Paper - The development of joints
| Embryology - 1 Mar 2026 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Haines RW. The development of joints. (1947) J. Anat. 81, 33-55.
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
The Development of Joints
By R. Wheeler Haines
Anatomical Department, St Thomas’s Hospital Medical School
Introduction
Since the classical work of Bernays (1878), Schulin (1879) and Hagen-Torn (1882), the general course of the development of joints has been well understood, and the work of Lewis (1902) and Bardeen (1905) has extended our knowledge by the description of carefully chosen series of human embryos. Many special studies bearing on the development of joints, some directly and some indirectly, have been published since, and the more important of these will be mentioned in the appropriate places in the text. But no general work based on an adequate series of preparations taken from the larger joints of man has appeared for many years, and it may be useful to present a simple account of the course of development, even if it involves the examination of stages already described and figured elsewhere. l ,
Recently, moreover, Walmsley (1940) and Whillis (1940) have given new accounts of the development of joints, somewhat at variance with those previously received. Walmsley found that the patella and femur, though originally developed quite separately, later became fused by their perichondria, and considered that this fusion played an essential part in the formation of the articular surfaces. Whillis found that in relatively late embryos, at stages long after the peripheral parts of the joint cavities had appeared, adjacent articular cartilages were joined together by a stratum of intervening cells, which eventually disappeared leaving the cartilages quite continuous with each other. Some of the specimens considered in this paper bear directly on the questions raised by these authors.
The series of embryos available covers the period from just before the first appearance of the joint rudiments at 10 mm. to the appearance of the cavities at 30-34 mm. with no serious gaps. Thereafter material is scanty, so that only a few remarks on later development can be offered and there is no discussion on the changing relationship of the articular surfaces and capsules in the late embryo and infant, a subject reviewed by -Schulin (1879), nor of the various mechanical theories put forward to account for the development of the shapes of the articular surfaces, fully discussed by Hesser (1926).
Interzones Of The Larger Joints, 11-14 mm
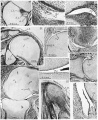
- All figures are shown on Plates 1-4, the figures being numbered consecutively.
The skeletal blastema appears in the fore-limb bud as a diffuse condensation of the mesenchyme at 7 mm., and is complete as far as the hand plate at 9 mm., while that of the hind-limb bud appears a little later, but is distinct by 9 mm. (reconstructions of Lewis, 1902, and Bardeen, 1905, embryos II, CLXIII). At 10 mm. (fig. 1) just before the first chondrifications become recognizable, the skeletal blastema is more clearly marked off from the surrounding tissues, but is still continuous in some places with the pre-muscle masses. The blastema of the upper arm is marked off from the extensor mass, but between it and the median nerve there is as yet no sign of differentiation of the intervening condensed mesenchyme. The blastema as a whole has the general form of the future skeleton (as shown in Lewis’s and Bardeen’s reconstructions), but though the general positions where the scapula and the long bones will appear later can already be distinguished, there is no sign of any morphological differentiation at these sites. The hind-limb of this embryo shows a similar structure, but the tissues are less well differentiated.
In a slightly older embryo (10.5 mm., fig. 2) the blastema is not so easy to distinguish from the surrounding tissues, for the muscle masses of the upper arm have become very dense and those of the lower arm ‘have now differentiated and are continuous with the blastema.) In the hind-limb of this embryo (fig. 3) the muscles are not yet differentiated in the distal segments, so the tibio-fibular part of the blastema and the foot plate stand out clearly from the neighbouring mesenchyme, while the femoral region is already obscured by the developing muscles, so that the structure resembles that of the fore-limb in the 10 mm. embryo. These specimens illustrate the late blastemal phase before the joints have appeared, but Murray (1926) and Fell & Canti (1934) have shown that in the chick at a similar stage the blastema is already determined as to the organs it is to form, though still morphologically undifferentiated.
The skeletal elements of the girdles and the larger bones appear as centres of chondrification in the interior of the blastema, one for each element at 11 mm. These were seen by Lewis (1902, embryo CIX), ‘who stated that the centres appeared at about the same time as the mesenchymal condensations for the main muscle masses of the limbs, but the two ‘embryos already described show that the muscle condensations precede the chondrifications. Each cartilaginous element is completely surrounded by blastemal tissues and is expanding by further chondrifications of these tissues, that is by appositional growth (Retterer, 1902). So the unchondrified blastema remaining between the cartilages gradually becomes thinned to form a series of plates, the interzones (Bruch, 1851, ‘Zwischenmasse’; Henke & Reyher, 1874, and Schulin, 1879, ‘Zwischenzone’; Whillis, 1940, and Walmsley, 1940, ‘articular discs’), and it is these plates that form the first morphological indication of the joints. ‘
By 12 mm. the cartilages at the shoulder and elbow (fig. 4) are already adopting their characteristic shapes and the interzones, though still relatively thick, are quite distinct, each formed by a mass of undifferentiated blastemal tissue between the cartilages. At the knee (fig. 5) the interzone is as yet indistinct, for the condylar region of the femur is still in the earliest (‘prochondral’) stage of chondrification, and the centre for the tibia is still very small. At the hip the pelvis is still represented by dense blastemal tissue continuous with that forming the upper end of the femur, and the interzone has not yet made its appearance. In Bardeen’s (1905) embryo CIX of 11 mm. chondrification of the hind-limb was more advanced than in my 12 mm. specimen or Petersen’s (1893) ‘S’ of 12-6 mm., for the centres for the pubis and ischium had already appeared. But no doubt there is great individual variation in development.
At 13 mm. the chondrification is more advanced and the skeletal elements have acquired some of their characteristic trochanters, condyles,. etc., while many of the muscles can now be identified individually and have been reconstructed (Lewis, 1902, embryo cLxxv). At the shoulder (fig. 7), elbow (fig. 8), hip and knee (fig. 10), each interzone is formed by a distinct plate of condensed tissue- whose cells pass without interruption into the cartilages on either side. Peripherally the interzone is continuous with the perichondrium of the skeletal elements, but while at the knee the perichondrium is demarcated from the neighbouring condensed tissues, particularly of the quadriceps tendon, by a layer of loose connective tissue, at the shoulder and hip the interzones, perichondria and condensed tissues are in unbroken continuity. Within the interzone there is no sign of differentiation; at the hip it is a ‘dense mass of sclero-blastema’ at 13 mm. (Lewis, 1902, embryo CLxxv), in other joints a ‘solid mass of mesenchyme’, and it remains for some time in this state while the other tissues of the joint develop round it.
At 13 mm. the interzones show little sign of the shape of the future articular surfaces. At the shoulder the head of the humerus is convex, but at the elbow, hip and knee the interzones are flat, and it would be difficult at this stage to say, for example, whether the upper ends of the femur and tibia were destined to carry convex or concave surfaces. But at 1.4 mm. the ends of the cartilages have assumed their characteristic shapes and the nature of the articular surfaces of the larger joints has become clear. The ulna, for instance (fig. 11, cp. fig. 8), has newly acquired olecranon and coronoid processes, and a curved interzone separates the convex trochlear surface of the humerus from the sigmoid notch of the ulna. The specimen corresponds closely to Lewis’s (1902) 14 mm. stage (embryo cLxIv).
The Carpal and Tarsail Regions
At 11 mm. (Lewis, 1902; Bardeen, 190,5; embryo CIX) and 124mm. (fig. 6), the carpal and tarsal regions are represented by masses of condensed blastema. At the wrist this is continuous proximally p_ with the radius and ulna, and distally with the metacarpals, and in this blastema the elements of the carpus are appearing as centres of more condensed tissue. At 13 mm. (figs. 9, 10) the carpus and tarsus have diverged in structure from the other parts of the skeleton, for each element is represented not by a chondrified centre in the interior of the continuous blastema, but by a separate condensation of the blastema itself, separated from the neighbouring elements by intervening sheets of rarefied tissue. By 14 mm. (fig. 12) the carpal and tarsal elements ‘are chondrified and are separated by dense interzones comparable in structure with those of the larger joints, and thereafter the course of development follows that of other regions.
