni to form two posterior fontanelles (fig. 39). Closure of the plates is complete, and the corpus sterni, especially the posterior half, has flattened and become less concave.
Fic. 38. Sternal rudiment at stage 6, from a wax reconstruction. The plates have fused except at the posterior end and the keel has formed along the line of union. The posterior lateral process has appeared. a.i.p. anterior lateral process; c.a. coracoid articulation; c.s. corpus sterni; k. keel; p.l.p. posterior lateral process; r. rib; s. spina sterni.
By the eleventh or twelfth day of incubation (stage 8) the embryonic development of the sternum is complete in all essential features (fig. 40).
The spina has continued to elongate relative to the corpus sterni and curves boldly upwards and forwards. At the back, the sternum bends slightly upwards to form a shallow ridge. In other respects the gross anatomy has not changed much since the previous stage. ‘The entire sternum is now cartilaginous. Except that the cartilage matrix continues to increase in density and amount, the histology remains almost unchanged during the rest of embryonic life. Chondroblastic hypertrophy and ossifica- tion do not occur until after hatching.
Fig. 39. Sternal rudiment at stage 7, from a wax construction. Closure of the sternal plates is complete and the posterior lateral processes have fused with the corpus sterni to form two fontanelles. a./.p. anterior lateral process; ¢.a. coracoid articulation; ¢.s. corpus sterni; f/. fonta- nelle; k. keel; p./.p. posterior lateral process; r. rib; s. spina sterni. c.s, corpus stern
The development of the keel. Keel formation, as stated above, begins about the ninth day of incubation (stage 5), when the sternal plates become sharply bent to form an upper, horizontal part (the corpus sterni), and a lower, vertical part (the dorsal portion of the keel).
The extreme anterior end of the keel at this stage consists of a single, unpaired mass of procartilage, from which the spina sterni arises. Farther back, but still anterior to the corpus sterni, the dorsal part of the keel is formed by the union of the sternal plates. At the level of the coracoid articulation, although the two halves of the corpus sterni are medially in contact, the two halves of the keel are bowed away from each other so that they are separated by a space which is elliptical in cross-section. ‘This space is filled with necrotic tissue into which chondrogenic cells are migrating in a transverse direction from the inner surface of each half.
With the exception of its extreme anterior end, the keel is always most developed towards the front of the sternum; the angle between the corpus sterni and the keel becomes increasingly obtuse towards the back and the keel itself shallower.
The keel seems to develop in two parts: (a) a dorsal part consisting of a pair of vertical plates which are directly continuous with the two halves of the corpus sterni, and (b) a ventral part in which the paired structure is less conspicuous. ‘The histo- logical appearance (fig. 12, Plate 34) suggests that cells are streaming downwards on either side from the perichondrium of the dorsal part, and are then sweeping inwards to the mid-line below the primary plates, where they form the ventral portion of the keel by infiltrating with chondrogenic tissue a vertical band of aggregated, degenerating cells which marks the mid-ventral line; this band of necrosis will be described and discussed in a later section.
Thickened intercellular fibres, orientated in a vertical, longitudinal plane, occur throughout the length of the keel (fig. 12, Plate 34) and are continued ventrally to the inner surface of the ectoderm. ‘Towards the hinder end of the sternum the thickened fibres are mostly longitudinal and vertical fibres become increasingly rare.
By about the tenth day of incubation (stage 6) the keel is fairly deep in front but becomes shallower as it passes backwards and ends about the level of the sixth pair of sternal ribs (fig. 38). Chondrification begins at this stage in the anterior and dorsal parts of the keel, which elsewhere is still unchondrified; this process is always less advanced in the keel than in the corpus sterni. Degenerate cells and the remains of blood vessels are still present inside the front part of the keel (figs. 13, 14, Plate 35). The apparent downward and inward migration of perichondrial cells to form a ventral extension of the keel can still be clearly seen. It is now possible (fig. 8, Plate 34) roughly to distinguish the boundary between the ventral part of the keel, consisting of compact procartilage, and the underlying less dense thickening which, as will be described later, is apparently formed by two cell streams from the pectoralis major muscles.
A few hours later (stage 7) the keel, which is deeper, has extended almost to the back of the sternum (fig. 39). The spina and all but the posterior third are chondrified. In the hinder part, the downward and inward migration of perichondrial cells seems to be still in progress, but elsewhere all signs of this process have disappeared and the perichondrium forms a continuous fibrous membrane over the surface of the cartilage. The most ventral part of the keel consists of closely packed cells, many of which are degenerate. In the forepart of the keel, just behind the spina (figs. 15, 16, Plate 35), the necrotic tissue formerly enclosed by the uniting plates has completely disappeared, but the cartilage cells by which it has been replaced are still irregular in shape and separated by wide intercellular spaces filled with rather lightly staining matrix. ‘The thickening immediately below the keel, apparently formed by the confluent cell streams from the pectoral muscles, has been obliterated by the downgrowth of the keel, the bottom of which now reaches the level of the ventral surface of the pectoralis major muscles.
By the eleventh or twelfth day (stage 8) the keel has reached its maximum length relative to the corpus sterni (fig. 40). In the interior of the front part of the keel, the cartilage cells are still widely separated by broad tracts of matrix which stains less deeply than that of the surrounding cartilage; this condition persists to the end of the incubation period, and in late embryos and newly hatched chicks a small, closed cavity of tubular form, probably containing fluid in life, appears in this region.
Tissue movements in the thoracic wall
The downgrowth of the dorso-lateral mesoderm. The dual origin of the sternum from a pair of widely separated lateral plates raises the interesting problem of how these plates eventually meet in the mid-line. It is unlikely that their meeting is due to their enlargement only. As described above, the sternal plates when first formed lie obliquely with their dorsal margins closer together than their ventral margins, but as they approach each other in later development they also rotate, first into a vertical, then into a sloping, and finally into an almost horizontal position. This rotation could not be due to mere expansion of the rudiments, and other factors must therefore be sought.
The histological results indicate that the downward movement of the sternal plates is only part of a generalized movement of the entire dorso-lateral tissue of the thoracic wall, correlated with degenerative changes in the ventral body wall.
When the sternal rudiment first appears as a mesodermal condensation continuous with the ventral end of the developing coracoid (stage 1), its ventral limit is lost in a mass of transversely flattened cells, apparently derived from both the muscles and the connective tissue of the dorso-lateral thoracic wall. These flattened cells, which have an amoeboid form, seem to be migrating downwards into the reticular mesenchyme of the ventral body wall. This apparent downward migration of the dorso-lateral tissue is not confined to the sternal region, but occurs throughout the length of the body cavity.
The median third of the original ventral body wall lying between the two masses of downward-moving tissue has by now begun to degenerate, and both ectoderm and reticular mesenchyme contain many necrotic cells. This degeneration extends behind the sternal rudiment in a less marked way to about the level of the third thoracic rib, and also in front of it into the coracoid region.
The ventral degeneration rapidly increases (fig. 2, Plate 33) as the downward move- ment advances, and becomes very pronounced in the reticular tissue at the level of the sternal ends of the coracoids, which at this stage project in front of the sternal articula- tion, and between the anterior parts of the sternal plates. The ectoderm covering this degenerate area is slightly thickened and itself contains degenerate cells, whilst the mesothelium on the inner surface has become thrown into small folds; this local thickening of the ectoderm and mesothelium indicates that the mesodermal necrosis has caused a contraction of the mid-ventral body wall in the anterior part of the thorax. Posteriorly the necrosis rapidly diminishes.
By the time the sternal plates have reached the vertical position (stage 2) the middle region of the ventral body wall has assumed a very remarkable appearance in the anterior part of the thorax. In front of the sternum at the level of the dorsal end of the sloping coracoid, the median ectoderm is nearly twice as thick as elsewhere, and a large proportion of the cells are necrotic. This strip of thickened degenerate skin narrows as it approaches the level of the sternum, and the cells of the stratum corneum become irregularly heaped to form a sharply projecting ridge (fig. 3, Plate 33). The strip disappears at a level slightly in front of the hind-end of the sternal articulation of the coracoid, where the skin again becomes normal. The loose mesenchyme which previously formed the ventral body wall has been almost entirely replaced by the invading streams of dorso-lateral tissue which have now met in the mid-ventral line to form a mass of flattened cells, some of which are degenerate.
Farther back, between the anterior ends of the sternal plates, the fused streams of flattened cells appear in transverse section as a thick, curved sheet uniting the sternal plates and overlying pectoral muscles of either side (fig. 10, Plate 34). The trough formed by this sheet and the two vertical sternal plates contains a mass of reticular tissue derived from the original mesenchyme of the ventral body wall and lined by much folded mesothelium. The enclosed reticular tissue is very compressed, and both cells and blood vessels are orientated roughly parallel with the plates and at right angles to the cells of the underlying sheet. Except near the mid-ventral line, a broad layer of the original loose mesenchyme separates the sheet of flattened cells from the skin.
The ventral sheet of tissue connecting the plates and pectoral muscles, though distinguishable throughout the length of the sternal rudiment, becomes much thinner and more diffuse towards the hinder end.
Cell degeneration occurs in the mid-ventral mesoderm to about the level of the first sternal rib, which in the living embryo gives the median body wall an opaque greyish
422 HONOR B. FELL ON THE ORIGIN AND
appearance when viewed by transmitted light (fig. 27a, Plate 38). Many degenerate cells are also seen in the mesoderm adjacent to the sternum and are mainly distributed along the inner surface of the anterior end of each plate. They are very numerous near and among the amoeboid cells composing the ventral margin.
Later development of the ventral body wall. During the period (stage 3) when the anterior ends of the plates are approaching each other preparatory to fusing, no very important changes take place in the body wall. The transverse sheet of flattened cells is more distinct posteriorly than before, and the median strip of thickened degenerate ectoderm described above, which disappears about the level of the hind-end of the coracoid articulation, reappears, though less conspicuously, behind the sternum.
When the anterior ends of the plates first unite (stage 4), they are still distinct a short way behind the extreme tip as two curved structures with their convex sides facing inwards (fig. 11, Plate 34). They enclose the now enormously compressed reticular tissue derived from the original ventral body wall, which contains many necrotic cells and degenerating blood vessels; the intercellular fibres of this compressed tissue are much thickened and confined to vertical longitudinal planes, rarely running at an angle to these planes. The subsequent fate of this tissue has already been described in connexion with the development of the keel.
The ventral body wall has greatly thickened throughout the length of the thorax. In transverse section it presents a very interesting feature. A narrow vertical band of rather densely crowded cells, a large proportion of which are necrotic, cuts right across the mid-line of the body wall from the skin to the pericardium. It can be traced forwards in front of the sternum to the level of the dorsal articulation of the coracoid and backwards, though becoming less obvious, to the yolk-stalk. In the living embryo it is extremely conspicuous, and the rather diffuse greyish band seen in younger embryos (stage 2) has now become a sharply marked line which, owing to the large number of degenerate cells present, is so opaque as to appear almost black when viewed by transmitted light.
This median band of degeneration may be interpreted as follows. There is no reason to suppose that tissue movement ceases after the invading streams of dorso-lateral mesoderm unite in the mid-ventral line. It probably continues until the sternal plates and pectoral muscles of either side come in contact. This continued downgrowth would involve an enormous accumulation of cells in the mid-line unless extensive degeneration took place in this region. It would seem, therefore, that when the down- growing tissues from either side meet in the mid-line, the median cells degenerate, thus forming the necrotic band described above, while more cells move down to take their place, like fresh troops being moved into the line of battle. The generalized nature of the degenerative process is shown by the fact that it begins well in front of the sternum and ends some distance behind it at the level of the yolk-stalk. It is not peculiar to the budgerigar, and has also been observed by the writer in the embryonic fowl and sparrow.
The degenerative changes in the epidermis of the mid-line have now spread back- wards as far as the yolk-stalk. At the previous stage (stage 3) this degeneration was most advanced at the level of the dorsal articulation of the coracoid, but the median epidermis now shows signs of recovery in this region; a basement membrane has formed, and the folds of the stratum corneum, which are longer and thinner than before, look as if they were being sloughed. At this stage the folding and necrosis are most pronounced over the forepart of the sternum, they gradually diminish as far as the hind-end of the sternum, when they again become conspicuous, and finally disappear at the level of the yolk-stalk.
It will thus be seen that degeneration passes down the mid-ventral epidermis as a wave, which synchronizes with the progressive closure of the sternal plates, and which is always greatest where the plates are about to unite. The ectodermal degeneration ‘is closely associated with that of the mid-ventral mesoderm and with the thickening and folding of the median pericardium and, like these processes, it is not confined to the sternal region, but occurs throughout the body wall as far back as the yolk-stalk.
Another curious feature of the body wall at this stage, which is not yet understood, is the appearance and orientation of the intercellular fibres as seen in Wilder prepara- tions. In the dorsal part of the mass of flattened cells connecting the sternal plates, the median intercellular fibres in the degenerating band are very thick and usually run longitudinally, whilst those to either side of the mid-line form a stout reticulum. In the ventral part of the band of flattened cells and in the subepidermal mesenchyme below it, the fibres, which are rather less coarse, have an oblique orientation, and in transverse sections of the embryo slope outwards from the mid-line to the epidermis; they often cross the long axes of the flattened cells almost at right angles, an unusual relationship of cells and fibres. Other finer, less conspicuous fibres form a network between the cells in the usual way. More posteriorly, where the plates are farther apart, these oblique fibres present an even more remarkable picture; they are very numerous and, while stouter and more abundant near the mid-line, they extend laterally almost as far as the ventral margins of the pectoral muscles. In the hinder part of the sternal region, the obliquely orientated fibres become less and less striking and are finally lost in the ordinary reticular network.
At the stage when keel formation begins (stage 5) the mid-ventral band of degenerate mesoderm is very thick and conspicuous ventral to the keel, where the two streams of cells from the pectoralis major muscles meet. Behind the level of the fourth sternal rib the plates are still separate, and the band of flattened cells connecting them is quite sharply demarcated from the overlying rather diffuse band connecting the pectoral muscles. The pericardium in the mid-line is now so folded and thickened that it forms a pronounced ridge which is highest in front. It is interesting to note that the median ectoderm has almost recovered from the degenerative changes seen at the previous stage, except over the hinder part of the sternum where the plates have not yet united; here the epithelium is still somewhat folded and degenerate.
By the time stage 7 is reached, when the plates have fused except at the extreme hind-end and a mesenchymatous keel extends along the line of union, degeneration in the mid-ventral mesoderm has greatly diminished beneath the forepart of the keel. Farther back, however, it remains pronounced, probably owing to the fact that the pectoral muscles are fairly widely separated in this region and are still connected by a thick band of flattened cells.
A few hours later (stage 8) (fig. 15, Plate 35), when union of the plates is complete, the pectoral muscles have come to occupy their final position in relation to the keel, and the band of degeneration has disappeared from the mesenchyme throughout the length of the sternal region. The ventral body wall now consists of normal skin, with rather thin epidermis and thick, vascular dermis, beneath which are the pectoral muscles attached to either side of the keel by fibrous connective tissue.
Discussion
The origin of the sternum. Histological observations on the normal embryo, as described above, afford no evidence that the sternum is formed by the ribs. The sternal plates are not at first in contact with the ribs, and the latter show no sign of cell proliferation taking place from their free ends. As the mesenchymatous sternal plate first appears in continuity with the coracoid, it would seem that the sternum is more nearly related to the pectoral girdle than to the ribs, and should thus be regarded as part of the appendicular and not of the axial skeleton.
Before any definite conclusion can be reached, however, it is necessary to know whether the sternal plates can develop in the absence of the rib rudiments and pectoral girdle, and if so where the presumptive sternal tissue is located in the undifferentiated body wall with reference to the wing bud. This information, however, cannot be obtained from a-morphological investigation and experimental methods are required.
The differentiation of the sternum. Previous work has shown (Murray 1926; Murray and Selby 1930; Fell and Robison 1929; Warren 1934; Fell and Canti 1934; Hamburger 1938) that the general form of the cartilaginous limb skeleton of birds is determined at a very early stage as a fairly strict mosaic, i.e. parts removed are not regenerated. The isolated rudiment is self-differentiating, and its shape does not depend on the influence of extrinsic factors. How far the sternal rudiment is also a self-differentiating mosaic structure can be ascertained only by experimentation.
A study of the normal morphogenesis of the sternum rather suggests that in the formation of the keel extrinsic factors may be at least partially involved. When the two halves of the sternum come in contact further expansion in a median direction would be prevented, and growth, by following the line of least resistance, might be expected to cause the sternum to bend downwards between the large paired masses of the pectoral muscles to produce the keel. Whether there is any truth in this hypothesis cannot be determined from morphological data alone.
Closure of the sternal plates. The results of this study have shown that the original mesenchyme of the ventral body wall degenerates and is replaced by downward- growing tissue from the dorso-lateral part of the thoracic wall, including muscle, connective tissue and the sternal plates. When the two streams meet in the mid-line a sharply defined median zone of degeneration appears which persists until both the sternal plates and the pectoral muscles have assumed their final positions.
This ventral cell degeneration, first of the original mesenchymatous body wall, and later of the downgrowing tissue derived from the dorso-lateral region, is correlated with shrinkage of the ventral body wall, as indicated by the deep folds which appear in both the mesothelium and the epidermis of the mid-line.
Whether expansion of the dorso-lateral tissue, combined with median degeneration, are the factors primarily concerned in the movement of this tissue towards the mid-line is uncertain. As already described, the histological appearance strongly suggests that there is an active, amoeboid migration of less differentiated cells of the dorso-lateral region. Ifsuch a migration really exists, it might pull the more differentiated material with it in a ventral direction.
Another possibility must also be considered, viz. that the sternal plates are pushed towards the mid-ventral line by the elongation of the ribs fused to their dorsal margins. Since the downgrowth of the dorso-lateral tissue is not confined to the sternal region, this fourth possibility seems unlikely, but can only be critically tested by experimental means. The whole problem of the movement of the sternal plates will be discussed at length in Part IV.
Summary (Part I)
- The sternum of the budgerigar first appears at about the sixth day of incubation as a pair of mesodermal condensations, each of which is continuous with the mesenchy- matous rudiment of the corresponding coracoid.
- The sternal divisions of the sternal ribs are not at first in contact with the sternal rudiments but fuse with them later. There is no evidence that the sternum is derived from the ribs.
- The mesenchymatous rudiments of the sternum enlarge and chondrify to form two widely separated plates on either side of the pericardium.
- An anterior lateral process grows out from the dorsal margin of each plate.
- The sternal plates move towards the mid-line, rotating from an oblique dorso- lateral to a horizontal ventral position, and fuse with each other. Fusion begins in front and extends backwards.
- The posterior lateral processes develop as curved outgrowths which grow back- wards to unite again with the hind-end of the corpus sterni, thus forming two fontanelles.
- The development of the keel follows the line of fusion of the plates and is always most advanced in the forepart of the sternum.
- The keel appears to develop in two parts; a paired dorsal part continuous with the two halves of the corpus sterni, and an unpaired ventral part produced by the downward and inward migration of the perichondrial cells of the upper part.
- The sternum does not ossify during embryonic Irfe.
- The original mesenchyme of the ventral body wall largely degenerates and is replaced by the downgrowing dorso-lateral tissue which includes connective tissue, the pectoral muscles and the sternal plates. This downgrowth is not confined to the sternal region.
- Where the two downgrowing streams of tissue from the dorso-lateral regions meet in the mid-line, a median tract of degeneration appears.
- This degeneration is correlated with a shrinkage of the mid-ventral body wall, as evidenced by the thickened and folded condition of the median ectoderm and pericardium. |
- The essential features of sternal development are complete by about the eleventh day of incubation.
Part II. Experiments on the Origin of the Sternum
Introduction
As stated in Part I, a study of the normal embryology of the avian sternum affords ‘*no evidence in support of the view that the sternum is formed from the distal ends of the ribs”’. It was decided to investigate the origin of the sternum by experimental means, to find whether the sternal plates could develop zn vitro in the absence of the rib rudiments, and if so to determine the position of the presumptive sternal tissue in the undifferentiated body wall. It was hoped that the results of these experiments would indicate whether the sternum should be regarded as part of the axial or as part of the appendicular skeleton.
Technique
The material consisted of budgerigar embryos of 4—5 days’ incubation and 34—4-day fowl embryos whose larger size made them more suitable than the budgerigar for experimenting with young stages.
All cultures made from the budgerigar embryos, with the exception of seven cultures of the axial region, were grown by the hanging-drop method on 1} in. square cover- slips over 3x 1} in. hollow-ground slides, in a medium composed of equal parts of plasma and extract of 9-10-day fowl embryos. The explants from the fowl embryos and the seven budgerigar explants mentioned above were cultivated by the watch- glass method (Fell and Robison 1929) on the surface of a clot consisting of four drops of plasma and four drops of embryo extract.
In most embryological experiments in vitro it is necessary to remove the ectoderm, which otherwise envelops the explants and, owing to its premature keratinization, becomes so impermeable that the enclosed tissue rapidly degenerates. ‘lo remove the ectoderm from the relatively thin lateral body wall before explantation was found to be impossible without hopelessly damaging the underlying tissue. In explants of the lateral body wall with the wing bud attached, the bud only was dissected free of ectoderm before explantation and the tissue was then placed in the culture medium with the ectoderm downwards, i.e. farthest from the cover-slip, in hanging-drop prepara- tions and upwards in watch-glass cultures. This prevented the skin from growing over the entire surface of the tissue, and after a certain period of cultivation, when growth and differentiation were more advanced, the ectoderm could be stripped from the body wall with a needle and fine cataract knife.
In most of the experiments camera lucida drawings of the living explants were made at intervals, and in others photographs were taken at the end of the culture period.
With the exception of five cultures of the axial region of the embryo, which were serially sectioned after fixation in 3°% acetic Zenker’s solution, the explants were fixed in 5°% acetic alcohol, stained either with thionin by the method described in Part I or more rarely with dilute haematoxylin, and were mounted whole in Canada balsam.
Except in those experiments where the entire axial region was cultivated as well as the lateral body wall, the bodies of the embryos from which the explants were taken were fixed as controls, either for serial sections in 3°, acetic Zenker’s solution or for whole mounts in 5° acetic alcohol. Sections were stained with haematoxylin followed either by erythrosin or chromatrop, and whole mounts by the thionin technique or with dilute haematoxylin, after which they were dehydrated, cleared and mounted in Canada balsam.
The relationship of the ribs to sternal development
Object of experiments. ‘To find whether the lateral body wall can form a sternal plate when removed from the embryo before the appearance of the rib rudiments, and cultivated in vitro.
Material and methods. The head and neck, the hind-end from just in front of the leg buds and the viscera were removed from a number of 4—5-day budgerigar embryos and 4-day fowl embryos. The lateral body wall with the wing bud attached was then cut off as a strip from one or both sides; in several experiments the axial mesoderm, by which is meant the somites and perichordal tissue, was also divided lengthwise, each part being explanted separately.
Most of the skin was removed from the explanted body wall of budgerigar embryos after 24 hr. growth, before the tissue had time to spread out into a thin sheet. The explants were then transferred to fresh medium and maintained for a further 3—6 days, when they were fixed and mounted whole. In the fowl embryos it was found that the skin should be removed by degrees, as otherwise too much of the underlying mesoderm was taken away from the less differentiated regions and the experiment wasconsequently spoilt. After 24 hr. cultivation, the ectoderm was therefore removed from the anterior part of the body wall only. The tissue was then changed to fresh medium and 24 hr. later, when growth and differentiation were more advanced, the rest of the skin was stripped off, and the culture was again transferred. After this, subcultures were made at intervals of 48 hr. in the usual way.
Five groups of experiments were made:
Group 1. Material: eleven 4—5-day budgerigar and twenty-one 4-day fowl embryos.
The lateral bédy wall with the wing bud was cut from one side of the body, the rest of the embryo being fixed and sectioned.
Group 2. Material: five 5-day budgerigar embryos.
Two explants were taken from each embryo: (1) the lateral body wall and wing bud of one side, (2) the adjacent somite region. The rest of the body was fixed and sectioned.
Group 3. Material: ten 4—5-day budgerigar and six 4-day fowl embryos.
Three explants were taken from each embryo: (1) the lateral body wall and wing bud of one side, (2) the middle region comprising the medullary cord, notochord and somites, (3) the lateral body wall and wing bud of the other side.
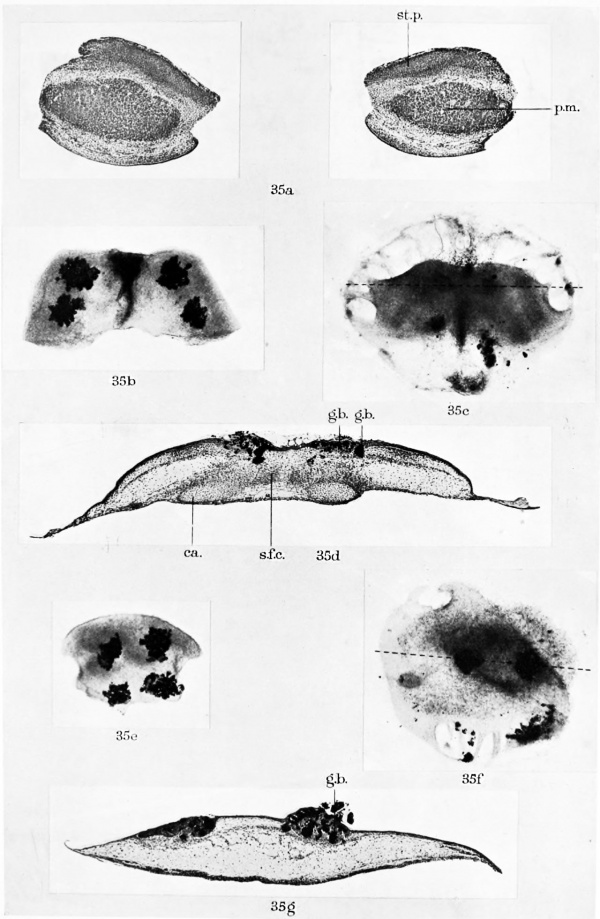
Fic. 41. Diagram of operation in experiments of group 4 (5-day budgerigar embryo). The strips removed for explantation are numbered in the same order as in the text. A, surface view. B, transverse section.
Group 4. Material: six 5-day budgerigar embryos.
Four cultures were made from each embryo: (1) the lateral body wall and wing bud of one side, (2) the adjacent somitic tissue and notochord (medullary cord largely removed), (3) the ventral half of the somitic region of the other side, (4) the other lateral body wall and wing bud (fig. 41).
It will be noted that in these experiments one strip of the embryo, that including the dorsal half of one somitic region, was not explanted.
Group 5. Material: one 5-day budgerigar embryo.
This experiment was exactly the same as those of group 4, except that the strip comprising the dorsal half of one somitic region, which was rejected in group 3, was here explanted, so that five explants were obtained from the single embryo instead of four.
Controls. Transverse sections of fourteen 4—5-day budgerigar embryos, used for the experiments in groups 1 and 2, showed that costal processes were present in one embryo only. The culture made from this chick was rejected. In another embryo from group 2, it was impossible to tell whether the rib rudiments had been formed or not, as the medullary canal and notochord with the perichordal tissue were missing ; the degree of development attained by the rest of the body, however, made it unlikely that the costal processes had appeared. No trace of the sternal plates was found in any of the controls, with the possible exception of the discarded specimen mentioned above.
The general structure of the skeletogenous tissue of the thorax and wing was briefly as follows. The sclerotomes formed a rather loose, perichordal sheath from which the still rather diffuse neural arches, two in each segment, extended upwards on either side of the spinal cord. The wing bud contained an axial condensation of mesoderm, the rudiment of the wing skeleton. Immediately below and behind the wing bud, the mesoderm underlying the skin of the lateral body wall was composed of rather densely arranged cells, orientated with their long axes at right angles to the ectoderm; towards the inner (pericardial) surface the mesoderm had the usual loose reticular structure. This subepithelial thickening of the mesoderm disappeared a short distance behind the wing, bud.
The embryos varied in their degree of development, but the range of variation was not very great. In the most developed chicks the neural arches and perichordal sheath were rather dense and more clearly defined, and the thickening of the subepithelial mesoderm in the lateral body wall, described above, had spread inwards and occupied most of the thickness of the body wall.
The twenty-one 4-day fowl embryos of group 1 (fig. 17a, Plate 35) were rather less developed than the budgerigar controls. Relative to the length of the body, the wing buds were shorter and contained only a very slight axial condensation. The sub- epithelial thickening of the mesoderm of the lateral body wall was still shallow and confined to the region immediately under the skin.
Examination of the controls of group 1 showed that in five of the budgerigar embryos the body wall removed for explantation had been cut off just below the ends of the somites, whilst in the remaining budgerigar and in all the fowl embryos the cut had been made across the ventral tips of the somites. In group 2 the entire somite region had been amputated except for the most median (perichordal) part of the sclerotome: the medullary cord on the operated side was almost and sometimes quite bare of mesoderm.
Results. The results of the five groups of experiments may be described together. Some part of the sternal plate, the coracoid, usually almost complete, part of the scapula, and the proximal part of the wing skeleton developed in thirty-eight out of fifty explants from budgerigar embryos, and in twenty-seven out of thirty-three fowl embryos. The comparatively large number of negative results were due to the technical difficulties of the work. Many explants had to be rejected owing to envelopment by ectoderm; others, as a result of damage inflicted when the skin was removed or when the thin, delicate tissue was detached from the clot, formed merely scattered fragments of cartilage which could not be identified.
No trace of ribs developed in a single explant from either the budgerigar or fowl embryos.
At the beginning of the culture period the explants of the body wall and wing bud consisted of an L-shaped mass, the short arm of the L being the wing and the long arm the lateral body wall. In the budgerigar embryos, after 24 hr. growth, the outline of the proximal part of the early procartilaginous wing skeleton could be distinguished. In the anterior part of the body wall, extending from the base of the wing skeleton, was an oval, rather ill-defined condensation of mesoderm which, by the third day, had formed a short coracoid, usually associated with the proximal fragment of a scapula, and the rudiment of a sternal plate, represented by an oblong mesodermal thickening which was less differentiated than the coracoid. By the fourth day the skeleton was usually chondrified throughout and the structure was very distinct. As a rule only the proximal part of the wing skeleton was present as the distal part was destroyed during the original dissection, but the elbow-joint between the humerus and proximal ends of the radius and ulna was clearly visible. ‘The shoulder-joint at the union of the coracoid, humerus and proximal fragment of the scapula and the joint between the coracoid and sternal plate were also very conspicuous. All the long-bone rudiments had lengthened considerably and the sternal plate, usually oval or triangular in form, had enlarged and had become sharply outlined from the surrounding muscle and connective tissue. Two days later (figs. 18a, e, Plate 36) the joints were less noticeable, as secondary fusion of the articular surfaces had begun, and eventually they disappeared.