The appearance of separate centres of condensation for the small bones of the carpus and tarsus is, of course, well known, and has often been figured, particularly in connexion with the study of the homologies of these elements, but the peculiar contrasts in the course of development of these and the long bones do not seem to have attracted attention. Probably they are of no morphological significance, but depend on the relatively late appearance of the cartilage in the carpal and tarsal elements.
Capsules and Synovial Mesenchyme, 16-19 mm
At 16 mm. the interzones of the larger joints (figs. 13, 14, 16) are still homogeneous and continuous with the perichondrium surrounding the cartilages. But on both flexor and extensor surfaces of the humero-radial joint curving strata of condensed tissue are seenvpassing between the articulating elements, bridging over the joint regions. There can be little doubt that these bulging sheets represent the fibrous capsules ‘and ligaments, though it is surprising to find them so early, for they have not been described in the human embryo at stages before 20 mm. (Bardeen,, 1905, embryo XXII). But this 16 mm. embryo is particularly well preserved, and the identification is supported by Hagen-Torn’s (1882) description of the capsules in rabbit embryos of 18-20 mm., and Carey’s (1922) figure of a pig of 19 mm.
The cells of the capsules are arranged longitudinally, while those of the perichondria near the joints have, as yet, no particular orientation, though towards the shafts of the cartilages the perichondria are already becoming differentiated in preparation for ossification. It will be convenient to speak, in describing this and later stages, of two parts of the perichondrium, the intra- and extra-capsular. Each interzone can then be said to be continuous at its periphery with the intra-capsular perichondrium, and this in turn to be continuous with the extra-capsular perichondrium.
Between the capsule and the extra-capsular perichondrium is a zone of less condensed tissue, as yet poorly developed, the synovial mesenchyme (of Luschka, 1858; vascular ‘mesochondrium’ of Hagen-Torn, 1882). This tissue will be concerned later with the formation of the synovial apparatus, including synovial and sub-synovial tissues, and in it intra-capsular structures such as ligaments will develop.
Anterior to the knee the quadriceps tendon is already developed at 13 mm. (fig. 10), and since this takes the place of a fibrous capsule, no separate capsule is developed here (fig. 16). At the shoulder (fig. 13) and hip the capsule cannot yet be distinguished from the dense tissues which will give rise to the fibro-cartilaginous labra and the tendons surrounding the_ joints. In the early stages of .skeletal development the vascular arrangements are simple, for the blastema itself is completely avascular, a point mentioned by Hagen-Torn (1882) and Retterer (1902), and illustrated in a 10 mm. rabbit embryo by Floderus (1915) in support of his contention that the blastema was of ectodermal origin. In the human 10 mm. embryo (fig. 1) vessels are seen passing between parts of the blastema which will later form the radius and ulna, but none ramify in the blastemal tissues themselves. Nor do they invade the early derivatives of the blastema, for the early cartilages, perichondria and interzones are quite free of blood vessels (cp. fig. 11), which come to form characteristic networks over the outer surfaces of the perichondria.
Here they are often difficult to distinguish, but in the well-fixed 16 mm. material they can be made out even in the monochromatic reproduction of fig. 13 as they cover the inner surface of the scapula, and they have been figured covering-the tibia of the 20 mm. pig by Carey (1922). No vessel enters the perichondrium itself until this layer alters in structure in preparation for the formation of periosteal bone. But small vessels do pierce the fibrous capsules of the joints and are seen in figs. 14 and 16, lying in the synovial mesenchyme on the inner side of the capsules, so that, as ‘Hagen-Torn (1882) has pointed out, at this stage the synovial mesenchyme (figured in a 32 mm. sheep embryo, his fig. 1) resembles the subcutaneous tissue in structure.
At 19 mm. the pattern of the interzone, synovial mesenchyme, capsule and perichondrium is more distinct (figs. 17 ,. 18, 19), and resembles that shown in Schulin-’s (1879, fig. 1) classical figure of an interphalangeal joint at 27' mm. This pattern is probably to be found at an appropriate stage in each joint, but it has seldom been figured (Hesser, 1926, figs. 2a, 3, shows it in the shoulder at 22 mm., and Whillis, 1940, fig. 1, in a toe at 30 mm.), and its significance has not been discussed. Where strong ligaments will later be associated with the capsule the structure of the embryonic tissue is peculiarly dense (fig. 19) but the ligaments and capsules cannot yet be distinguished from each other. All the interzones are still perfectly homogeneous dense structures composed of tightly packed rounded cells, as in Lewis’s (1902) 18 and 20 mm. embryos (XvII and XXII), and the cells are undergoing frequent mitotic division (Retterer, 1902, fig. Xv, 17' mm. guinea-pig). On their surfaces the interzones are chondrifying, so that at this stage they form important growth zones for the skeleton.
The Three-Layered Interzone and Intra-Capsular Differentiation, 21-30 mm
At 21 mm. the interzones of some of the joints have lost their homogeneous character. At the elbow (fig. 21) both humero-radial and humero-ulnar joints are distinctly three layered, with two dense chondrogeneous layers (‘chondrogenen Schichten’ of Bernays, 1878), destined to form the articular surfaces of the joint, separated by an intermediate loose layer. This intermediate layer of the interzone is continuous peripherally with the synovial mesenchyme, and the two are similar in structure, but the interzonal tissue is, and always remains, avascular. Its histological structure has been studied in detail and illustrated in a 30 mm. sheep embryo by Retterer (1902). j p j
The chondrogenous layers are continuous peripherally with the intracapsular perichondrium, and form with it a complete investment for the end of each skeletal element. These layers persist to a much later stage of development, and at this later stage are often spoken of as true perichondria, which are said to cover the articular surfaces in the embryo (e.g. Bardeen & Lewis, 1901, 30 mm. embryo CCXXII). There is no fundamental objection to such a usage at this stage of development for there is no histological distinction between the chondrogenous layers and the intra-capsular perichondrium, and no sharp topographical distinction can be made once the interzone has become three layered. But since at an earlier stage the interzone seems to be a separate organ, a conception supported by the experimental work of Fell & Canti (1934), it is convenient that its derivatives should be distinguished from neighbouring structures throughout development. Further, the statement that in the embryo the articular surfaces are covered by sheets of ‘perichondrium’ seems to have misled some workers into the belief that typical perichondria of adult type, provided with abundant coarse collagenous fibres and blood vessels, were to be looked for in embryonic joints. Such a mistake could hardly have arisen if Bernays’s conception of chondrogenous layers of the interzone had become established.
The shoulder joint at 21 mm. shows an early stage of the loosening of the middle layer of the interzone (fig. 20), reported by Simon (1923) at 18 mm., though the interzone is still homogeneous in Hesser’s 22 mm. embryo (1926) both at the shoulder (‘his fig. 3) and at the hip (his fig. 18). The capsule of the shoulder and the tendons nearby are now differentiated from the dense mesenchyme seen at an earlier stage, but the synovial mesenchyme is scanty. At the knee (fig. 22, andHesser, 1926, fig. 19, 22 mm.), dense interzonal tissue still intervenes between each femoral condyle and the corresponding articular surface of the tibia. By 23 mm. the interzone of the shoulder (fig. 23) has attained the typical three-layered structure and the fibro-cartilaginous labrum is now conspicuous. This latter is continuous centrally with the chondrogenous layer of the interzone which covers the glenoid surface of the scapula and peripherally with the perichondrium of the neck of the scapula, and would appear to be developed from the interzonal tissue. The continuity with the interzone is very clear in a 24 mm. embryo (fig. 24), in which the staining is unusually sharp. At the hip and knee the interzones are still dense and homogeneous.
At 26 mm. the interzones of the knee are in the earlythree-layered stage (fig. 25, and Langer, 1929, 26 mm. embryo), and the menisci are indicated as faint independent condensations in the synovial mesenchyme.
At 29 mm., just before the joint cavities appear, the distinction of the layers of the interzone becomes sharper (fig. 29). In the intermediatelayer the cells become flattened and lie with their surfaces parallel to the surfaces of the interzone, and at the same time the matrix of this tissue becomes clearer in preparation for liquefaction. At the shoulder (fig. 28), whose development at this stage is a little in advance of that of the other joints, the synovial mesenchyme on either side of the joint is actually breaking down and small cavities are already formed. Towards the centre of this joint the interzonal tissues present a peculiar appearance, for the intermediate layer of the interzone is represented not by a loose tissue, but by a dense layer in which the flattened cells are close-packed and often aggregated into clumps. If these cells were absent it would seem as if the interzone had reverted from a threelayered to a homogeneous structure, yet it is improbable that such very different structures as those shown in figs. 28 and 29 should co-exist in the same preparation. It is more likely that the appearances at the shoulder joint are artifacts due to the compression of the soft intermediate layer of the interzone by the expansion of the cartilages during the preparation of the material, and a pressure fold is actually seen crossing the neck of the scapula near the joint. A similar contrast’ in structure has been figured without comment in the humero-ulnar and humero-radial joints at 27 mm. by Hesser (1926, figs. 7a, b) and here also the pressure folds are well developed in the sections figured.
By 29 mm. all the larger joints have reached the stage in which the interzone is three layered. ,At the shoulder this was attained at 23 mm. (fig. 23) and at the elbow at 21 mm. (fig. 21), and Hesser (1926) found it at the elbow at 25 mm. (his fig. 6) and _27 mm. (his fig. 7). The interzones of the wrist and most of the intercarpal joints are three layered at 25 mm. (Hesser, 1926, fig. 10), and all the interzones of the fingers by 27 mm. (Hesser, 1926 ; cp. my fig. 20 and Floderus, 1915, fig. 35). Those of the toes are still homogeneous at 30 mm. (Floderus, 1915, fig. 22; Whillis, 1940, fig. 1), but three layered at 32 mm. (Hesser, 1926, fig. 32). The hip is three layered at 30 mm. (Hesser, 1926, fig. 32; Bardeen, 1905, embryo CCXXVII), the knee at 26 mm. (Langer, 1929), the ankle and talocalcanean joints at 27 (mm. (Hesser, 1926, figs. 26, 30), and the smaller intertarsal joints, still homogeneous at 27 mm., are three layered at 32 mm. (Hesser’s fig. 27; cp. my fig. 42). The interzone between the temporary cartilage in the fibrocartilage of the wrist (possibly a primary element of the carpus, Thilenius, 1896 and Corner, 1898), and the styloid process of the ulna never passes beyond the homogeneous stage, which is still retained at 49 mm. (fig. 48). Hesser has described a three-layered stage in the intervertebral and costo-vertebral joints at 25 mm., and- it is reasonable to suppose that all synovial joints pass through this stage.
Yet, though Bernays (1878) described the three-layered structure in detail, he failed to illustrate it, and it has often passed unnoticed by later authors. Whillis (1940) ignored it altogether, and indeed his fig. 6, from a wrist at 30 mm., shows interzones which appear to be homogeneous-.4 But since Hesser’s (1926) 25 mm. and my 30 mm. (fig. 34) and 34 mm. embryos show the threelayered structure clearly there can be little doubt that the appearances illustrated by Whillis are due to artifacts and are of similar nature to those illustrated in my fig. 28. In the interphalangeal joints, too, Whillis did not find the three-layered stage at the time when it was presumably present (his fig. 2), though it certainly does occur in these joints (Hesser, 1926, fig. 32, and my fig. 37 ).
The intra-capsular structures are, so far as is known, all formed from condensations of the synovial mesenchyme (Bernays, 1878; Hagen-Torn, 1882),. At the shoulder the long head of the biceps can be distinguished as a condensation Within the fibrous capsule at 20 mm. (Simon, 1923) and 22 mm. (Neale, 1937), and the course of embryological development gives no suggestion of any migration of the tendon through the capsule, such as is believed, from anatomical evidence, to have occurred in the course of phylogeny. Similarly, the ligamentum teres of the hip is believed to have migrated from its reptilian position outside the joint, Where it functioned as a collateral ligament, to its mammalian position inside the joint cavity (Moser, 1893), yet in the rabbit it arises in situ as a condensation which can be recognized at 15 mm. (Schuster, 1880) and 18 mm. (Hagen-Torn, 1882), while in the human embryo it is more difficult to distinguish from other condensed tissues at early stages, but it has been figured in a vascular synovial mesenchyme at 22, 30 and 34 mm. (Schuster, 1880; Moser, 1893; Bardeen, 1905; cp. my fig. 38).
At the knee the intercondylar interval is filled with an abundant vascular synovial mesenchyme, and in this tissue the cruciate ligaments appear as poorly marked condensations, first visible at 20 mm. (Bardeen, 1905, embryo CCXXIX; Walmsley, 1940, fig. 1; cp. my fig. 26). The menisci appear as independent condensations separate from the femur, tibia and capsules (Langer, 1929, and my figs. 25, 39), not as_ secondary derivatives of the capsule, as suggested by Luschka (1855). The intra-capsular part of the tendon of the popliteus, studied in great detail by Moser (1892,unillustrated), appears in its definitive position in a loose mesenchymal tissue (fig. 41), and the general cavity of the knee joint gradually extends both above and below the lateral meniscus so as to surround the tendon in later development. The fibrocartilaginous disc of the wrist joint (Whillis, 1940, fig. 6, and my fig. 48), and the interosseous ligaments of the carpus (fig. 49) are, at the time of their formation, surrounded by an abundant loose vascular mesenchymal tissue, though later they are intimately related to the joint cavities.
The Joint Cavities and Synovial Tissues at 30 mm and Onwards
At 30 mm. (figs. 31, 32) the middle layer of the interzone and the inner portions of the synovial mesenchyme are softening, and in part already broken down to give the first joint cavities. The process of liquefaction was studied carefully by Retterer (1896) in material specially fixed for the purpose from the bursae mucosae, where the preparations were not complicated by the presence ' of cartilage or bone, and later (1902) he confirmed his conclusions by the study of typical synovial joints. He found that the intercellular ground substance was liquefied so as to leave a cellular network composed of scattered strands containing irregularly disposed nuclei. Someaof the cells were eventually destroyed, but most became attached to one or other of the walls of the cavity, and persisted as its lining. This account is confirmed by special preparations of well-fixed material made by Dr Hughes (fig. 50), in which the synovial surface is ragged, and strands of tissue float out from the surface into the synovial cavity. Some of the cells in the interior of the cavity appear to be dying, and the synovial fluid contains cellular debris.