In the explants taken from fowl embryos the mesodermal rudiment of the sternal plate did not appear until about 24 hr. after that of the coracoid; for this reason it was necessary to leave the skin covering this part of the explant intact for a longer time than in the budgerigar cultures. In the first experiment with fowl material, the ectoderm with an adherent layer of mesoderm was removed from the entire body wall 24 hr. after explantation, when the coracoid condensation was already present. As a result of this procedure, either no sternal plate developed or only a small fragment, showing that the presumptive sternal tissue must be superficially located at this early stage. In the next experiment the ectoderm was first removed from the anterior part of the body wall only; after 2 days’ cultivation a second mesodermal thickening had formed immediately ventral to the developing coracoid which had already begun to chondrify, and proved to be the rudiment of the sternum. Eight of the twelve cultures in this experiment developed a large part of the sternal plate (fig. 174, Plate 35) and smaller fragments differentiated in the remaining four.
The explants of various parts of the axial region, included in groups 2-5, did not reach a very advanced stage of anatomical development. A fragment of the sternal plate occurred in one axial explant only. This culture, from group 2, was of the somitic region (region 2), and a small part of the lateral body wall had been included; although well-formed ribs appeared, they did not develop in association with the fragment of sternal plate, from which they were widely separated by a broad area of segmented muscle.
Explants of the complete axial region (group 3) usually formed two parallel bars of cartilage, often incomplete, one on either side of a rather convoluted and degenerate nerve cord; these bars represented fragments of the vertebral column. In some cultures the cartilage developed a series of tooth-like projections, apparently the remains of the vertebral arches. Adjacent toeach of the rods a band of segmented muscle differentiated _ which often showed quite strong spontaneous contractions. The tissue beneath the nerve cord was usually very degenerate, but sometimes the perichordal sheath chon- drified and showed traces of segmentation in the form of a series of constrictions.
In group 2, where part of the somitic region from one side was cultivated, segmented muscles readily developed. In one culture five elongated ribs appeared between the muscle segments, in addition to several irregular nodules of cartilage lying in a curved line to one side of the ribs and probably representing fragments of the vertebral column. In another culture a long rod of cartilage developed with distinct traces of segmenta- tion, from which projected a number of narrow rods, probably costal, lying in the septa of the segmented muscles. The remaining somitic explants contained rather irregular nodules of cartilage distributed along a curved line on one side of a broad area of segmented muscle bands.
Two explants of the most median region (region 2) cultivated in group 4, each formed a set of four ribs lying in the intermuscular septa, and also a cartilaginous vertebral column with a distinct segmented structure. Of the other median regions of groups 4 and 5, one showed a number of elongated nodules probably homologous with ribs, and all contained a partly segmented, cartilaginous rod (figs. 18, c, Plate 36) representing the vertebral column. One explant of the ventral half of the somitic region (region 3) from groups 4 and 5 formed three oblong pieces of cartilage which perhaps corresponded with ribs, two contained no cartilage (fig. 18d, Plate 36) and the remainder gave rise to a few small nodules with a linear arrangement.
Conclusion. The sternal plates are not derived from the ribs.
The localization of the presumptive sternal tissue
Method of defining the position of the sternal tissue. The following experiments were undertaken to determine the site of the presumptive sternal tissue in relation to the wing bud. Different areas of the lateral body wall were excised from early fowl embryos and their developmental potencies investigated by cultivation im vitro. To render the results obtained with different embryos as nearly comparable as possible, all transverse cuts were made with reference to the intersomitic septa (fig. 42).
In most 34—4-day fowl embryos the wing bud has an expanded base from which the main part of the bud projects, and in the following descriptions of operations, references to the front and hind margins of the bud apply to this projecting part only, not to the base. In the large majority of embryos of this age, the projecting part of the bud occupied a region of the body wall approximately 4 somites in length, one intersomitic septum being almost in a line with the front margin and another with the hind margin of the bud. The expression “first intersomitic septum behind the posterior margin of the bud”’ refers to the septum which is in a line with or slightly behind the posterior margin, and similarly with the first intersomitic septum in front of the bud.
Unfortunately this system of defining the explants is not always quite precise, owing to the individual variation among embryos of the same age. In the least developed chicks there is no distinction between the base and the distal part of the bud, and the complete wing rudiment may occupy an area of body wall 5 somites in length. On the other hand, in the most highly developed embryos the proximal part of the wing bud above the base narrows, and may extend for 34 somites only. In more than two-thirds of the embryos, however, the wing buds were almost exactly 4 somites in width.
Sertes 1
Object of experiments. To find the posterior boundary of the presumptive sternal tissue in the undifferentiated body wall.
Material and methods. Fragments of the body wall ae to the somites were removed from twelve 4-day fowl embryos and cultivated by the watch-glass method. ‘The rest of the trunk was fixed in acetic alcohol, stained with dilute haematoxylin or by the thionin technique and mounted whole as a control. The tissue was explanted with the skin surface uppermost, and was changed to fresh medium after 24 hr. in vitro; 48 hr. later it was freed from skin and again transferred. In explants which included the wing bud, the ectoderm was dissected from the axial mesoderm of the bud before cultivation. ‘he explants were grown for periods ranging from 4 to 7 days and were then fixed in acetic alcohol, stained with thionin, and mounted whole.
Two groups of experiments were made (referred to by number).
Controls. ‘The embryos were rather under-developed for their age; in most the limb buds were still short and rounded, though in a few they had just begun to elongate. The defects left by the excision of the explants were sharply defined and the cuts were accurately located. The extreme tip of the myotomes may have been included in some of the cultures, but it was not possible to make certain of this.
Experiments and results. Group 1. Six embryos. In four the wing buds occupied an area of body wall 4 somites in length, in the fifth 3} somites, whilst the sixth control was rejected as the corresponding cultures were discarded. Two explants were taken from each embryo and were grown for 4 days (fig. 43):
Fic. 42. Diagram of 4-day fowl embryo Fic. 43. Diagram of operation in series 1, prepared for operation. The four somites group 1, showing the two regions A and B of the wing-bud region, which in the fol- removed from the lateral body wall for ex- lowing experiments were used as reference explantation.
marks for the transverse cuts, have been numbered.
A. A region extending from the level of the first intersomitic septum behind the wing bud backwards to the level of the fourth intersomitic septum behind the bud.
B. A region from the opposite side of the embryo extending backwards from the fourth to the sixth intersomitic septum behind the wing bud.
One pair of explants was later rejected owing to accidental damage.
The five explants of region A all formed a small flat plate of cartilage usually prolonged at one end into a short curved spike (fig. 19a, Plate 36), but the five explants of region B (fig. 194, Plate 36) formed no cartilage.
This result showed that the presumptive sternal tissue does not extend for more than 3 somites behind the posterior margin of the wing bud.
Group 2. ‘Three explants were taken from each of six embryos and were grown for 7 days (fig. 44):
C. A region extending from the first intersomitic septum in front of the wing bud backwards to the first intersomitic septum behind the bud, the bud being included.
D. A region from the same side as A, extending from the first to the fourth inter- somitic septum behind the wing bud.
E. A region from the opposite side, extending from the third to the sixth intersomitic septum behind the wing bud.
The six explants of region C (fig. 20a, Plate 36) all formed a large fragment of the sternal plate, a coracoid, usually part of the scapula and the proximal part of the wing skeleton (humerus and fragments of the radius and ulna). Those of region D (fig. 206, Plate 36) formed a small flat plate of cartilage usually with a small spike at one end similar to that of the explants of region A, group 1. This projection probably represented one of the posterior lateral processes. Five of the six explants of region E formed no cartilage (fig. 20c, Plate 36) but a small nodule developed in the sixth.
These results define the posterior border of the sternal tissue still more precisely. The fact that one explant of region E developed a little cartilage whilst all those of region D formed cartilage shows that the posterior boundary of the presumptive sternal tissue lies near the third intersomitic septum behind the wing bud.
Conclusion. The posterior border of the presumptive sternal tissue is near the level of the third intersomitic septum behind the wing bud.
Sertes 2
Object of experiments. To find the anterior boundary of the presumptive sternal tissue in the undifferentiated body wall.
Material and methods. Fragments of the body wall from 34-day fowl embryos were prepared and cultivated as in series 1, and at the end of the culture period were stained and mounted whole by the technique described. After excision of the explants the trunk was fixed in acetic alcohol, stained with dilute haematoxylin and mounted whole as a control.
Six groups of experiments were made and the cultures were grown for 8 days.
Controls. Vhe average stage of development was about the same as in series 1.
Experiments and results. Group 1. Four embryos. In three the wing-bud area was 4 somites long and in the fourth 5 somites. ‘Two explants were taken from the same side of each embryo (fig. 45):
F. A region of the lateral body wall stretching forwards from the first to the sixth intersomitic septum in front of the wing bud and bounded dorsally by a cut level with the ventral border of the base of the bud.
G. An L-shaped explant: the long arm of the L was a narrow strip of tissue im- mediately dorsal and parallel to explant F, extending forwards from the first to the sixth intersomitic septum in front of the bud, and including the tips of the myotomes and part of the expanded base of the wing bud; the short arm of the L was much wider and included the tips of the myotomes, the wing bud and the lateral body wall from the first intersomitic septum in front of the wing bud to the first septum behind it. Explant G was thus exactly complementary to explant F.
None of the explants of region F developed cartilage (fig. 21a, Plate 37). Those of region G (fig. 216, Plate 37) all formed a large fragment of the sternal plate, with which a coracoid (incomplete in one explant) articulated, three developed a piece of scapula and all formed part of the wing skeleton, i.e. a humerus and fragments of the radius and ulna.
The absence of cartilage in the explants of region F showed that the presumptive sternal tissue does not extend in front of the wing bud.
Fic. 44. Diagram of operation in series 1, = Fic. 45. Diagram of operation in series 2,
group 2. Three regions C, D and E were _ group 1. Two regions F and G were re-
removed for explantation. The entire wing moved for explantation. The line ab
bud was included in explant C. marks the transverse boundary between explants F and G.
Group 2. Three embryos. In two the wing-bud area was 4 somites long, and in the third 34 somites. Two complementary explants (H and I) were taken from the same side of each embryo. The explants differed from those of group 1 only in that the transverse cut ab (fig. 45) was at the level of the second intersomitic septum behind the anterior margin of the wing bud (i.e. 1 somite farther back than the corresponding explants of group 1), and the anterior border of the short arm of region H was therefore at the same level.
No cartilage appeared in the three explants of region H. As in group 1 all the explants of region I formed a fragment of the sternal plate, a coracoid, an incomplete scapula and part of the wing skeleton.
Since no cartilage differentiated in the explants of region H, it is clear that there is no presumptive sternal tissue beneath the anterior part of the wing bud for the length of 1 somite behind the front margin of the bud.
Group 3. Two embryos. In one the wing-bud area was 4 somites long and in the other 5 somites. Two complementary explants (J and K) were taken from the same side of each embryo. The explants were similar to those of groups | and 2, except that the transverse boundary ab (fig. 45) between them was at the level of the third inter- somitic septum behind the anterior margin of the wing bud, i.e. 1 somite farther back than in group 2.
Of the first pair of explants (from an embryo with 5-somite wing buds), that of region J formed no cartilage whilst that of region K developed a large piece of the sternal plate, a coracoid, a fragmentary scapula, a humerus and part of the radius and ulna. In the other pair, from an embryo with 4-somite wing buds, the explant ofregion K (fig. 22), Plate 37) formed a large piece of the sternal plate, the dorsal half only of the coracoid, which was well separated from the sternal plate, a fragment of the scapula and part of the wing skeleton. Explant J (fig. 22a, Plate 37) developed two small nodules of unequal size, separated by a joint, which probably represented the ventral end of the coracoid missing in explant K and a fragment of the sternal plate. This result, which required confirmation, suggested that the anterior border of the presumptive sternal tissue in an embryo with 4-somite wing buds lies near the third intersomitic septum behind the front margin of the bud.
Group 4. Four embryos. In two embryos the wing-bud area was 4 somites long, in one more than 4} and in the fourth more than 3} somites. Two complementary explants (L and M) were taken from the same side of each embryo.
L. A region of the lateral body wall immediately below the attachment of the wing bud and extending from the first intersomitic septum in front of the wing bud to the first septum behind it.
M. The wing bud and ventral tips of the myotomes, from the first intersomitic septum in front of the bud to the first behind it.
Of the explants of region L, no. 1 formed a single, very small nodule of cartilage, no. 2 (fig. 23a, Plate 37) a large, flat, curved plate associated with another smaller nodule, no. 3 a flat, bean-shaped plate with which a long, narrow nodule articulated, and no. 4 a single large plate.
Of the explants of region M, no. 1 formed most of the humerus and part of the radius and ulna, no. 2 (fig. 236, Plate 37) a humerus, part of the radius and ulna and most of the coracoid, and nos. 3 and 4 the humerus, part of the radius and ulna and about half the coracoid.,
It will be seen that the skeletal structures developed by regions L and M respectively were not fully complementary to each other in nos. 1 and 4. Thus in no. 1 the entire coracoid and most of the sternal plate were missing from both L and M, and in no. 4 half the coracoid failed to appear in either explant, although a large part of the sternal plate developed in L. In nos. 2 and 3, however, the parts missing in explant M were present in explant L. In no. 2L the ventral end of the coracoid was missing but was probably represented in 2M by the small nodule of cartilage adjacent to the large flat nodule which was obviously part of the sternal plate. Similarly, in no. 3 the half- coracoid absent from 3M occurred, articulated with a fragment of the sternal plate, in 3L. The loss of cartilage in nos. 1 and 4 was probably due to damage inflicted on the explants during the rather delicate operation of removing the ectoderm.
Group 5. Four embryos. The wing-bud area was approximately 4 somites long in all the embryos. Two complementary explants (N and O) were removed from the same side of each embryo (fig. 46):
N. A section of the lateral body wall immediately TT below the base of the wing bud, stretching from the first intersomitic septum in front of the anterior margin of the bud to the first septum in front of the hind margin of the bud.
O. The entire wing bud and the tips of the myotomes from the first intersomitic septum in front of the bud to the first septum behind it, together with a transverse strip of the lateral body wall, 1 somite in width, extending from the first intersomitic septum in front of the hind margin of the wing bud (linecd) to the first septum behind the bud.
No. 1N developed a large flat plate with which an elongated nodule articulated, no. 2 N formed nocartilage, no. 3N a large flat plate and a small oval nodule, and no. 4N a similar plate with which an oblong cartilage articulated. Fic. 46. Diagram of operation
The four explants of region O closely resembled each N a: aoe fe ene other. All developed a large flat cartilage which was entire wing bud was included in obviously part of the sternal plate, and which was well explant O. The line cd marks the
transverse boundaryin the lateral separated from the other skeletal structures, one- to two- body wall (beneath the wing thirds of the coracoid, a humerus and part of the radius bud) between explants N and O. and ulna.
In no. 2, explant N, probably owing to experimental damage, formed no cartilage in spite of the fact that one-third of the coracoid was missing from explant O, but in the other three pairs explant N almost perfectly complemented explant O. Thus all the explants of region N in these pairs developed two nodules of cartilage: one broad and plate-like representing the anterior part of the sternal plate, which was associated with a second, oval or elongated nodule representing the ventral end of the coracoid, while the explants of region O, in addition to part of the wing skeleton, developed the dorsal portion of the coracoid and a fragment of the posterior half of the sternal plate.
The experiments of groups 3 and 4 showed that the lateral body wall below the anterior part of the wing bud contains no presumptive sternal tissue for a distance of 1 and possibly 2 somites behind the anterior margin of the bud. The experiments of group 5 showed that in the body wall below the posterior part of the wing bud, sternal tissue is present for a distance of more than | somite from the hind margin of the bud, since part of the sternal plate developed in both explants N and O. In an embryo with a 4-somite bud, therefore, in the light of the above results the anterior border of the presumptive sternal region must lie near the level of the middle (third) inter- somitic septum of the wing-bud region.