As the cavities develop the sections become more difficult to interpret. The humero-radial (fig. 32) and humero-ulnar (fig. 33) joints are taken from neighbouring sections of the same serial preparation, yet they look very different. Between the humerus and radius is loose liquefying tissue, and each articular surface is clothed by its own chondrogenous layer, while the humerus and ulna appear on the contrary to be joined by a homogeneous interzone in which the cells are compressed into a single dense’layer. But since at both earlier .(fig. 29) and later (fig. 36) stages a three-layered structure of the interzonal tissues may be observed at the humero-ulnar joint, there is little doubt that the type of appearance shown in fig. 33 is the result of an artifact caused by pressure between the articulating elements. Cartilages are notorious for their liability to expand and contract as they pass from one solution to another both. in the cellodion and paraflin techniques, and pressure folds and even overlapping of the articular surfaces are all too common in the sections. At this stage it is relatively easy, however, to decide what appearances are natural and what due to artifact, but in later stages the difficulties are enormously increased.
At 34 mm. the cavity of the humero-radial joint is well formed (fig. 35) but still contains a few cells in its interior, and a separate joint cavity is forming between the head of the radius and the annular ligament, but the humeroulnar joint (fig. 36) is less advanced,,with occasional isolated areas of liquefaction in the loosened tissue. At the hip (fig. 38) the cavity is A spreading round the head of the femur and the ligamentum teres lies in the synovial mesenchyme accompanied by conspicuous blood vessels which will later supply the cartilage canals of a large part of the head of the femur (Haines, 1933). At the knee the menisci are now sharply differentiated and joint cavities have developed between the anterior parts of the menisci and the femoral condyles (fig. 39 and Floderus, 1915, fig. 42). Reconstructions showing the precise distributions of these cavities have been made by Langer (1929).
The joint between the patella and femur calls for special comment. At 13 mm. (fig. 10) the quadriceps passes to the tibia as a band of dense tissue quite distinct from the femur, and separated from it by a layer of loose uncondensed tissue. At 20 mm. (Walmsley, 1940, fig. 1) the patella is differentiating as an aggregation of round cells in the quadriceps mass, and at 23 mm. it is more distinct (his figs. 2, 3), but the arrangement of the quadriceps tendon and its relation to the femur are unchanged. At 32 mm. (Walmsley, fig. 5; Hesser, 1926, fig. 22b) the patella is separated from the femur by very loose tissue but there are as yet no joint cavities separating them, and in a sheep embryo of 52 mm. total length, though the patella was not yet ossified, Kazzander (1894) found a similar tissue (his ‘ stratum intermedium’) between the femur and quadriceps tendon. So far there is no difliculty in the accounts, though the femoro-patellar joint differs from the more typical joints inthat no dense interzone was found at any stage, so that the loose tissue which separates the parts at 32 mm. has persisted as such from an early stage of development. But now both Kazzander and Walmsley describe a disappearance of the loose tissue between the patella and femur so that their perichondria become fused, a condition shown in Kazz_ander’s sheep embryo of 57 mm., and Walmsley’s human embryos of 35 and 38 mm., and this is interpreted by Walmsley as indicating a late secondary formation of an interzone (his ‘ articular disc’), similar to those of other joints, and he suggests that this structure plays an important "part in the definition of the articular surfaces. Yet in my 34 mm. preparation (fig. 40) there is no fusion whatever between the patella and femur; instead there is a broad layer of loose tissue in which a small but typical joint cavity has appeared, and Hesser’s (1926, fig. 25a) 40 mm. specimen has a similar structure but the joint cavity is larger. Walmsley’s fig. 4 would appear to have been taken from poorly fixed material, and the frequency with which artifacts have been seen to appear in developing joints wherever the dense articulating elements are separated by soft liquefying tissue makes it probable that Kazzander and Walmsley have been misled by similar artifacts due to compression of the loose tissues between the patella and femur.
Judging from my series the cavities of the larger joints may be said to appear at or soon after the onset of periostial ossification in the long bones, an estimate supported by other workers. Black (1934) found a small split of the shoulder at 32-5 mm., Kessel (1927) found the iliopsoas bursa present but the cavity of the hip joint absent at 28 mm., and Moser (1893) found a cavity absent at 30 mm. but present at 34 mm. At the knee Langer (1929) found no cavities at 28 mm., Bardeen (1905) and Floderus (1915) early cavities at 30 mm., and Langer several cavities at 37 mm. In the smaller joints the cavities appear later. At 34 mm. the ankle and intertarsal joints still have typical threelayered interzones though the synovial tendon sheaths have already appeared (fig. 42), and Hesser (1926) found a similar structure at 40 mm.
Dehiscence of the Articular Surfaces
There is no particular difficulty in the determination of the time of the first appearance of the joint cavities in the synovial mesenchyme but as regards the time of final separation of the cartilages there are very wide discrepancies between the observations of various authors, and no precise estimates can be abstracted from the literature. This state-of affairs is due to the difficulties of interpretation of preparations from joints, and these must now be considered in some detail.
The humero-radial joint has been followed through stages with a homogeneous interzone (figs. 4, 14) and a three-layered interzone (fig. 21), through the stage of early liquefaction (fig. 32) to the stage of full separation at 34 mm. (fig. 35), when the cavity is clear but for a few scattered strands of cells, and other preparations examined in the course of this investigation agree well with those illustrated. But at 45 mm. (fig. 43), though the joint cavities are perfectly distinct peripherally, the more central parts of the articular,surfaces of the humerus and radius appear to be joined by cartilage. The general preservation of the specimen is good, and there is nothing to suggest at first sight the presence of a major artifact. Yet a high-powered View (fig. 44) shows that near the region of contact each articular surface is covered by a thin, deeply staining, fibrillar layer, similar to that illustrated in Hammar’s (1894) classical figure of articular cartilage from a newly born subject, and lying in and below the fibrillar layer are flattened cells, contrasting with the more rounded cells deeper in the cartilage. Where the cartilages meet, the fibrillar layers fuse to form a single sheet which can be found throughout the area of apparent fusion, and on either side of this sheet are flattened cells. Some of these cells appear to be degenerating, and small masses, probably debris derived from such cells, are found‘ in and near the fibrillar layer. The curvatures of the articular surfaces are irregular, for the surfaces suddenly bend towards each other just before they meet and then pass into a smooth curve over the region of contiguity.
Comparing this arrangement with that seen earlier it would appear that the chondrogenous layers of the interzone, already inconspicuous at 34 mm., have now become completely chondrified, so that their identity is lost, and that the cuticular layers and flattened cells are the remnant of the intermediate loose layer. The changes of curvature in the articular surfaces suggest that during the preparation ‘of the sections the cartilages, originally separate, swelled so as to press against each other, and in the more central regions of the joints became stuck together, and that later the cartilages shrank away from each other except where they were attached. On the other hand the peculiar shapes might be due to a primary continuity partially broken by tearing due to shrinkage.
Hultkrantz (1897) referred to the difficulties met with in the interpretation of joint sections. He said that a priori it would seem that there should be no ‘difficulty in determining the presence or absence of a joint cavity, but when in a single serial preparation the cavity appeared open in one section and closed in a neighbouring section it was difficult to decide whether there had been a secondary fusion of the cartilages or a tearing apart of originally continuous structures. This kind of variation is illustrated in the 45 mm. embryo. In the third metacarpo-phalangeal joint, for instance (fig. 46), I the cartilages are apparently united, in the fifth (fig. 47 ) from the same series of sections they are separate, yet it is most unlikely that the fifth digit would really be in advance of the third in its development. Material from more advanced embryos presents similar difficulties.