Group 6. Three embryos. The wing-bud area was 4 somites long in all the embryos. Two complementary regions (P and Q.) were explanted from the same side of each embryo.
The explants differed from those of group 5 only in that the transverse cut ed (fig. 46), between explants P and Q, was made at the level of the second intersomitic septum in front of the hind margin of the bud, 1.e. 1 somite anterior to the corre- sponding cut in group 5.
Nos. 1 P and 2P (fig. 24a, Plate 37) both developed a long club-shaped nodule, the narrow end of which articulated with a second, very small, flat nodule; no. 3 P formed a small oval nodule which articulated with another minute piece of cartilage.
Nos. 1 Q and 2Q (fig. 244, Plate 37) both formed a large fragment of the sternal plate, an hour-glass-shaped coracoid, the middle of the shaft being defective, a humerus and fragment of the radius and ulna. No. 3Q developed a large piece of the sternal plate, a coracoid with an apparently complete shaft, a humerus and a fragment of the radius.
In this group fully complementary structures differentiated in all three pairs of explants. The presumptive coracoid tissue in nos. 1 and 2 must have been split longitudinally by the transverse cut, since the part of the shaft missing from the explants of region Q appeared in those of region P (cf. figs. 24a and 246, Plate 37). In no. 3, where the shaft of the coracoid was complete in explant Q, the larger of the two small nodules in explant P probably represented part of the ventral end of the coracoid and the smaller a fragment of the sternal plate. It will be noted that whereas the explants of region P all contained a large piece of the sternal plate, those of region Q. contained only a very small fragment. This showed that only a small part of the presumptive sternal tissue occurs in region Q,, 1.e. in the lateral body wall anterior to the middle intersomitic septum of the wing-bud area.
Conclusion. ‘The anterior border of the presumptive sternal tissue lies near the middle intersomitic septum of the wing-bud region.
Series 3
Object of experiments. To find the ventral boundary of the presumptive sternal tissue in the lateral body wall.
Material and methods. A strip of the lateral body wall, from just in front of the wing bud to about the fourth intersomitic septum behind it, was removed at different levels from one side of each of twelve embryos. The rest of the trunk was fixed and sectioned as a control. ‘The strips were cultivated by the watch-glass method, freed from ectoderm in the usual way, and after 6 days in vitro were fixed in acetic alcohol, stained with dilute thionin and mounted whole.
Three groups of experiments were made.
Controls. As described in Part I in the case of the budgerigar embryo, there is a thickening of the subepidermal mesoderm of the lateral body wall in the 3}—4-day fowl embryo, which extends a short distance ventral to the wing bud (figs. 25a, 6, Plate 37). The cells of this thickening, which is similar to and continuous with the subepidermal tissue of the wing bud, are orientated with their long axes at right angles to the ectoderm. For purposes of description this thickening has been adopted as a “‘land- mark” to which the position of the dorsal cuts can be referred.
The embryos were at about the same average stage of development as those of series | and 2 and showed the usual individual variation.
Experiments and results. Group 1. Four embryos. Region R. The dorsal cut was well below the subepidermal condensation (figs. 25a, b, Plate 37) in all the embryos and passed through the ventral reticular tissue.
No cartilage differentiated (fig. 25c, Plate 37). The explants grew well, and produced a mass of actively contracting smooth muscle.
Group 2. Four embryos. Region S. The dorsal cut passed through the outer end of the condensation.
The explants formed no cartilage but only smooth muscle and connective tissue.
Group 3. Four embryos. Region T. The dorsal cut was made immediately below or very close to the base of the wing bud, and thus included all or nearly all the subepidermal condensation.
The controls of nos. 1 and 2 showed that the embryos were less developed than nos. 3 and 4. In no. 1 the dorsal cut had been made immediately below the base of the bud and the explant developed a large oval plate of cartilage. The dorsal cut in no. 2 (fig. 26a, Plate 38) was slightly below the base of the bud; the explant (fig. 26, Plate 38) formed the ventral end of the coracoid which articulated with a large piece of the sternal plate. In no. 4, the most developed embryo of the series, the dorsal cut was almost, but not quite, against the base of the bud; the ventral part of the coracoid and a large fragment of the sternal plate differentiated in the explant.
The results of this series of experiments show that the presumptive sternal tissue lies immediately below the base of the wing bud and does not extend very far ventral to the bud, since the most dorsal explants (group 3) alone formed sternal cartilage.
Conclusion. he ventral border of the presumptive sternal tissue lies a short distance below the base of the wing bud.
Discussion
As stated in the introduction to Part II, the experiments described above were undertaken in the hope that they would show whether the sternum should be regarded as part of the axial or as part of the appendicular skeleton. The results obtained strongly suggest that from the developmental standpoint the sternum is related to the appendicular skeleton.
It has been demonstrated experimentally that the sternum is not derived from the ribs, since explants of the undifferentiated lateral body wall developed sternal plates in the complete absence of the ribs. When the differentiation of the sternal plate was studied in living cultures which included the wing bud, the sternum always developed in apparent continuity with the procartilaginous coracoid. It does not originate from the coracoid rudiment, however, since experiments have shown that the posterior part of the sternal plate can develop from the undifferentiated body wall when isolated from the anterior part and the adjacent coracoid. Hommes’ conclusion (1921) that the sternal rudiment originates independently both of the ribs and of the coracoid rudiment has thus been verified experimentally.
The localization experiments showed that the presumptive sternal tissue occupies a restricted area of the body wall, approximately 4 somites in length, lying slightly below the base of the wing bud and extending backwards a distance of about 2 somites behind the bud and forwards 2 somites in front of the hind margin of the bud (fig. 47). These results emphasize still further the intimate association which exists between the presumptive sternal tissue and the rudimentary appendicular skeleton of the wing.
Fic. 47, Camera-lucida drawing of a 4-day fowl embryo (lateral view) showing the site of the presumptive sternal tissue (p.s.t.) in the lateral body wall.
Summary (Part II)
- The origin of the sternum was investigated experimentally by removing fragments of the undifferentiated thoracic wall from early budgerigar and fowl embryos, and studying their developmental potencies during cultivation in vitro.
- The lateral body wall and wing bud, when removed from the embryo before the appearance of the costal processes, formed a sternal plate and part of the appendicular skeleton but no ribs.
- Explants of different parts of the axial region from the same embryos formed cartilage showing various degrees of segmentation; in a few cultures ribs developed but were not associated with a sternal plate.
- These results showed that the sternal plates are not derived from the ribs.
- The posterior and anterior boundaries of the presumptive sternal tissue in the 4-day fowl embryo were found by excising fragments of the lateral body wall, the position of the fragments being defined with reference to the intersomitic septa, and cultivating them in vitro.
- The posterior border of the presumptive sternal tissue is near the level of the third intersomitic septum behind the wing bud.
- The anterior border lies near the middle intersomitic septum of the wing-bud region,
- The ventral boundary was found by removing strips of the lateral body wall at different levels beneath the wing bud and growing them in vitro. The rest of the body was fixed and sectioned to show the exact position of the dorsal cut.
- The ventral border lies a short distance below the base of the wing bud.
- From the developmental standpoint the sternum is more nearly related to the appendicular than to the axial skeleton.
Part III. Experiments on the Differentiation of the Sternum
Introduction
The experiments described in Part III were undertaken to investigate the factors controlling the anatomical development of the sternum, and in particular to ascertain how far the development is regulated by intrinsic and how far by external agencies. This was attempted by studying the differentiation in vitro of the isolated sternal rudiment and of parts of the rudiment.
Technique
The explants were obtained from 7—9-day budgerigar embryos and were grown by the watch-glass method, as sterna from fowl embryos of this age were too large for tissue-culture work. Daily camera-lucida drawings were made of nearly all the cultures, and others were periodically photographed.
Most of the explants were fixed in 2-3 °% acetic Zenker and serially sectioned, but a few were fixed in acetic alcohol and mounted whole. To prevent contraction and distortion, it was found best to fix the tissue on the clot, from which it was removed immediately after coagulation and transferred to a tube of fixative. Sections were
stained with safranin and picro-indigo-carmine, or with haematoxylin followed by either erythrosin or chromatrop. The whole mounts were stained with dilute haematoxylin.
A wax reconstruction was made from the serial sections of one explant of the complete sternum.
The development in vitro of the entire sternal rudiment
Object of experiments. To study the developmental capacity of the sternal rudiment when isolated from the pectoral muscles, viscera, ribs and blood supply.
Material and methods. The degree of development of the sterna used ranged from stages 1 to 4 (Part I). Thirty-three cultures were made, of which two were discarded owing to accidental damage, and the rest were grown for various periods from 8 hr. to 23 days. The explants were fixed in acetic Zenker’s solution and serially sectioned with one exception, which was fixed in acetic alcohol and mounted whole.
The explants were prepared as follows. The embryo was decapitated, and the trunk was opened down the back and carefully eviscerated. Half the costal region of either side was cut off and the ventro-lateral body wall laid with the skin surface upwards. The skin was dissected away completely (embryos of stages 2—4) or partially (embryos of stage 1), the pectoral muscles were removed except for a few tags, and finally the remains of the ribs were cut off at or near their articulation with the sternal plates. ‘The ventro-lateral body wall, either to the level of the yolk-stalk or slightly behind it, was then explanted with the ventral surface upwards on the clot in the watch-glass. ‘Thus, in the following description of the development of the sternal rudiment zn vitro, the upper surface of the explant as it lay on the clot was morphologically the ventral surface.
In the earlier experiments the culture medium consisted of four drops of embryo extract mixed with four drops of plasma. The comparatively large explants liquefied the clot so quickly that sometimes they were transferred to fresh medium every 24 hr., which gave better results than the usual 48 hr. interval but involved too frequent disturbance of the tissue. It was later found that a clot composed of four drops of embryo extract and eight drops of plasma was much more satisfactory, and made it possible to subcultivate every 48 hr. without causing excessive liquefaction.
Observations on the living cultures. Most of the sternal rudiments were between stages 2 and 3 (Part I) when first explanted, i.e. the plates were well separated and the keel had not begun to develop (fig. 27a, Plate 38). The original explants were usually rather less than 0-25 sq. cm. in area, and the cartilaginous sternal plates appeared as clear, translucent regions each occupying about one-third of this area. The curved inner borders of the plates were more widely separated behind than in front; the posterior margin could not be distinguished, as the plates merged gradually into the more opaque tissue of the posterior body wall. The tissue between the plates formed a fairly thin sheet, down the middle of which ran the conspicuous line of degeneration described in Part I.
After 1-2 hr. incubation the whole explant had greatly contracted. Contraction then ceased and the tissue began to expand owing to the amoeboid migration of the marginal cells.
Although the explant enlarged, the sternal plates did not move apart, but instead began to approach one another as in normal development (fig. 27), Plate 38). ‘That this approximation of the plates was not due merely to expansion by growth was shown by the fact that the distance between their outer (costal) margins diminished as well as that between their inner (ventral) margins. The front-ends of the plates came in contact first, after which the hind-ends continued to move inwards until the outer edges of the plates, which originally sloped away from each other, became parallel or might even converge posteriorly. After 48 hr. growth the plates were usually in contact for about two-thirds of their length (fig. 27c, Plate 38).
Correlated with the movement of the sternal plates was a great thickening of the intervening tissue, which became heaped up into a broad, thick, median ridge con- taining enormous numbers of opaque, degenerate cells. In cultures where the skin had been almost or completely removed, these necrotic cells drifted offinto the medium in a loose cloud, so that after 2 days’ cultivation the ridge had become much smaller.
The form of the sternum also developed. The hind-end gradually became visible as a dense area of procartilage with a bilobed hind margin (fig. 27c, Plate 38), the outer lobe being the rudiment of the posterior lateral process.
After 2 days’ growth the explants were transferred to fresh medium and much of the surplus connective tissue was removed, partly to prevent the formation of a tough fibrous coat which distorted the growing sternum by resisting its expansion, and partly to facilitate the nutrition and respiration of the cartilage. ‘The keel developed during this passage and its formation was readily observed. In normal development, as described in Part I, the forepart of the keel in front of the corpus sterni is formed by the lateral fusion of the two plates, which in this region do not rotate into a horizontal position as they do farther back. In the explants, probably owing to their being laid flat on the surface of the clot, fusion of the anterior ends of the plates occurred along the inner margins only. Thus, instead of a vertical, double plate being formed as in the normal embryo, merely a rather shallow arch of cartilage was produced in the cultures. At the level of the corpus sterni the keel developed better and a sharply projecting ridge appeared, extending to the end of the line of fusion. The anterior and posterior lateral processes had lengthened relative to the rest of the sternum, and the plates, which were almost flat when first explanted, had acquired to some extent their normal curvature, so that they had become convex to the clot.
The sternum did not develop much farther during subsequent cultivation (fig. 27d, Plate 38). ‘The posterior lateral processes grew longer and occasionally showed a slight inward curvature, but they never united with the corpus sterni to form the two fon- tanelles. The keel also elongated slightly. Fusion of the plates never reached the hind-end, and a triangular gap always separated them for the posterior third or fourth of their length.
As compared with the normal embryonic sternum at an equivalent stage of develop- ment, the explanted sternum was always very broad in proportion to its length, and its keel was relatively much shallower (fig. 27e, Plate 38). The part corresponding to the spina was also abnormally short in relation to the corpus sterni. It was interesting that the coracoid articulation, so conspicuous in the normal embryonic sternum, did not develop in vitro, although its rudiment was present at the time of explantation. ‘The groove gradually flattened out as cultivation proceeded, and finally became indis- tinguishable.
When the sternal rudiment was explanted at a slightly later stage when the anterior ends were nearly or quite in contact (stage 4, Part I), development zm vitro advanced farther. The anterior ends, though not fully united laterally, became almost vertical and formed a conspicuous keel and spina in front of the corpus. Fusion of the plates extended farther back but never quite to the hind-end. The posterior lateral processes also grew longer and more curved than in explants of younger rudiments, but never succeeded in fusing with the corpus. As in the younger sterna, the coracoid articulation failed to develop and disappeared. The general proportions of the sternum showed the same type of abnormalities as those described above but to a lesser degree, so that the explants approximated more nearly to the normal.
The cultures could not be maintained in a healthy state for more than about 14 days in vitro, and after about 3 weeks became completely necrotic. Growth was always greatly subnormal in vitro, and the explanted sterna only enlarged by about 50°% of their original size.
FAiistological development. The sterna explanted at stages 2 and 3 will be described first. Transverse sections through the extreme anterior end of such an explant, fixed after 6 hr. cultivation, showed a dense median mass of tissue occupying about three-fifths of the diameter of the explant, from either side of which the sternal plates projected. About half of each plate was embedded in the mass, the other half being covered by a perichondrium only. In this region, which corresponded with that part of the sternum anterior to the corpus sterni, the plates sloped upwards from the clot towards the mid-line and merged medially with the undifferentiated tissue composing the middle of the explant.
Slightly farther back in the region of the corpus sterni, the first rudiment of the keel could be very faintly seen. The median edge of each plate, consisting of extremely early procartilage, had become bent in a ventral direction (fig. 28, Plate 39). This bent part represented the rudiment of half the keel. It was only distinguishable from the surrounding tissue by the fact that the cells covering its outer surface, though not yet forming a definite perichondrial membrane, were orientated parallel with the surface of the procartilage, i.e. at an angle to the surface of the explant, whilst the cells of the surrounding undifferentiated tissue were orientated parallel to the surface of the explant. The chondrogenic cells of the keel rudiment were compressed in a dorso-ventral direction, and their long axes were thus at right angles to the future perichondrial cells covering the outer surface of the keel. There was no visible boundary between the medial surfaces of the two keel rudiments and the undifferentiated tissue between them.