Other authors have accepted the apparent absence of a cavity at its face value. Schulin (187 9) described the cartilages of the elbow at 70 mm. and Floderus (1915) those of a metacarpal-phalangeal joint at 50 mm. as still united, and Whillis (1940) speaks of ‘complete cartilaginous union at later stages. ‘There can, however, be no doubt that articular surfaces can become adherent in sections prepared with ordinary care. Dr Richardson’s stock series at University College from the joint of a kitten that had been running about for some days, though very well fixed and prepared, shows. numerous local adhesions, and my own preparations from adult material of agile South African lizards show similar appearances, which must be artifacts. A dissection of the joints of the 45 mm. embryo with the aid of a dissecting microscope showed the cartilages of all the large joints completely free from each other, and this was confirmed by examination of older embryos. Though some doubt may remain, it is consistent with the evidence to believe that the intermediate layer of the interzone is always liquefied soon after the synovial mesenchyme, so that dehiscence is complete by the 34 mm. stage in the shoulder and humero-radial joints (figs. 31, 35), by 44 mm. at the knee (Hesser, 1926, fig. 25a; cp. my fig. 45) and probably at the hip (open in my .45 mm. embryo), by 45 mm. at the metacarpo-phalangeal joints (fig. 47) and ankle joints (my 45 mm. embryo, not yet in Hesser’s 40 mm. embryo), while the wrist joints and carpal joints do not dehisce until after 50 mm. (fig. 49).
Completion of the Synovial Cavity
When the joint cavities are first formed they extend only a short distance into the synovial mesenchyme, and have none of the complex recesses they will develop later. In the humero-radial joint, for instance (figs. 32, 35), the cavity follows the articular surface of the. capitulum of the humerus, but does not yet include the head of the radius. The cavity between the annular ligament and head of the radius is formed separately, and is seen in the course of liquefaction at 34 mm. (fig. 35) and fully developed and continuous with the main synovial cavity of the extensor surface, of the joint at 45 mm. (fig. 43). With the extension of the cavity over the humerus and radius, parts of the synovial mesenchyme are demarcated as synovial folds (fig. 43; cp. Lucien, 1904, folds of the knee), which become progressively more conspicuous with the spread of the cavities (fig. 54; cp. Henke & Reyher, 1874, fig. 25, metacarpo , phalangeal joint), and are prominent features at birth (Grant, 1931 ; Santo, 1937 ).
At the knee the early cavities follow the condyles of the femur (Hesser, 1926, 40 mm. embryo and my fig, 45), not the tibia, so that at this stage the menisci are separate from the femur, but joined to the tibia by loose synovial mesenchyme, liquefying in my 45 mm. embryo, and destroyed in Floderus’s (1915) 50 mm. specimen. Though the menisci were recognizable aspdistinct condensations of the synovial mesenchyme before the synovial cavities appeared (fig. 25), their ‘final separation from the articular cartilages resembles closely that of the synovial folds of the humero-radial joint, an observation which supports Grant’s (1931) conclusion that the two structures are morphologically comparable with each other. The femoro-patellar cavity, first seen at 34 mm.
(fig. 40), was found continuous with the condylar cavities at 47 mm; by Langer (1929), and he and Moser (1892) have discussed the later expansion of these cavities and the inclusion of the ‘ supra patellar’ bursa as a part of the knee joint.
The Articular Surfaces
Hesser (1926) has collected together the various theories that have ascribed the shapes of the articular surfaces to the direct or indirect results of embryonic movements, and has rejected them on the grounds that the shapes are already developed at a stage when the interzones are still homogeneous, and little or no movement could occur. It is, however, possible that the appearance and later extensions of the ’ joint cavity may be associated with embryonic movement, for in the rat embryo angular movements of the elbow joint are seen at 13 mm., at a stage when the interzone is three layered (Blincoe, 1928). But the course of development of the malleolo-incudal joint, where movements must be small or absent before birth, appears quite similar to that of the other joints of the body (Urbantschitsch, 1880).
At the time of their first appearance the synovial cavities are formed partly in the tissues of the interzone and partly in the synovial mesenchyme (figs. 32, 35), so that the articular surfaces are composed centrally of the chondrogenous layers of the interzones, and peripherally of tissues of similar constitution which were originally part of the intra-capsular perichondrium. With_ the further spread of the synovial cavities (figs. 43, 54), a large proportion of the perichondrium becomes chondrified so as to form articular cartilages, and the greater parts of the articular surfaces of most joints are formed in this way. Near the margins of the articular surfaces there is a zone of transition between the articular surfaces and perichondrium (fig. 56), which progresses over the embryonic cartilages so long as the articular surface is spreading, but eventually becomes stationary to form the transition zone of the adult, described in detail by Key (1932).
The histological structure of the articular surfaces varies at different ages and in different joints. At the stage of liquefaction (fig. 32) the tissues forming the surfaces are not yet chondrified, so that the chondrogenous layers of the interzones are still recognizable as_such, and similarly, where the cavities are forming in the synovial mesenchyme the articular surfaces are still covered by perichondrium, as is the head of the radius where it articulates with the annular ligament (fig. 43). With full chondrification the remains of the liquefied tissues of the interzone or synovial mesenchyme come to form, as we have seen, a thin fibrillar layer overlying the cartilage, containing flattened cells some of them pyknotic or reduced to debris (fig. 44). Eventually these flattened cells disappear, so that now if the articular surfaces have come into contact in the course of preparation of the material there is no striking change in cell type as the structure of the cartilage is followed from one articulating element to another. This is the stage figured by Whillis (1940) in an interphalangeal joint at 125 mm. and in a newborn rat to illustrate union of the articular cartilages by ‘ primitive cartilage’. At earlier stages, ‘however, artifacts of this nature have been found to occur with some frequency, and the appearances in Whillis’s preparations (similar to those illustrated in my figs. 58, 59) were probably also due to such artifacts. Floderus (1915, figs. 29, 30) has illustrated complete joint cavities in the rat embryo at 18 mm., and the recognition that apparent union of the cartilages may be due to artifact solves the paradox that embryonic movements are well developed at a time when the cartilages may be apparently joined.
The fibrillar layers persist till birth (cp. fig. 55), at which time the mainvmass of the cartilage is still homogeneous in appearance. The development of the various layers of the adult articular cartilages (sliding, transitional, pressure and basal), described by Benninghoff (1925) and others, must occur later and has not yet been studied. ' In some joints the articular surface is formed notof hyaline but of fibrocartilage, and this distinction is quite evident in the embryo, e.g. the glenoid surface of the scapula is covered with a layer of fibro-cartilage at 66 mm. (fig. 51). The acromio—clavicular joint at this stage is largely filled by a mass of dense fibro-cartilaginous tissue, still attached to the clavicle, but separated from the acromion by a fully developed joint cavity. Later much of ‘this tissue will be separated from the clavicle to form the inter-articular disc, but some will be left to form the fibro-cartilaginous sheet covering the clavicle itself. The jaw joint is similar in its development, for here at 47 mm. and 65 mm. Hesser (1926) found a joint space below the disc, but none between theldisc and the temporal bone, and here again the articular surfaces are covered with fibro-cartilage (Luschka, 1858).
Synovial and Extra-Synovial Structures
The structure of the synovial surface of any particular region depends, as was pointed out by Langer (1929) and others, on the rate of spread of the synovial cavity, as well‘ as on the nature of the substratum on which the surface lies. At the time of the first appearance of the joint cavity the synovial surfaces are ragged (fig. 32), but as soon as active extension of the cavity slows down, even temporarily, the surface is smoothed off and is covered with a layer of synovial cells (figs. 35, 43, 45). By the 60-70 mm. stage most of the joints have attained the proportions they will have in the adult (fig. 51), and the The development of joints synovial surfaces are formed of one or two layers of typical synovial cells lying on the vascular substratum (fig. 52). In the smaller joints and in the recesses of the larger joints, however, there is still evidence of extension in this and later stages, for the dorsal recesses of the inter-phalangeal joints at 125 mm. (fig. 53), and the recess of the humero-radial joint which projects distally to the annular ligament at 66 mm., still show the typical ragged surfaces which indicate advancing liquefaction.