Towards the hind-end of the coracoid articulation the keel became still more indefinite, and near the hind-end of the sternum, where the plates were widely separated, it could not be seen.
The histological structure of the dense median mass of tissue, from which the plates projected, presented some interesting features. At the level of the most anterior part of the sternal plates there was a median cleft in the dorsal surface of the explant, which extended into the tissue as deep as the ventral surface of the plates. It appeared to be a fold and was probably formed by the contraction of the tissue and the approximation of the plates. The median line of degeneration which passed into the top of the cleft was wider than in the normal body wall, especially near the ventral surface of the explant, and was being invaded by normal cells from either side.
Farther back, at the level of the coracoid articulation (fig. 28, Plate 39), the dorsal cleft disappeared. The inner surface of each half of the keel was continuous with a broad, very dense band of flattened cells which appeared to be streaming inwards and slightly downwards (dorsally) to the median line of degeneration, which here was much narrower than elsewhere. At the tip of each keel rudiment the flattened cells covering the outer surface of the procartilage had a similar orientation towards the mid-line as in normal development.
As the hind-end of the sternum was approached, the sheet of tissue between the plates became thinner and the band of flattened cells uniting them more sharply defined from the less compact tissue above and below it.
Except at the extreme hind-end of the sternal rudiment, where a small patch of healthy muscle occurred on either side, removal of the pectoral muscles had been almost complete and only a few degenerate fibres remained.
After 24 hr. zn vitro, chondrogenesis was more advanced throughout the sternum and the cartilage contained many mitotic figures. As described above, the extreme anterior ends of the plates were much closer together at this stage, and as a result of this approximation the cleft in the dorsal surface of the explant was nearly or quite obliterated, and the median band of degeneration much reduced in width. The central mass of tissue from which the plates projected was usually narrower and thicker than before, and was fairly sharply differentiated into a broad dorsal zone of densely packed transversely flattened cells uniting the median edges of the plates, and a ventral zone of about equal width, composed of a rather loose connective tissue containing many degenerate cells.
The structure of the forepart of the corpus sterni had not changed much. The two halves of the keel were almost in contact medially and, though not yet chondrified, were more clearly defined from the surrounding tissue, which had become looser and less cellular. The two keel rudiments (fig. 29, Plate 39), as in normal development, enclosed between them necrotic tissue which was being invaded by amoeboid cells streaming inwards from the inner surface of each rudiment. There was the same apparent inward migration of the external perichondrial cells which has already been described, but this was much less marked than in the normal keel at a similar stage of development. It was impossible to distinguish the ventral end of the keel from the overlying mass of non-chondrogenic flattened cells. The sternal plates were more curved than at the time of explantation, thus confirming what was observed in the living cultures.
Keel formation extended farther back than at the previous stage, but the halves of the keel were abnormally widely separated. A broad median band of necrosis was present in the intervening tissue, into which normal cells seemed to be migrating.
Towards the hind-end of the sternum there was no trace of the keel, and the histological structure of the explant had altered little.
In 2-day cultures, the anterior ends of the plates were usually nearly or quite in contact. The halves of the foremost part of the keel, which had begun to chondrify, had elongated in a vertical direction and had also increased in diameter until each was thicker than the horizontal portion of the plate. Each half-keel had a rounded, club- shaped form in transverse section. The mass of tissue occupying the middle of the explant was smaller than before, and the median band of degeneration was much more restricted throughout the length of the sternum.
By about the third day the two halves of the keel fused. In the forepart of the sternum, as described in the living cultures, only the edges fused, so that a shallow arch of cartilage was formed (fig. 30a, Plate 39) instead of a vertical, double plate as in the normal. Farther back the two rudiments, club-shaped in cross-section, united to make a single keel (fig. 305, Plate 39), which in section was usually almost semicircular. The perichondrium covering the ventral surface of the keel was always very thick and dense. Its appearance suggested that the perichondrial cells, which, in the normal embryo, would have infiltrated the median band of degeneration extending below the keel between the pectoral muscles, had been prevented from doing this in culture by the absence of the pectoral muscles and the intermuscular septum, and had therefore accumulated on the ventral surface of the keel. The sternal plates were covered on both sides by a layer of connective tissue.
The keel continued to elongate ventrally (fig. 27e, Plate 38) until about the sixth day in vitro. Sometimes the connexion between the keel and one of the plates was resorbed, so that the keel appeared to be derived from one plate only; all stages in this process occurred. ‘Towards the front of the sternum the keel was broad and oval in cross- section, but farther back it narrowed and in section became first oblong and then triangular, the apex of the triangle being ventral. As already stated, it never developed in the posterior third of the sternum, where the plates did not fuse but were united by a band of fibrous tissue only.
As noted in the living cultures, sterna explanted at stage 4 (Part 1), when the anterior ends of the plates were already in contact, developed further in vitro than those explanted at stages 2 and 3. After 24 hr. cultivation, a well-developed keel, which in front was beginning to chondrify, had appeared, and lateral fusion of the two halves was well advanced. ‘The interior of the keel contained necrotic tissue heavily invaded by normal cells. The keel rudiment extended farther back than in cultures of young rudiments. By the fourth day the keel had chondrified throughout its length.
Conclusions
- The approximation ofthe sternal plates, keel formation, the appearance of the posterior lateral processes and the curvature of the corpus sterni do not depend on the presence of the pectoral muscles, viscera, ribs or blood supply.
- The processes of anatomical development are not completed in vitro.
- The coracoid articulation is not self-differentiating 7n vitro.
The development in vitro of half the sternal rudiment
Object of experiments. ‘To find whether the sternal plates can develop independently of each other, with special reference to keel formation.
Matertal and methods. ‘The sterna of the embryos used were at stages 2—3 (Part I). The ventro-lateral body wall was removed from the embryo and denuded of muscle and skin exactly as in the previous experiments. It was then cut in half, and in one set of experiments (group 1) one half was fixed and sectioned as a control and the other explanted, and in the other set of experiments (group 2) both halves were explanted, one with the dorsal surface uppermost and the other with the ventral surface uppermost.
The explants were grown by the usual watch-glass method, on a clot composed of eight drops of plasma and four drops of embryo extract. They were fixed at intervals of 1-8 days.
Both controls and explants were fixed in acetic Zenker’s solution and serially sectioned, with the exception of one pair of explants which were fixed in acetic alcohol, stained with dilute haematoxylin and mounted whole.
Experiments and results. Group 1. Half the ventro-lateral body wall was explanted, and the other half was fixed and sectioned as a control. ‘Ten explants were made, four of which were grown with their dorsal surface uppermost and the rest with their ventral surface upwards.
Four of the control sternal plates (fig. 32a, Plate 40) were at stage 2 (Part I), and were almost flat with no trace of the keel rudiment. Five were between stages 2 and 3; anteriorly the plates were slightly concave and the ventral edge very slightly bent outwards, the first indication of keel formation; the rest of the plates was nearly flat. The last control was rather more advanced than stage 3, and the anterior curvature and the bending of the ventral edge were more pronounced.
The explanted halves showed as great a capacity for self-differentiation as the entire sternum, and the majority developed a half-keel and the normal curvature of the corpus sterni, during cultivation.
Two explants were fixed after 24 hr. in vitro. The curvature of the plates was much more pronounced than in the corresponding controls and extended much farther back. A keel rudiment had also appeared in the front half of the plate. The ventral edge of the plates had become bent at right angles to the corpus sterni, as in normal develop- ment, but a second almost right-angled bend occurred a short distance ventral to the first (fig. 31, Plate 39), so that most of the keel, instead of projecting vertically, lay nearly parallel with the surface of the clot. The keel consisted of early procartilage which was not yet sharply defined from the surrounding tissue, and the general orientation of cells in the keel region was similar to that already described in early cultures of the complete sternum. The isolated plates formed a longer keel rudiment than the explants of the complete sternum. This was probably due to the fact that in the half-sterna the keel was better nourished than in the complete sterna owing partly to its peripheral position in the culture, and partly to the fact that a large part of it lay parallel with the culture medium instead of projecting vertically away from it as in the complete sterna. The posterior lateral processes were visible, though still mesenchymatous. Many necrotic cells occurred in the connective tissue in the morphologically median part of the explant. There was usually more connective tissue on the ventral than on the dorsal side of the sternal plate, especially in the keel region.
Seven cultures were fixed after 2 days’ growth, four of which (fig. 326, Plate 40) were explanted with the dorsal surface upwards. As compared with the control halves and with the 24 hr. cultures, the curvature of the plates had increased and chondrification was more advanced. The proximal part of the keel was chondrified but the distal part was still procartilaginous, though now sharply defined from the loose reticular tissue around it. In the three explants grown with the ventral surface upwards the keel had the two right-angled bends described above, but in the two grown with the dorsal surface upwards (fig. 32), Plate 40) the keel had a much more normal appearance. In these cultures the second angle was very obtuse, so that the entire width of the keel projected at an angle to the corpus sterni.
Two cultures were grown for 8 and 6 days respectively. Their structure was much less normal than in the younger cultures owing to secondary distortion occurring during the later stages of growth. One had a well-formed keel but no curvature of the corpus sterni, whilst the other showed the usual curvature but the keel was almost in a line with the corpus.
Group 2. Both halves of the sternum were grown side by side, one plate being dorsal side upwards and the other ventral side upwards. The rudiments when first explanted were at stages 2 and 3 (Part I). Eight pairs of explants were cultivated.
The results were essentially the same as in group 1.
Two pairs were fixed after 2 days in vitro. Each half showed a distinct curvature and a well-formed keel which was bent at a rather sharper angle in the half grown dorsal side upwards.
Five pairs were cultivated for 3 days. One half of one pair was damaged and formed no keel. Secondary distortion had already begun to appear; the curvature of the corpus sterni, though still distinct, was somewhat reduced, and the angle between the keel and the corpus was more obtuse than in the 2-day cultures. As before, the keel was more sharply bent in the halves explanted with the dorsal surface upwards.
One pair was fixed after 8 days in vitro and mounted whole. Each half was strongly concave, but in both the keel, though more distinct in that grown dorsal side upwards, was greatly flattened.
Conclusions
- An isolated sternal plate has as great a capacity for self-differentia- tion as the entire sternal rudiment.
- Each half of the keel develops independently in response to factors intrinsic in the sternal plate.
- In the isolated sternal plates conditions in vitro are not favourable for maintaining either the curvature of the corpus sterni or the angle between the corpus sterni and the keel.
The development in vitro of the ventral body wall
Object of experiments. To study the developmental capacity of the ventral body wall between the sternal plates.
Material and methods. The sterna of the embryos used were at stages 2-3 (Part I). The ventro-lateral body wall was isolated exactly as in the previous experiments, but the skin and muscle were not removed. ‘Two longitudinal but converging cuts were made, one just ventral to the inner border of the pectoral muscles of one side and the other near the median line of degeneration. These cuts extended backwards to the hind-end of the sternum or a little beyond it, and forwards to within a short distance of the front of the sternal plates. ‘This roughly triangular flap of body wall was then detached and explanted in a watch-glass in the usual way, with the skin surface uppermost. The rest of the ventro-lateral body wall was fixed and sectioned as a control.
Eight cultures were made. After 2 days’ growth the skin was removed from the explants, which were transferred to fresh medium, They were grown for 4—7 days and were then fixed and sectioned.
Both cultures and controls were fixed in acetic Zenker’s solution and sections were stained with haematoxylin and chromatrop.
Controls. As described in Part I, at stages 2 and 3 each sternal plate ends ventrally in a fringe of undifferentiated cells which is directly continuous with a sheet of flattened cells stretching right across the ventral body wall. It is impossible to dis- tinguish histologically how far the chondrogenic tissue extends into the band of flattened cells. In most of the controls (fig. 33a, Plate 40) a variable amount of this fringe of undifferentiated tissue had been removed with the explant, and in one embryo a small part of the chondrified area had also been taken.
Results. Three of the eight cultures formed no cartilage. The controls of these three explants showed that the lateral cut just missed the fringe of undifferentiated cells in two of the embryos, but in the third, which was considerably less developed, it was difficult to tell whether the fringe had been touched or not. One of the explants was lost during dehydration, but in sections of the remaining two the tissue was seen to consist of very loose connective tissue with much finely fibrillar intercellular material, and scattered multi-nucleated myoblasts.
In all the other five cultures (fig. 335, Plate 40) cartilage appeared as a thin rod of varying length which usually became rather twisted and distorted during the later stages of cultivation. Sections showed typical hyaline cartilage and a loose fibrous stroma containing scattered myoblasts. In all the controls the fringe of amoeboid cells had been wholly or partly removed (fig. 33a, Plate 40). The longest and widest rod was formed in the explant derived from the control mentioned above, in which a small part of the chondrified region of the plate had been removed as well as the undifferentiated fringe. .
From these results it was clear that the chondrogenic tissue extends only a very short way below the chondrified part of the sternal plates, since cartilage only appeared in the explants if the lateral cut almost touched the chondrified region.
Conclusions
- Chondrogenic tissue extends only a very short way below the chondrified part of the sternal plates.
- The transverse band of flattened cells uniting the sternal plates is not chondrogenic.
Discussion
The results of the above experiments have shown that the sternal rudiment has a considerable capacity for self-differentiation. Thus the approximation of the sternal plates, keel formation, the development of the posterior lateral processes and the curvature of the corpus sterni can take place in the isolated rudiment explanted in vilro.
The problem of the movement of the sternal plates will be considered fully in Part IV and therefore will not be discussed here.
In view of previous experimental work on the embryonic skeleton (Murray 1926; Fell and Robison 1929; Murray and Selby 1930; Warren 1934; Hamburger 1938) which has shown that the general form of bone rudiments is determined at a very early stage, the posterior lateral processes might be expected to appear in the isolated sternum during cultivation in vitro.
It is more surprising, however, that the curvature of the corpus sterni and the ventral bending of the keel should appear not only in the explants of the complete sternum but also in cultures of a single sternal plate. From a study of normal embryonic development it might have been thought that the curvature of the corpus sterni was caused by the pressure of the growing viscera. This is disproved by the fact that it appears in vitro in the complete absence of the viscera, and regardless of whether the sternum is explanted with the dorsal or with the ventral surface uppermost.
On morphological evidence alone, it seemed likely that the formation of the keel was related to the growth and development of the pectoral muscles with which it is so intimately associated, and, as indicated in Part I, the writer herself at first inclined to this opinion. Experiment showed, however, that the keel can develop in the absence of these muscles, but this result did not preclude the possible influence of other extrinsic mechanical factors. Thus, as suggested in Part I, the continued expansion of the sternal plates after they had met in the mid-line might easily explain the right- angled bending of the growing edges to form a keel in spite of the absence of the pectoral muscles. This view also became untenable when it was found that each sternal plate could independently form a half-keel. As already stated, there was usually more connective tissue on the dorsal than on the ventral side of the explants. This was more conspicuous in the earlier experiments, and it was theoretically possible that it might somehow influence keel formation, though how it could do so was obscure. In later experiments, however, the original dissection was much cleaner, so that the difference between the two sides was less marked and sometimes very slight. Keel formation, however, was unaffected.
Thus the keel, like the curvature of the corpus sterni, must develop in response to factors intrinsic in the sternal plates. How this is achieved, whether by increased cell division in certain areas, by orientation of the cells, or by greater deposition of inter- cellular substance in certain regions, is not known; but the problem is being investigated further.