Where the synovial surface overlies dense structures such as ligaments and tendons without the intervention of loose connective tissue, the synovial tissues are poorly developed. The inner surface of the annular ligament, for instance, is separated from the head of the radius by a layer of loose synovial mesenchyme at 30 mm. (fig. 32), and when this liquefies its remnant forms a single layer of synovial cells covering the inner surface of the ligament at 34 mm.: (fig. 35) and 45 mm. (fig. 43), and similar cells can be seen covering the upper surface of the menisci at the knee (fig. 45), and the inner surfaces of the volar accessory and collateral ligaments of the digital joints (figs. 46, 47). This synovial stratum can still be distinguished at laterstages (fig. 56), but becomes progressively less differentiated, and in the adult can hardly‘ be distinguished (Petersen, 1930;, Key, 1932).
In the earlier stages the synovial cells lie directly on the loose synovial mesenchyme, but later there may be some condensation of the connective tissue to form a distinct tissue, the stratum sub-synovial (fig. 56). In most joints this is indistinct, but at the knee it is eventually well developed in the region of the intercondylar septum, and can be dissected away as a continuous sheet. It is, of course, formed much later than the fibrous capsule for it is barely visible at 45 mm., whereas the fibrous capsule appears at 16 mm. (fig. 16), and it does not in any way deserve the name of ‘ true’ capsule, given it by Higgins (1896).
The fibrous capsules and ligaments all appear as mesenchymal condensations (fig. 14, capsule of humero-radial joint; fig. 19, collateral ligaments of digital joints), Where tendons or ligaments are developed in close association with the capsule they are not at first (distinguishable from it, but later their histological structure becomes differentiated. In a humero-radial joint from a specially well fixed rat embryo of 20 days (fig. 57), the radial collateral ligament, passing, as it does in this animal, between the humerus and radius, already‘ shows the characteristic spiral twisting of its fibres, and its cells are cuboidal in shape and arranged in longitudinal rows between the straight fibre bundles of the ligament. The same kind of structure is seen in the tendons of origin of the muscles arising from the humerus, but the fibrous capsule of the joint has quite a different structure with small flattened cells, either scattered or arranged in short rows between the wavy fibre bundles. The sharp contrast in gross structure between ligaments and fibrous capsules has beendescribed in the adult (Haines, 1944); this specimen shows that the contrast is recognizable in the microscopic structure and is developed in embryonic life.
At the time of liquefaction the interzone is of about the same thickness throughout its extent, so that the articular surfaces, when they are first defined, are necessarily congruent (figs. 32, 33). Later the surfaces develop their characteristic incongruencies so that highly curved convexities always articulate with less curved concavities (figs. 51, 53, 54), and the area ‘of contact is relatively reduced, probably to satisfy the demands of lubrication (MacConaill, 1932). The gaps between the articular surfaces are filled partly by the synovial fluid and partly by the folds carved out from the synovial mesenchyme by the expansion of the synovial cavities. Later, in most joints, synovial villi are found (figs. 54, 55). Retterer (1902) considered that these villi were developed directly from the cells found on the rough synovial surfaces at the stage of liquefaction, but since at an intermediate stage the synovial surface is smoothed off this cannot be so, and they are presumably new ingrowths from the synovial tissues.‘ Bennett, Wainei & Bauer (1942) have shown that the villi continue to grow throughout life and Meyer (1922) that they appear in chronically inflamed synovial bursae,’ but very little is known of their development or significance.
The Embryological Approach to the Morphology of Joints
Baer (1887), working on the chick, made the fundamental observation that in general each element of the appendicular skeleton was originally laid down as a separate cartilage, and that the unchondrified tissue between the. elements formed the joints. Bruch (1851), who gave the first detailed account of the development of the joints themselves, described the formation of the synovial tissue and joint cavities and suggested that the fibrous capsule was a continuation of the perichondrium across the joint region. Schulin (187 9) confirmed this suggestion. His fig. 1, a section from an inter-phalangeal joint of a 26 mm. foetus, shows a structure similar to my fig. 19, with a homogeneous interzone, a fibrous capsule enclosing the synovial mesenchyme and intra- and extra-capsular perichondrium. All these structures he derived from the blastema, so that he considered the synovial mesenchyme as blastemal tissue loosened in preparation for the formation of the joint cavity. Lewis (1902) and Bardeen (1905) accepted Schulin’s suggestions withoutquestion and they are found in all modern text-books concerned with joint morphology. 1 Yet there are considerable difficulties in the way of accepting Schulin’s ideas. If the fibrous capsule really represented the perichondrium it ‘would’ be surprising to find another layer, the intra-capsular perichondrium, enclosed within it. The perichondrium near the larger oints is perfectly clear at 12 mm. (fig. 4), while the fibrous capsule is still absent at 14 mm., and when it does appear, by 16 mm. (fig. 16), its cells are arranged longitudinally while those of the perichondrium are rounded. Further, Hagen-Torn’s (1882) discovery that the synovial mesenchyme was vascular, a point confirmed by my figs. 14 and 16, led him to assert that it was a part of the general mesenchyme, cut off by the development of the joint capsule, and not a part of the avascular blastema, so that he saw in the fibrous capsule a completely extra-blastemal formation. Unfortunately his work was poorly illustrated and has never been discussed. My own work supports Hagen-Torn’s theory rather than Schulin’s, for where the development of the joints is uncomplicated by neighbouring structures it is clear that the capsules are new formations. The tissue filling the bend of the elbow and knee at 12 and 13 mm. is loose (figs. 4, 10), and in it the capsules appear at 16 mm. (figs. 14, 16). At 16 mm. the metacarpophalangeal joints are surrounded by loose tissue which fills the indentations between the two cartilages (fig. 15), and the capsules appear in this tissue (fig. 19). It is difficult to believe that in either of these cases the synovial mesenchyme can be blastemal in origin though in the case of the shoulder (fig. 20), when the synovial mesenchyme is less abundant, its origin cannot be determined with certainty.
It is possible that the simpler joints without synovial cavities or synovial apparatus, such as are found commonly in fishes (Haines, 1942) and have survived in the joints between the body and cornua of the mammalian hyoid, are blastemal in origin, but the more complex synovial joints, first developed in the jaw joints of gnathostomes, include derivatives from the surrounding general mesenchyme.
Summary
- The development of the joints of the limbs is described from the time of their first definition at 11 mm. to the time of the appearance of the synovial cavities of the larger joints at 30-34 mm., and some stages from later embryos are discussed.
- The joints first appear as interzones, which are formed from the remains of the skeletal blastema between the cartilages. The interzones form the more central parts of the articular cartilages and synovial cavities.
- Each interzone passes through a three-layered stage with two chondrogenous layers and an intermediate loose layer, and a stage where the intermediate layer breaks down and the chondrogenous layers become fully chondrifiedi.
- The development of the fibrous capsules as condensations in the extra blastemal tissue near the joints cuts off a part of the general mesenchyme to form the synoviallmesenchyme, and a part of the perichondrium to form_ the intra-capsular perichondrium.
- The synovial mesenchyme gives rise to the more central parts of the synovial cavities, the synovial and sub-synovial tissues and all intra-capsular structures including ligaments, tendons and fibro-cartilages.
- The intra-capsular perichondrium is partly transformed into the more peripheral parts of the articular cartilage, while the remainder persists throughout life.
- The interzones of the larger joints appear at 11-12 mm., their fibrous capsules at about 16‘ mm., the interzones become three layered. at 21=-26 mm., liquefaction at the synovial mesenchyme begins at 30-34 mm., and dehiscence of the articular cartilages is complete by the 40 mm. stage but is delayed at the wrist and ankle. A ‘
- When the synovial cavities are first formed the remnants of the liquefying tissue at first cover the articular surfaces of the cartilages, but soon are reduced to form narrow cuticular layers containing a few flattened cells, and before birth all the flattened cells disappear.