Although keel formation is caused by intrinsic factors in the sternal plate, the subsequent development of the keel is readily affected by extrinsic, mechanical factors. Thus, the half-keel formed by an isolated single plate, though bent at nght angles to the corpus sterni in the normal way, acquired a second, abnormal bend which brought the ventral part of the keel to an oblique or even horizontal position. ‘The second bend was much sharper in those half-sterna which had been explanted with the ventral surface uppermost and in which the keel would have had to grow against gravity in order to maintain the vertical. Moreover, nearly all the half-sterna became distorted after the first 2-3 days in vitro, so that the curvature of the corpus sterni and the angle between the corpus and the keel tended to flatten and disappear. It may therefore be concluded that although the potency for keel formation is inherent in each half of the sternal rudiment, fusion of the plates and possibly also the presence of the pectoral muscles provide the optimal conditions for the expression of this potency.
The articular groove for the coracoid, as described above, does not continue to develop in vitro but rapidly disappears during cultivation. This result agrees with that of Niven (1933), who worked on the avian patella. She noted “‘the absence of the development of the concave articular structure for articulation with the condyles of the femur’’, and suggested that “‘the ‘negative’ surface thus possibly differs from a ‘positive’ surface like the head of the femur, in that it requires for its development the presence of the corresponding surface’’. The present results support Niven’s view.
It has been shown that although most of the chief anatomical processes concerned in the development of the sternum begin and progress some way during cultivation, they are never completed. Thus an explanted sternum at an advanced stage of histological differentiation retains a fairly early embryonic form; it is also much smaller than a normal sternum of the same age. A similar phenomenon was previously observed in the development of the leg skeleton in vitro (Fell and Landauer 1935), where the explanted bone rudiments “‘still had a short, stumpy appearance as com- pared with a normal limb at the same stage of development”’. It was concluded that ‘this effect. ..was due to the fact that the conditions zn vitro retarded chondrification. As the gross anatomical form of the long-bones is largely the result of differential growth, retardation of the growth rate also involved retardation of anatomical develop- ment, so that in vitro the limb skeleton chondrified at an abnormally early stage of anatomical differentiation.”” ‘This explanation almost certainly applies also to the subnormal anatomical development of the explanted sternum. From this it appears that the shape of the sternum depends partly on the relationship of growth rate to differentiation rate, and disturbances of this relationship produce abnormalities of form.
Summary (Part III)
- The ventro-lateral body wall, including both sternal plates, was denuded of skin and of the pectoral muscles and cultivated in vitro by the watch-glass method.
- During cultivation the sternal plates moved together and fused in the mid-line: a small keel and the posterior lateral processes appeared, and the corpus sterni developed its normal type of curvature.
- The coracoid articulation did not continue to develop im vitro but gradually disappeared.
- Growth of the explanted sternum was greatly subnormal.
- Histological differentiation zn vitro was much less retarded than growth, so that the sternum chondrified at a comparatively early stage of its anatomical development. As a result the explants failed to complete their anatomical development.
- Explants of half the ventro-lateral body wall containing only one sternal plate were cultivated. ’
- Each isolated sternal plate formed half the keel, a posterior lateral process and half the corpus sterni with the normal type of curvature.
- The distal part of the half-keel tended to become flattened on the clot.
- Explants of the ventral body wall between the sternal plates were made.
- Chondrogenic tissue was found to extend only a very short way below the chondrified part of the sternal plates.
Part IV. The Closure of the Sternal Plates
Introduction
Of the factors concerned in the growth and development of the thorax, four must now be discussed in their relation to the closure of the sternal plates. ‘These factors are:
- The expansion of the sternal plates.
- The elongation of the ribs.
- The shrinkage of the mid-ventral body wall.
- The movement of the dorso-lateral tissue of the thoracic wall.
Of these, some have already received consideration in Parts I and III (pp. 420, 425 and 443); others require additional experimental evidence before they can be correctly evaluated.
(1) The expansion of the sternal plates. It is obvious that if the plates grew more rapidly than the intervening ventral body wall they would eventually meet in the mid-line. This simple explanation of their union is unlikely, however, because, as already pointed out in Part I, it would not account for the rotation of the plates from an oblique to a vertical and finally to a horizontal position (cf. figs. 5-7, Plate 33 and figs. 8, 9, Plate 34). That union is not due primarily to the expansion of the plates was finally proved by the experiments described in Part III, in which the ventro-lateral body wall containing the sternal plates was grown zn vitro with the result that the plates moved to the mid-line and fused. Displacement of each plate as a whole was demonstrated by the fact that the distance between the outer (costal) margins diminished as well as that between the ventral margins; had union occurred by expansion alone, the latter distance only would have lessened.
(2) The elongation of the ribs. The ribs fuse with the sternal plates fairly early in development, and their growth keeps pace with the downward movement of the plates.
It was theoretically possible, as stated in Part I, that the elongating ribs might push the sternal plates towards the mid-line. This possibility, however, was eliminated by the experiments quoted above, since the sternal plates in explants of the ventro-lateral body wall moved to the mid-line in the complete absence of the ribs.
(3) The shrinkage of the mid-ventral body wall. ‘The mid-ventral body wall shrinks and degenerates throughout the length of the thorax, and the degeneration disappears shortly after the sternal plates have united.
‘T'wo stages in the degeneration of the ventral body wall are distinguishable (see Part I): (a) A diffuse degeneration in the median third of the original mesenchymatous body wall when the dorso-lateral tissue first begins to move downwards. Why the original body wall exhibits this extensive degeneration is not known. (6) Degeneration in a median tract when the two bands of flattened cells growing downwards from the dorso-lateral region meet in the mid-line. This tract rapidly narrows to form a sharply marked vertical band of necrosis. ‘The second period of degeneration persists until the sternal plates and pectoral muscles meet in the mid-line and is correlated with a very rapid shrinkage of the ventral body wall. Such a local contraction of the mid-ventral thoracic wall would cause the dorso-lateral tissue from either side to move towards the mid-line and would also tend to rotate it to a horizontal position. Thus the union of the plates could be explained on the hypothesis that the median shrinkage and degeneration occur spontaneously, and in some way pull the sheets of flattened cells and the sternal plates towards the mid-line. On this view the mid-ventral shrinkage would be the primary factor in the movement of the plates.
(4) The movement of the dorso-lateral tissue of the thoracic wall. The entire dorso-lateral tissue of the thoracic wall, including muscle, connective tissue and the sternal plates, moves towards the mid-ventral line, and there is histological evidence that this move- ment is due, at least in part, to an active migration of the less differentiated cells.
As described in Part I, the sternal plate and pectoral muscles of either side are continuous ventrally with a sheet of flattened amoeboid cells. These two sheets of tissue move downwards through the degenerating mesenchyme of the original ventral body wall and meet in the mid-line. The vertical median tract of degeneration men- tioned above then appears covered by a narrow strip of necrotic and much-folded ectoderm (fig. 3, Plate 33).
If there is no active cell migration it is very difficult to understand why, when once the sheets have fused, the ventral body wall separating the plates and pectoral muscles should continue to shrink and finally disappear, and why the contraction and degenera- tion in the sheet of flattened cells should become largely restricted to a narrow, mid- ventral band.
The view that there is an active streaming of cells from either side towards the mid-ventral line accords better with the known facts. Such a streaming of tissue would produce the median accumulation of cells described in Part I and, as previously stated, local degeneration might be expected to occur under such overcrowded con- ditions. It is necessary to assume, however, that the two streams of amoeboid cells pull the sternal plates, and probably the pectoral muscles also, with them towards the mid-line. Unless this happened, the two streams of flattened cells would not become rapidly shorter, which is the observed fact; they would only become increasingly scanty and diffuse, which would mean that the ventral body wall would not shrink | and the sternal plates would remain apart. The displacement of differentiated tissue by the amoeboid migration of less differentiated cells is a familiar feature of tissue cultures, as will be described later, and it would not be very surprising if a similar phenomenon should occur in normal development.
Whether it is considered that the union of the sternal plates is due to spontaneous median shrinkage and degeneration, or whether the alternative view is accepted that the plates are pulled towards the mid-line by an active streaming of cells from either side, either hypothesis rests on the two assumptions that there is a movement of undifferentiated tissue in the ventral body wall in a median direction, and that this movement does not depend on the presence of the differentiated lateral tissue (pectoral muscles and chondrified part of the sternal plates). On the first hypothesis the move- ment would be passive, due to the contraction of the degenerating tissue in the mid-ventral line, and on the second hypothesis it would be active, due to the amoeboid migration of the individual cells. The first set of experiments described below were designed to test the accuracy of these assumptions, and to find whether the un- differentiated tissue of the ventral body wall can move in a ventral direction in the absence of all or most of the differentiated lateral tissue.
The second hypothesis stated above involves a third assumption, viz. that the sternal plates are pulled in a ventral direction by the amoeboid migration of the adjacent undifferentiated cells. The second set of experiments was made in order to find whether the sternal plates can be displaced in this way.
Technique
Budgerigar embryos of 7—9 days’ incubation were used. ‘The explants were cultivated by the watch-glass method in medium composed either of four drops of plasma and four drops of embryo extract or of eight drops of plasma and four drops of embryo extract, the latter mixture being the better. Subcultivation, which always involves a periodic contraction of the explant, was impracticable in experiments in which the movements of structures and of vital marks had to be measured and compared, so that all observations were made on the undisturbed culture during the first 2 days of growth.
The explants were drawn with the aid of a camera lucida and sometimes photo- graphed at intervals during the culture period.
Tissue movements in the ventral body wall
Object of experiments. ‘To find whether the undifferentiated tissue in the ventral body wall can move towards the mid-line in the absence of all or most of the lateral differentiated tissue.
Material and methods. The ventral body wall was excised from nineteen 7—8-day embryos and explanted with the skin surface uppermost. The pectoral muscles were excluded from all the explants, the sternal plates also were completely excluded from two, and the remainder contained only the extreme ventral (unchondrified) margins of the plates. The rest of the dorso-lateral body wall was fixed in acetic Zenker’s solution and sectioned to determine the exact boundaries of the part removed for cultivation.
Six control cultures were made of the skin and subcutaneous tissue of the costal region, and were grown with the skin surface uppermost.
In order to compare tissue movements in the experimental and control explants, two or more patches of sterile gas black were laid one behind the other on either side of the explant a short distance within the margin. The patches of one side were more or less level with those of the opposite side, thus forming a series of paired marks (figs. 35, e, Plate 41). Some of the carbon particles of each patch floated off the tissue a few hours after explantation, when the medium began to liquefy. The remainder, however, were quickly incorporated with the tissue (figs. 35d, g, Plate 41) and were carried with it, so that gross tissue movements could be readily followed by observing the behaviour of the marks.
Both sets of cultures were maintained for 48 hr., after which they were fixed in acetic Zenker’s solution and sectioned. Ten of the explants were drawn immediately after being laid on the clot, again after an interval of 1—2 hr., to allow for the initial contraction to take place, and then on the first and second days of cultivation. The second drawing was used as the standard for comparison with the third and fourth. Nine cultures were photographed 1-2 hr. after explantation and again shortly before fixation.
Results. Although the explants of the ventral body wall greatly increased in circum- ference, the carbon marks moved towards the mid-line provided enough dorso-lateral tissue had been included (figs. 356, c, Plate 41). In the controls, on the other hand, the carbon marks moved apart or remained stationary (figs. 35¢, /, Plate 41).
In fifteen explants of the ventral body wall, sections of the corresponding dorso- lateral region showed that the extreme ventral edges of both sternal plates had been included (fig. 35a, Plate 41). The carbon patches in these cultures moved rapidly in a median direction, often reaching the mid-line; in one culture, only three marks could be studied, as the third failed to become attached to the tissue and floated away. The edges of the plates developed as narrow rods of cartilage which approached each other (fig. 35d, Plate 41), and sometimes came in contact. In one explant, one lateral cut just missed the ventral edge of one plate, but the edge of the opposite plate was included; the carbon marks moved inwards almost to the mid-line. Another explant contained only the posterior part of the ventral edge of one plate and was cut well below the sternal plate on the opposite side; both pairs of carbon marks at first moved inwards, but later, as the explant expanded, the anterior pair moved apart. In one explant, both cuts were made immediately below the margins of the plates, and the marks moved medially almost to the mid-line. In the remaining culture, both lateral cuts were well below the sternal plates; the marks at first approached one another slightly, then moved apart again with the further expansion of the tissue.
In the fifteen controls the carbon patches usually moved widely apart in a peripheral direction though a few pairs of marks remained almost stationary. In one culture one of the four carbon patches failed to adhere to the tissue, but the remaining three moved apart in the usual way. Carbon marks approached each other in two cultures only. In one of these exceptional explants two patches came in contact during the first day’s growth, owing to local contraction of the ectoderm due to damage caused during dissection, and later failed to separate again. In the other, two marks became in- corporated in a single cord of ectoderm which split off from the rest of the skin and contracted.
The histology of the cultures was studied by serial sections. As described in Part I, the sheet of flattened cells in the ventral body wall of the normal embryo is double, and consists of an inner layer connecting the ventral edges of the sternal plates, and an outer layer which is less compact and connects the two masses of pectoral muscle. This double structure was usually more conspicuous in the cultures than in the normal body wall. A median zone of degeneration occurred in the anterior part of the explant only, where the flattened cells formed a stout, continuous sheet across the mid-line (fig. 35d, Plate 41), but even here the zone was abnormally broad and diffuse. Farther back the sheet of flattened cells gradually lost its membranous structure as it approached the mid-line, and there was no sign of a definite median tract of degeneration; necrotic cells occurred at random among the scattered flattened cells. As in the normal embryo, superficial to the sheet of flattened cells was a fairly thick layer of reticular connective tissue derived from the original ventral body wall; this was covered by ectoderm. It was interesting that this layer always extended laterally far beyond the outer margins of the sheet of flattened cells (fig. 35d, Plate 41) ; the latter was usually bounded externally by two thin rods of cartilage—the edges of the sternal plates. Thus the enlargement of the explants during life was due to the peripheral expansion of the superficial tissue only.
In section, the control cultures (fig. 35g, Plate 41) were seen to consist of loose reticular tissue similar to the superficial layer of the body-wall explants with sometimes a little skeletal muscle.
In both the experimental and control cultures some of the carbon particles were embedded in the ectoderm, whilst others had sunk into the underlying tissue.
The above results showed clearly that in the ventral body wall there is a general movement of tissue in a median direction.
This was demonstrated by the paired carbon marks which moved towards the mid-line in the explants of the ventral body wall but towards the periphery in the control cultures. That this movement also involved the ectoderm and superficial connective tissue was shown by the fact that all the marks in the ventral body-wall cultures moved inwards even when they were embedded in the skin and adjacent tissue, and not only those incorporated in the sheet of flattened cells. Whether the superficial tissue moved inwards of its own accord or was carried along by the under- lying sheet of flattened cells is uncertain. Probably, however, the latter is true, because at the periphery, where it was not under the influence of the layer of flattened cells, the superficial tissue spread outwards over the medium like an ordinary tissue culture, so that it finally extended far beyond the outer margins of the inward-moving sheet of flattened cells.
Although the tissue moved in a median direction in the explanted ventral body wall, the movement was usually neither so rapid nor so extensive as in normal development. Thus the ventral edges of the sternal plates, represented in the cultures by two rods of cartilage, did not always come in contact, and the accumulation and degeneration of cells near the mid-line, so conspicuous in the normal body wall, only occurred in the anterior part of the cultures. This was probably due largely to the outward migration of the superficial tissue at the periphery of the explant, which would tend to counteract the inward movement of the sheet of flattened cells. The results indicated that a certain minimal quantity of the dorso-lateral tissue must be included in the explant, as other- wise movement towards the mid-line is completely cancelled by the outward movement of the peripheral tissue.