- At the stage of liquefaction, and wherever the joint cavities are spreading rapidly, the synovial surface is ragged, and strands of cells project into the synovial cells of the joint, but when extension is arrested, even temporarily, a smooth layer of synovial cells demarcates the cavity.
- The synovial folds are part of the synovial mesenchyme dissected out by the secondary extensions of the cavities as they spread over the cartilages . The villi are formed as secondary ingrowths of the synovial tissues.
- A revision of joint development supports the morphological conceptions of Hagen-Torn ‘rather than those of Bruch and Schulin.
I have to thank the workers who have so generously placed their material at my disposal: Profs. Appleton (‘H 19, 23, 27, 29, 30, 43, 49, 58, 66, 125, 190’ of C.R. lengths corresponding to their numbers) and Boyd (‘H 23’, 13 mm. ;. ‘H 8’, 14 mm.; ‘mx’, 24 mm.; ‘H 11 ’, 26 mm.), Dr Hughes (specially prepared 60 mm. human and 20-day rat embryos), Profs. Kirk (‘series 1’, 16 mm.; ‘ series 8 ’, 21 mm.), Lucas Keene (‘ 2387’, 10 mm.), Smout (‘ 5% weeks ’, 10-5 mm.; ‘6 weeks’, 12 mm.) and West (34 mm.).
References
BAER, K. E. v. (1837). Ueber Entwiclcelungsgeschichte der Thiere, II. Theil. Konigsbergz Borntrager.
BARDEEN, C. R. (1905). Amer. J. Anat. 4, 265.
BARDEEN, C. R. & LEWIS, W. H. (1901). Amer. J. Anat. 1, 1.
BENNETT, G. A., WAINE, H. & BAUER, W. (1942). Changes in the Knee Joint at various Ages.
New York: Commonwealth Fund.
BENNINGHOFF, A. (1925). Z. Zellforseh. 2, 783.
BEBNAYS, A. (1878). Morph. J. b. 4, 403.
BLACK, B. M. (1934). Anat. Rec. 60, 333.
BLINCOE, H. (1928). Anat. Rec. 40, 277.
BRUCE, C. (1851). Denlcschr. Schweiz. naturf. Ges. 12, 14.
CAREY, E. J. (1922). J. Morph. 37, 1.
CORNER, E. M. (1898). J. Anat., Lmzd., 32, 272.
FELL, H. B. & CANTI, R. G. (1934). Proc. Roy. Soc. B, 166, 316.
Fnonmms, B. (1915): Kgl. Swenslc Vetenslc. Aloud. Handl. N.F. 53, 416.
GRANT, J. C. B. (1931). Brit. J. Surg. 18, 636. _
HAGEN-TORN, O. (1882). Arch. milcr.-anat. 21, 591.
HAINES, R. W. (1933). J. Anat., Lorcd., 68, 45.
HAINES, R. W. (1942). J. Anat., Lond., 77, 12.
HAINES, R. W. (1944). J. Anat., Lond., 78, 44.
HAMMAR, J. A. (1894). Arch. milcr.-amat. 43, 266, 813.
HENKE, P. J. W. & REYHER, C. (1874). S.B. Alcad. Wise. Wien (Abt. 3), 70, 217.
HESSER, C. (1926). Morph. J. b. 55, 489.
HIGGINS, H. (1896). J. Anat., Lond., 30, 289.
HULTKRANTZ, J. W. (1897). Das Ellbogengelenlc und seine M echanilc. Jena: fischer.
KAzzANDER, G. (1894). Arch. Anat. Physiol., Lpz., p. 161.
KEssEL, F. (1927). Morph. Jb. 58, 413. .
KEY, J. A. (1932). ‘The synovial membranes of joints and bursae.’ In Cowdray, Special cytology, 2nd ed., vol. 2. New York: Hoeber.
LANGEB, M. (1929). Z. ges. Anat. 1. Z. Anat. EntwG'esch. 89, 83.
LEWIS, W. H. (1902). Amer. J. Anat. 1, 145.
LUCIEN, M. (1904). Bibliogr. anat.‘13, 133.
LUSCHKA, H. (1855). Arch. Anat. Physiol. wise. Med. p. 481.
LUSCIIKA, H. (1858). Die Helbegelenkedes menschlichen Korpers. Berlin.
MACCONAILL, M. A. (1932). J. Anat., Lond., 66, 210.
MEYER, A. W. (1922). J. Bone Jt Surg. 4, 491.
MOSER, E. (1892). Morph. Arb. 1, 147.
MOSER, E. (1893). Morph. Arb. 2, 36.
MURRAY, P. D.-F. (1926). Proc. Linn. Soc. N .S.W. 51, 187.
NEALE, R. M. (1937). Anat. Rec. 67, 205.
PETERSEN, H. (1893). Arch. Anat. Physiol., Lpz., p. 67.
PETERSEN, H. (1930). ‘Die Organe des Skelettsystems.’ In v. Mollendorfl‘, Handb. milcr.-anat. Mensch. Berlin: Springer.
RETTERER, E. (1896). J. Anat., Paris, 32, 256.
RETTERER, E. (1902). J. Anat., Paris, 38, 473, 580.
SANTO, E. (1937). Anat. Anz. 85, 223.
SCHULIN, K. (1879). Arch. Anat. Physiol., Lpz. (Anat. Abt.), p. 240.
SOHUSTER, H. (1880). Mitt. embryol. Inst. Wien 1, 199.
SIMON, S. ('l923). S.B. Aloud. Wiss. Wien (Abt. 3), 130/31, 61.
THILENIUS, G. (1896). Morph. Arb. 5, 1.
UBBANTSOHITSCH, V. (1880). Mitt. embryol. Inst. Wien, 1, 229.
WALMSLEY, R. (1940). J. Anat., Lond., 74, 360.
WHILLIS, J. (1940). J. Anat., Lond., 74, 277.