Conclusions.
- There is a general movement of undifferentiated tissue in the ventral body wall in a median direction.
- This movement does not depend on the presence of the lateral, differentiated tissue (pectoral muscles and chondrified part of the sternal plates).
Cell migration in relation to the movement of the sternal plates
Object of experiments. To find whether the sternal plates can be displaced by the amoeboid migration of adjacent cells when shrinkage of the ventral body wall is eliminated.
In order to differentiate between traction of the plates by the spontaneous shrinkage of the mid-ventral body wall and traction by the amoeboid migration of neighbouring cells, the ventro-lateral body wall was cut in half and the two halves were explanted with their dorsal (costal) margins facing inwards and their ventral margins facing outwards. If the plates are normally displaced by a stream of cells migrating in a ventral direction, they should move apart when orientated with their ventral margins outwards.
Material and methods. The ventro-lateral body wall, divested as far as possible of skin and muscle in the usual way, was removed from a number of 7—8-day embryos and cut in half along the mid-line. The two halves were explanted on the clot ventral surface upwards, with the ventral edges facing outwards (fig. 34a, Plate 40). In some cultures the ribs were removed completely, in others stumps of varying length were left attached to the plates. The amount of soft tissue left on the dorsal margins was also deliberately varied, so that in some pairs of plates it was nearly all removed whilst in others much was left. Thirty-one explants were made; in five of these, observations were discontinued after 24 hr. and in the rest after 2 days.
As controls to these experiments, the ventro-lateral body wall was prepared as before and cut in half longitudinally, but the two halves were orientated with their ventral surfaces facing each other in the normal way. Seven of these pairs were arranged so that the dorsal surface of one half was upwards and the ventral surface of the other downwards, the front-end of one sternal plate thus being level with the hind-end of the other. Two of these were observed for 24 hr., the rest for 2 days. Twenty pairs had the usual orientation, both plates being placed with the dorsal surface upwards (fig. 34¢c, Plate 40). One of these was studied for 24 hr., the rest for 2 days.
Most of the explants were fixed in acetic alcohol and mounted whole but some were fixed in acetic Zenker’s solution and sectioned.
Results. Twenty-nine of the thirty-one pairs of plates orientated back to back moved apart (figs. 34a, b, Plate 40), two remained stationary, but none moved together. Of the seven controls arranged with the ventral edges inwards, but with one half dorsal side upwards and the other half ventral side upwards, in two the plates moved towards each other and partially united, in one the plates approached but did not fuse, in two they remained stationary and in two they moved apart.
Of the twenty pairs orientated in the normal way with the median edges facing inwards and the ventral surface upwards in both, eleven moved towards each other and united (figs. 34¢, d, Plate 40), seven approached each other but failed to unite and two remained stationary.
Thus the plates moved apart in 94°, of the experimental cultures and in only 7% of the controls, whilst they moved towards each other in 0°% of the experimental cultures and in 78°, of the controls.
In the halves placed back to back, migrating connective tissue cells rapidly bridged the gap between the plates, and after about 12 hr. growth had formed a continuous sheet of tissue connecting them. The plates moved apart fairly slowly during the first day and much more rapidly during the second day. They sometimes moved through a distance greater than the width of one plate. The distance through which they travelled showed no correlation with the amount of connective tissue left attached to their dorsal borders, indicating that the separation was not due merely to the growth of the intervening tissue. The tissue of the ventral body wall, peripherally situated in the cultures, spread out considerably on the clot, so that the diameter of the explant greatly increased. In one of the two explants where the plates remained stationary, two opposite rib stumps had fused, thus binding the plates to each other.
Fusion of the halves usually proceeded more slowly in the controls, where the ventral edges faced each other, owing perhaps to the fact that there is extensive degeneration in the mid-ventral region as described in Part I, so that the cells probably migrated less actively than those of the dorsal margins of the plates. When too much ectoderm was left on the median body wall, the halves sometimes failed to unite after 24 hr. or more and then became joined only by a very thin sheet of cells. In other cultures union was delayed or impaired by unusual softness and liquefaction of the clot, a condition which is always exaggerated by the presence of degenerate cells or of ectoderm. Such delayed or incomplete fusion occurred in all the six controls where the plates failed to approach each other. As in the experimental cultures, the explants as a whole expanded greatly.
In sections of the explants, the histological structure of the tissue extending from both the ventral and dorsal sides of the plates was seen to be essentially the same and consisted of loose, fibrillar, connective tissue.
Conclusion.
The sternal plates can be displaced by the amoeboid migration of neighbouring cells when shrinkage of the ventral body wall is eliminated.
Discussion
The first set of experiments recorded above has shown that there is a movement of undifferentiated tissue in the ventral body wall towards the mid-line, and that the movement does not depend on the presence of the undifferentiated lateral tissue. ‘The results provide no evidence, however, as to whether the movement is active, due to the amoeboid migration of the component cells, or passive, due to the spontaneous shrinkage of the mid-ventral body wall.
The second set of experiments demonstrated that the sternal plates can be displaced by cell migration, since they moved wide apart when explanted back to back. Under these conditions the possibility that the movement of the plates is due to spontaneous shrinkage of the mid-ventral body wall is eliminated. The obvious criticism of this result, viz. that the plates were forced apart by growth of the intervening tissue, 1s almost certainly unfounded, because, as stated above, the distance through which the plates moved was quite unrelated to the amount of tissue connecting them; the quantity of tissue left attached to the dorsal (costal) borders of the plates was purposely varied within wide limits to test this point.
There is nothing inherently improbable in the idea of differentiated tissue being displaced by the amoeboid migration of undifferentiated cells because, as already pointed out, this phenomenon occurs in most tissue cultures. Thus if a lump of embryonic skeletal muscle is grown in vitro, the undifferentiated cells rapidly wander into or over the medium, pulling the muscle fibres with them, until in a few days’ time the thick rounded mass of tissue has spread out into a wide thin sheet. Similarly in explants of the mesonephros, the tubules become largely uncoiled and dragged out with the zone of emigrating cells. Sheets of epithelium are often displaced by the amoeboid movement of the peripheral cells, and a small sheet may wander into the medium and become completely detached from the explant.
The results of the experiments described in Part [V have demonstrated the truth of two of the three assumptions upon which the second hypothesis was based, and have adduced strong evidence in support of the third. Thus they have shown (a) that there is a movement of undifferentiated tissue in the ventral body wall in a median direction, (b) that this movement does not depend on the presence of the differentiated lateral tissue, and (c) that under appropriate experimental conditions the sternal plates are pulled in a ventral direction by the amoeboid migration of the adjacent undifferentiated cells. It is probable, therefore, that the second hypothesis affords a correct explanation of the union of the plates, in which case the primary factor in causing union is the streaming of undifferentiated cells in a ventral direction, and the secondary factor is shrinkage and degeneration of the mid-ventral body wall.
Summary (Part IV)
- Results recorded in Parts I and III, which related to the closure of the sternal plates, were discussed ; these results showed that union is due neither to the expansion of the plates nor to the elongation of the ribs.
- The ventral body wall containing only the extreme ventral edges of the sternal plates was explanted in vitro, and carbon marks were placed on the skin just within either lateral border. The explants greatly increased in circumference, but the marks moved rapidly towards the mid-line. As controls, sheets of skin and subepidermal tissue from the costal region were explanted and marked with carbon patches in the same way; the marks moved wide apart.
- These experiments showed (a) that there is a general movement of tissue in a median direction in the ventral body wall, and (b) that this movement does not depend on the presence of the differentiated lateral tissue (pectoral muscles and chondrified part of the sternal plate).
- The ventro-lateral body wall was cut in half longitudinally and the two halves were arranged with their dorsal (costal) margins facing inwards and their ventral margins facing outwards. The sternal plates became united dorsally by a sheet of connective tissue, but instead of approaching each other they moved apart. In controls in which the two halves of the ventro-lateral body wall were arranged with their ventral margins facing inwards in the normal way, the halves united and the plates moved together as usual.
- These results showed that the sternal plates can be displaced by the amoeboid migration of adjacent cells.
- In general it was concluded that the union of the sternal plates is probably due primarily to the active streaming of undifferentiated cells towards the mid-line, and secondarily to shrinkage and degeneration in the mid-ventral body wall.
Summary of General Conclusions
The origin of the sternum
1. The sternum is formed independently of the ribs and of the coracoid rudiment.
Evidence: the sternal rudiment can develop in vitro in the absence of both ribs and coracoid.,
2. The presumptive sternal tissue occupies a narrow strip of the lateral body wall, 4 somites in length, extending from the middle intersomitic septum of the wing-bud region to the third intersomitic septum behind the wing bud, and lying immediately ventral to the base of the bud.
Evidence: observations on the developmental potencies of different regions of the undifferentiated, lateral body wall when isolated and grown in vitro.
3. The sternum should probably be regarded as part of the appendicular skeleton.
Evidence: the close association of the presumptive sternal tissue with the wing bud.
The differentiation of the sternum
1. The anatomical development of the sternum is largely controlled by factors intrinsic in the sternal rudiment.
Evidence: (a) The posterior lateral processes appear in the isolated sternal rudiment during cultivation in vitro.
(b) As in normal development, the explanted sternal plates become slightly concave during cultivation although the viscera are absent.
(c) The keel forms in vitro in the absence of the pectoral muscles and a half-keel is formed by each sternal plate in the absence of the opposite plate.
2. Extrinsic factors may be important in providing the optimal conditions for the expression of potencies inherent in the rudiment.
Evidence: the half-keel which develops in vitro from a single isolated plate is not vertical, but acquires a second bend which brings the ventral part of the keel to an oblique or even horizontal position ; this suggests that union of the sternal plates, though not the cause of keel formation, may be necessary for the normal development of the keel.
3. The maintenance of the normal anatomical form probably depends partly on extrinsic factors.
Evidence: explanted sterna usually lose some of the normal structure they have developed during the early stages of cultivation and later become flattened and distorted.
4. The articular groove for the coracoid is not self-differentiating zn vitro.
Evidence: the rudiment of the groove rapidly flattens out in vitro.
5. The shape of the sternum depends partly on the relationship of growth rate to differentiation rate, and disturbances of this relationship produce abnormalities of form.
Evidence: (a) In vitro the growth rate of the sternum lags behind the normal much more than the differentiation rate, so that an explanted sternum at an advanced stage of histological differentiation is very much smaller thananormalsternum of thesame age.
(b) ‘The anatomical development of the sternum is also much retarded in vitro. The chief anatomical changes begin and progress to some extent, but they are never completed in culture. The explant thus retains permanently a fairly early embryonic form.
The closure of the sternal plates
1. Union of the sternal plates is not due primarily to their expansion.
Evidence: (a) Expansion alone would not cause the rotation of the plates from an oblique to a vertical and finally to a horizontal position, such as occurs in normal development.
(b) In explants of the ventro-lateral body wall, the sternal plates unite in the normal way and an actual displacement of the plates in a medial direction has been demon- strated.
2. Displacement of the plates is not due to the elongation of ribs fused to their dorsal margins.
Evidence: in explants of the ventro-lateral body wall, the plates move together in the complete absence of the rib rudiments.
3. The union of the sternal plates is probably due primarily to the active amoeboid migration of neighbouring undifferentiated cells towards the mid-line, and secondarily to shrinkage and degeneration in the mid-ventral body wall.
Evidence: (a) In normal development the entire dorso-lateral tissue of the thoracic wall, including muscle, connective tissue and the sternal plates, moves towards the mid-ventral line.
(b) The histological appearance suggests that this movement is due at least in part to an active migration of the less differentiated cells.
(c) In the normal embryo the mid-ventral body wall shrinks and degenerates throughout the length of the thorax; the degeneration disappears shortly after the sternal plates have united.
(d) When explants of the ventral body wall, including only the extreme ventral edges of the sternal plates, are marked with carbon patches and grown in vitro, the results show (1) that there is a general movement of tissue in a median direction, and (2) that this movement does not depend on the presence of the differentiated, lateral tissue (pectoral muscles and chondrified part of the sternal plates).
(e) Under appropriate experimental conditions the sternal plates can be widely displaced solely by the amoeboid migration of adjacent cells, shrinkage of the ventral body wall being eliminated.
References
Bruch, C. 1852 N. Denkschr. schweiz. Ges. Naturw. 12.
Fell and Canti 1934 Proc. Roy. Soc. B, 116, 316.
Fell, H. B. and Landauer, W. 1935 Proc. Roy. Soc. B, 118, 133.
Fell, H. B. and Robison, R. 1929 Biochem. J. 23, 767.
Gladstone, R. J. and Wakeley, C. P. 1932 J. Anat., Lond., 66, 508.
Goette, A. 1877 Arch. mikr. Anat. 14, 502.
Hamburger, V. 1938 J. exp. Zool. 77, 379.
Hoffmann, C. R. 1879 Niederl. Arch. Zool. 5, 31.
Hommes, J. H. 1921 “Over de ontwickkeling van de clavicula en het sternum van vogels en Zoogdieren.”’ Groningen,
Lillie, F. R. 1919 ‘‘The development of the chick.” Henry Holt and Co.
Lindsay, B. 1885 Proc. Zool. Soc. Lond. p. 684.
Murray, P. D. F. 1926 Proc. Linn. Soc. N.S.W. 2, 187.
Murray, P. D. F. and Selby, D. H. 1930 Arch. Entw Mech. Org. 122, 629.
Niven, J. S. F. 1933 Arch. EntwMech. Org. 128, 480.
Parker, W. K. 1868 Ray Society.
Paterson, A. M. 1903 ‘The human sternum.” Three lectures delivered at the Roy. Coll. Surg., Eng.
Rathke, H. 1838 Arch. Anat. Physiol., Lpz.
Warren, A. E. 1934 Amer. J. Anat. 54, 449.
Description of Plates
|
b.v. blood vessel ¢. coracoid ca. cartilage dc. degenerate cells ect. ectoderm ep.r. epidermal ridge g.b. gas black h. heart hu. humerus 1.f. intercellular fibres k. keel l.b.w. lateral body wall |
my. myoblasts pch. perichondrium p.m. pectoral muscles r. rib sc. scapula s.f.c. sheet of flattened cells st.d. sternal division of rib st.p. sternal plate v.d. vertebral division of rib v.e.k. ventral extension of keel w.b. wing bud |
Plate 33
Fic. 1. Transverse section of the thorax of a 64-day embryo (stage 1). The mesenchymatous sternal plate and first sternal rib of one side are seen. Note the sharp outline of the sternal division of the rib, which is not in contact with the sternal plate. (Zenker; safranin and picro- indigo-carmine. x 99 diameters.)
Fic. 2. Transverse section of the same embryo as that shown in fig. 1, showing extensive cell degeneration in the ventral body wall. ( x 648.)
Fic. 3. Transverse section of an 8-day embryo (stage 2) at the level of the sternal articulation of the coracoid, showing the mid-ventral skin. The median epidermis is very degenerate and the stratum corneum has become deeply folded to form a well-marked ridge. (Zenker; safranin and picro-indigo-carmine. x 648.)
Fic. 4. Transverse section of the same embryo as that shown in fig. 3. Part of one sternal plate is seen. The plate, most of which is chondrified, ends ventrally in a fringe of undifferentiated amoeboid cells. ( x 540.)
Fic. 5. Transverse section of the same embryo (stage 1) as that shown in figs. 1 and 2, at the level of the sternal articulation of the first sternal rib. The sternal plates are widely separated and their ventral margins are farther apart than their dorsal margins. (x 18.)
Fic. 6. Transverse section of a 7-day embryo (stage 2), at the level of the sternal articulation of the first sternal rib. The sternal plates, though still widely separated, are now vertical. They are connected ventrally by a sheet of flattened cells. (Zenker; safranin and picro-indigo-carmine. x 18.)
Fic. 7. ‘Transverse section of an 8-day embryo (stage 4), at the level of the sternal articulation of the first sternal rib. ‘The sternal plates have begun to draw together, so that the ventral margins are now closer to each other than the dorsal margins. (Zenker; safranin and picro-indigo-carmine. x 18.)
Plate 34
Fic. 8. Transverse section of a 9-day embryo (stage 6), at the level of the sternal articulation of the first sternal rib. The sternal plates have fused and the keel has begun to develop. Immediately below the keel is a mass of tissue apparently formed by the two cell streams from the pectoralis major muscles. (Zenker; safranin and picro-indigo-carmine. x 18.)
Fic. 9. Transverse section of an 11-day embryo (stage 8), at the level of the sternal articulation of the first sternal rib. The keel has chondrified and enlarged greatly. (Zenker; safranin and picro-indigo-carmine. x 18.)
Fic. 10. Transverse section of the same embryo (stage 2) as that shown in figs. 3 and 4, at the level of the extreme anterior ends of the sternal plates. A thick sheet of flattened cells unites the sternal plate and pectoral muscles of one side with those of the opposite side. Note the folded pericardium, thickened epidermis along the mid-ventral line, and the compressed appearance of the tissue between the plates. ( x 90.)
Fic. 11. Transverse section of the body wall of a 9-day embryo (stage 4), showing the anterior ends of the sternal plates connected ventrally by a thick sheet of flattened cells. The tissue between the plates is very dense and compressed and contains many degenerate cells. (Zenker; haema- toxylin and erythrosin. x 90.)
Fic. 12. Transverse section of a 9-day embryo (stage 5) at the level of the sternal articulation of the coracoid, showing the apparent downward and inward streaming of the perichondrial cells to form the keel. Below the keel is another condensation, probably derived from cells streaming inwards from the pectoral muscles. Note the thick intercellular fibres running vertically in the interior of the keel downwards to the epidermis. (Zenker; Wilder’s silver method, carmalum and light green. x 126.)
Plate 35
Fic. 13. Transverse section through the same embryo (stage 6) as that shown in fig. 8, at the level of the anterior end of the sternum. The tissue enclosed between the two halves of the keel is necrotic. A ventral extension of the keel is beginning to form, apparently by the downward and inward migration of perichondrial cells from the dorsal region. (Zenker; safranin and picro- indigo-carmine. x 90.)
Fic. 14. Transverse section through the keel of the same embryo as that shown in fig. 13. Note the necrotic tissue enclosed by the two halves of the keel. (Zenker; safranin and picro-indigo- carmine. x 342.)
Fic. 15. Transverse section of a 9-day embryo (stage 7), showing the anterior part of the keel. The necrotic tissue enclosed by the halves of the keel has disappeared and been replaced by amoeboid chondroblasts and lightly staining cartilage matrix. (Zenker; safranin and picro- indigo-carmine. x 126.)
Fic. 16. Part of the same section as that shown in fig. 15, showing the amoeboid chondroblasts in the interior of the keel. (Zenker; safranin and picro-indigo-carmine. x 342.)
Fic. 17. a. Transverse section of a 4-day (control) fowl embryo through the wing-bud region. One bud and the adjacent lateral body wall have been removed for explantation. (Zenker; haematoxylin and erythrosin. x31.) 5. The wing bud and lateral body wall removed from the embryo shown in fig. 17a, after 6 days’ cultivation (group 1, p. 428). Part of the wing skeleton, a fragment of the scapula, the coracoid and the sternal plate have developed during cultivation. Note the complete absence of ribs. (Acetic alcohol; dilute thionin. x 31.)
Plate 36
Fics. 18a-e. Photographs of five living explants obtained from the same 5-day budgerigar embryo and cultivated in vitro for 5 days (group 5, p. 429). ( x18.) a,e, the wing bud and lateral body wall from either side. Each culture has formed part of the wing skeleton, a coracoid and a sternal plate. 6, the perichordal region and the somites adjacent to explant a. An incomplete and rather distorted vertebral column has differentiated. c, the dorsal half of the somitic region from the same side as explants d and e. A rod of cartilage has developed showing tooth-like projections probably representing the vertebral arches. d, the ventral half of the somitic region adjacent to explant e. No cartilage has differentiated.
Fics. 19a, 6. Photographs of two living explants taken from opposite sides of the same 4-day fowl embryo and cultivated in vitro for 4 days (series 1, group 1, p. 432). ( x18.) a, the lateral body wall from the first to the fourth intersomitic septum behind the wing bud (region A). The posterior end of the sternal plate has been formed in vitro. 6, the lateral body wall from the fourth to the sixth intersomitic septum behind the wing bud (region B). No cartilage has appeared.
Fics. 20a-c. Photographs of three living explants from the same 4-day embryo, grown in vitro for 7 days (series 1, group 2, p. 433). ( x18.) a, the living wing bud and the lateral body wall from the first intersomitic septum in front of the bud to the first septum behind it (region C). The proximal part of the wing skeleton, part of the scapula, the coracoid and a large part of the sternal plate have developed. 6, a region of the lateral body wall from the same side as a, extending from the first to the fourth intersomitic septum behind the wing bud (region D). The posterior part of the sternal plate has differentiated. c, a region of the lateral body wall from the opposite side, extending from the third to the sixth intersomitic septum behind the wing bud (region E). No cartilage has formed.
Plate 37
Fics. 21a, 6. Photographs of two living explants from the same 4-day fowl embryo, cultivated in vitro for 8 days (series 2, group 1, p. 434). (x 18.) a, the lateral body wall from the first to the sixth intersomitic septum in front of the wing bud (region F). No cartilage has formed. 6, an L-shaped explant from the same side as a (region G). The long arm of the L was a narrow strip immediately dorsal and parallel to explant a and extending from the first to the sixth intersomitic septum in front of the bud; the short arm included the wing bud and the lateral body wall from the first intersomitic septum in front of the wing bud to the first septum behind it. The proximal part of the wing skeleton, the coracoid and a large part of the sternal plate have differentiated.
Fics. 22a, 6. Photographs of two living explants taken from the same side of a 3}-day fowl embryo and cultivated in vitro for 8 days (series 2, group 3, p. 435). ( x18.) The explants were similar to those of figs. 21a, b, except that the transverse boundary between them was at the level of the third intersomitic septum behind the front margin of the bud, i.e. two somites farther back than in the preceding pair of explants. a (region J), two small nodules representing the ventral end of the coracoid and part of the sternal plate have differentiated. 5 (region K), the explant has formed the proximal part of the wing skeleton, part of the scapula, the dorsal half of the coracoid and a large fragment of the sternal plate.
Fics. 23a, b. Photographs of two living explants taken from the same side of a 34-day fowl embryo and cultivated in vitro for 8 days (series 2, group 4, p. 436). (x18.) a, the lateral body wall immediately below the wing bud, extending from the first intersomitic septum in front of the wing bud to the first septum behind it (region L). The ventral end of the coracoid and a large fragment of the sternal plate have developed. b, the wing bud and ventral tip of the myotomes from the first intersomitic septum in front of the bud to the first behind it (region M). The proximal part of the wing skeleton and most of the coracoid have formed during cultivation.
Fics. 24a, b. Photographs of two living explants taken from the same side of a 34-day fowl embryo and grown in vitro for 8 days (series 2, group 6, p. 438). (x 18.) a, the lateral body wall im- mediately below the wing bud, extending from the first intersomitic septum in front of the anterior margin of the bud to the second intersomitic septum in front of the hind margin of the bud (region P). Part of the shaft of the coracoid and a small piece of the sternal plate have differen- tiated. 6, the entire wing bud together with a transverse strip of the lateral body wall extending from the second intersomitic septum in front of the hind margin of the bud to the first septum behind the bud (region Q). The explant has formed part of the wing skeleton, an incomplete coracoid and part of the sternal plate.
Fic. 25. a, transverse section of a 34-day (control) fowl embryo through the wing-bud region. On the left, part of the lateral body wall below the subepidermal condensation has been removed for explantation. (Zenker; haematoxylin and eosin. x31.) 6, transverse section of the lateral body wall of the same embryo showing the subepidermal condensation of mesoderm of the operated side. The cells are orientated at right angles to the ectoderm and the cut is below the condensation. (Zenker; haematoxylin and eosin. x 288.) c, photograph of the living explant taken from the embryo shown in fig. 25a (region R), after 6 days’ cultivation. No cartilage has appeared. ( x 18.)
Plate 38
Fic. 26. a, transverse section of a 34-day (control) fowl embryo through the wing-bud region. On the right, the lateral body wall has been removed slightly below the base of the wing bud and explanted in vitro. (Zenker; haematoxylin and eosin. x31.) 5, the explant taken from the embryo shown in fig. 26a, after 6 days’ cultivation (region T). The ventral end of the coracoid and a large piece of the sternal plate have developed. (Acetic alcohol; dilute thionin. x 18.)
Fics. 27a—-d. Photographs of a living explant of the ventro-lateral body wall from a 9-day budgerigar embryo (stage 3, Part I). The pectoral muscles and all but the proximal stumps of the ribs have been removed and the tissue has been laid on the clot with the ventral surface upwards. (x 22.) a, explant after 80 min. incubation, i.e. after the initial contraction of the tissue had taken place. The sternal plates are widely separated and their costal margins diverge posteriorly. Note the opaque, median band of degeneration in the ventral body wall. 4, the same after 24 hr. incubation. The plates are closer together and their costal margins are now parallel. c, same after 2 days’ incubation. The plates are now in contact anteriorly and the posterior lateral processes are clearly seen. Note the numerous degenerate cells floating in the medium. d, same after 6 days’ incubation. The costal margins of the plates now converge posteriorly and the plates are in contact for about two-thirds of their length. A keel has developed during cultivation. ¢, transverse section of the same explant after 6 days’ growth showing the keel which has developed in vitro. (Zenker; haematoxylin and chromatrop. x 40.)
Plate 39
Fic. 28. Transverse section of the sternal rudiment from an 8-day budgerigar embryo (stage 3, Part I) after 6 hr. in vitro. The first sign of keel formation is distinguishable as a ventral bending of the median edge of each sternal plate. A thick sheet of flattened cells connects the two plates and a vertical, median band of degeneration cuts right across the explant. (Zenker; haematoxylin and chromatrop. x 99.)
Fic. 29. Transverse section of the sternal rudiment from an 8-day budgerigar embryo (stage 2, Part I) after 24 hr. in vitro. Chondrogenesis is more advanced and the keel is better developed. (Zenker; haematoxylin and chromatrop. x 99.)
Fics. 30a,4. Transverse sections of the sternal rudiment from a 7-day budgerigar embryo (rather younger than stage 2, Part I) after 3 days’ cultivation. a, section through the anterior end. Only the median edges of the sternal plates have fused, forming a shallow arch in place of a sharply projecting keel. 5, section through the region of the coracoid articulation. The keel is now fully chondrified and at this level is almost circular in cross-section. Note the dense perichondrium on the ventral surface of the keel. (Zenker; haematoxylin and chromatrop. x 99.)
Fic. 31. Transverse section of an isolated sternal plate from an 8-day budgerigar embryo after 24 hr. cultivation (stage 3, Part I). The plate was explanted with its ventral surface uppermost. The developing keel has formed two right-angled bends (1 and 2) so that its proximal part is vertical and its distal part parallel to the clot. The corpus sterni has acquired an almost normal curvature (convex to the surface of the clot) during cultivation. (Zenker; haematoxylin and chromatrop. x 99.) 2
Plate 40
Fics. 32a, 6. Transverse sections of the sternal plates from an 8-day budgerigar embryo (stage 2, Part I). The ribs and pectoral muscles were removed and the ventro-lateral body wall was cut in half down the mid-line, one half (a) being fixed as a control, and the other (6) being explanted in vitro with its dorsal surface upwards. a, control plate fixed immediately after dissection. The plate is almost flat and has no keel. (Zenker; haematoxylin and chromatrop. x 99.) 6, explanted plate after 2 days’ cultivation. The normal curvature of the corpus sterni (concave to the clot) and a half-keel have developed in vitro. The keel projects from the corpus sterni much more sharply than in plates grown with the ventral surface upwards (cf. fig. 31). (Zenker; haematoxylin and chromatrop. x 99.)
Fic. 33. a, transverse section of the ventro-lateral body wall of an 8-day budgerigar embryo (stage 2, Part I). Half the ventral body wall, including the undifferentiated ventral margin of one sternal plate, has been removed for explantation. (Zenker; haematoxylin and chromatrop. x 32.) 6, the fragment of ventral body wall removed from the previous specimen after 4 days’ cultivation. The explant contains connective tissue, muscle and a thin rod of cartilage repre- senting the ventral margin of the sternal plate. (Zenker; haematoxylin and chromatrop. x 99.)
Fic. 34. Photographs of two living cultures of the ventro-lateral body wall from 8-day budgerigar embryos (stage 3, Part 1). ( 13.) a, the body wall has been cut in half down the mid-ventral line and the two halves arranged with the costal margins of the sternal plates facing each other. Photographed shortly after explantation. 6, same explant after 48 hr. growth. The costal margins are united by a sheet of connective tissue but the sternal plates have moved widely apart. c, a similar explant in which the body wall has been cut in half down the mid-ventral line, but the two halves have been arranged with the ventral margins of the plates facing each other in the usual way. Photographed at the beginning of cultivation. d, same explant after 48 hr. growth. The sternal plates have moved together and fused in the mid-line in the normal manner.
Plate 41
Fig. 35. a, transverse section through the ventro-lateral body wall of an 8-day budgerigar embryo (stage 2, Part I). The ventral body wall, including the ventral margins of the sternal plates, has been excised and explanted in vitro. (Zenker; haematoxylin and chromatrop. x51.) b, the ventral body wall removed from the embryo shown in fig. 35a, photographed 2 hr. after explantation. Four patches of gas black have been laid on the surface of the tissue. ( x 22.) c, same explant photographed after 48 hr. in vitro. Note that the four patches of gas black have moved almost to the mid-line. The dotted line indicates the level of the section shown in fig. 35d. (x 22.) d, section of the same explant, fixed after 2 days’ growth. Some of the particles of gas black are incorporated in the skin and some in the underlying connective tissue. The edges of the sternal plates are seen as two rods of cartilage connected by a thick sheet of flattened cells. Note that the margin of the explant extends laterally far beyond the cartilaginous rods. (Zenker; haematoxylin and chromatrop. x51.) e, explant of supra-costal tissue from the same embryo and grown in the same watch-glass as the preceding explant of ventral body wall. Four patches of gas black have been laid on the ectoderm. Photographed shortly after explantation. ( x 25.) J, same explant after 48 hr. in vitro. The carbon marks have not moved together. The dotted line indicates the position of the section shown in fig. 35g. (x 22.) g, section of the same explant fixed after 2 days’ cultivation. Note the carbon particles in the ectoderm and subjacent tissue. (Zenker; haematoxylin and chromatrop. x 51.)
Cite this page: Hill, M.A. (2024, April 28) Embryology Paper - The origin and developmental mechanics of the avian sternum. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Paper_-_The_origin_and_developmental_mechanics_of_the_avian_sternum
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G