Explanation of Plates
Plate 1
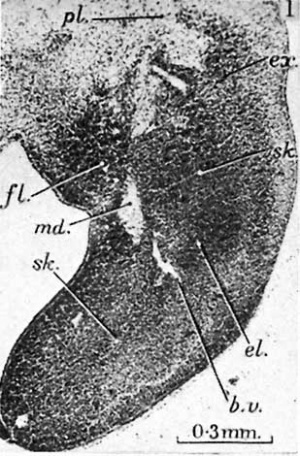
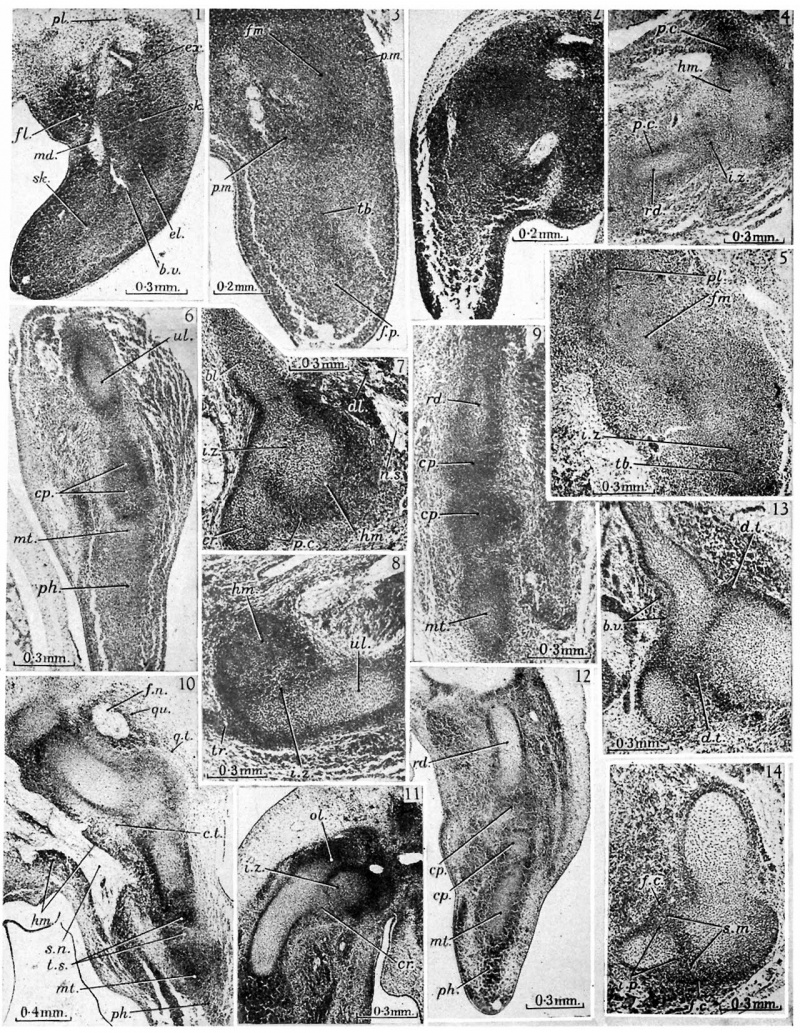
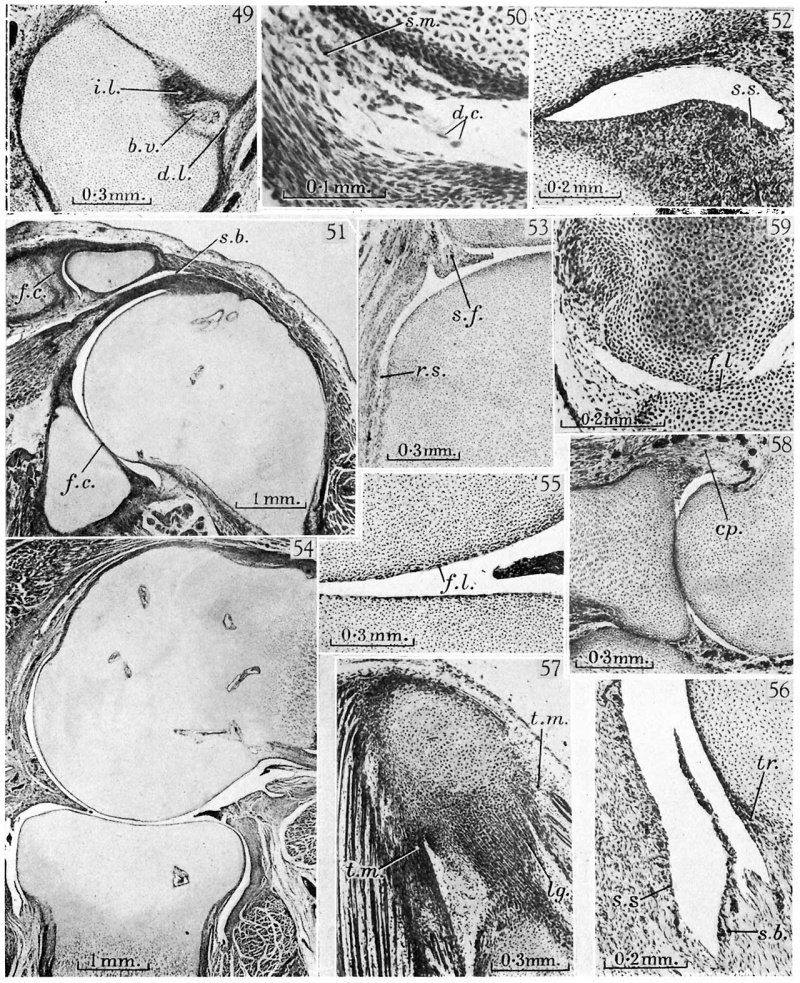
fig. 1. 10 mm. Lucas Keene’s ‘2387’, 50.4. Fore-limb. The skeletal blastema (sIc.) shows clearly in the humeral, elbow (el.) and fore-arm regions, while distally it fades away towards the marginal vein. The pre-muscle masses of the upper arm on both flexor (fl.) and extensor (e:c.) aspects, the brachial plexus ( pl.) and median nerve (md.) are visible. A blood vessel of the interosseous group (b.v.) pierces the blastema. V
fig. 2. [10-5 mm. Smout’s 5} weeks embryo, 11.1.4. Fore-limb. The pre-muscle masses of the lower arm have now differentiated, and the boundaries of the skeletal blastema are less easy to follow, as the condensations for the skeleton and the muscles now form a continuous mass.
fig. 3. 10-5 mm. Smout’s 5} weeks embryo, 16.1.4. Hind-limb. The skeletal blastema of the femoral (fm.), tibial (tb.) and foot-plate (f.p.) regions are distinguishable. The pre-muscle masses of the thigh (p.m.) are more condensed than the general mesenchyme, but those for the more distal part of the limbs have not yet appeared.
fig. 4. 12 mm. Smout’s 6 weeks embryo, 31.3.5. \Humero-radial joint. The lower end of the cartilaginous humerus (hm.) is assuming its characteristic shape and the shaft of the radius (rd.) is well developed, but the ends are not yet chondrified. A thick interzone (i.z.) separates the two chondrifications and gives the first indication of joint formation. Peripherally the interzone is continuous with the dense mesenchyme surrounding the cartilages which now forms their perichondria (p.c.). Distally the blastema of the radius is quite continuous with that of the carpal region and with the condensed tissue which will form the muscular and tendinous apparatus. ,
5. 12 mm. Smout’s 6 weeks embryo, 49.2.5. Thigh. The shaft of the femur (fm.) is well developed, but that of the tibia (tb.) is barely recognizable as a small centre of chondrification. The knee appears as a thick interzone (i.z.) but at the hip the femoral tissues are continued without interruption into the dense blastema of the pelvis (pl.).
6. 12 mm. Smout’s 6'weeks embryo, 33.1.5. Carpal region. The shaft of the ulna (ul.) is well formed and distinct from the muscles on each side, and the carpal region is formed by a dense mass of blastema in which the carpal elements are appearing as regions of greater density (cp.). The metacarpal region shows early centres of chondrification (mt.), but passes without any break into the blastema for the phalangeal region (ph.) and the dense tissues destined to form the flexor and extensor apparatus.
7. 13 mm. Boyd’s ‘ H 23 ’, 24.1.5. Shoulder joint. The scapula with its characteristic blade (bl.) and coracoid (cr.) is well chondrified, and is separated from the head of the humerus (hm.) by an interzone»(i.z.). The interzone is continuous at its margins with the perichondrium (p.c.) of the scapula and humerus, and through this with the very dense tissues surrounding the head of the humerus in which the tendons of the short muscles of the joint and the long head of the biceps will differentiate. The muscular belly of the deltoid (dl.) and its nerve supply (n.s.) are quite distinct. ,
8. 13 mm. Boyd’s ‘H 23’, 23.3.4. Humero-ulnar joint. The trochlear region of the humerus (hm.) is separated from the ulnar (ul.) by a thick interzone (z'.z.). The ulna has not -yet developed its olecranon and coronoid processes, though the triceps (tr.) is quite distinct, and there is little indication of the future shape of the joint surfaces.
9. 13 mm. Boyd’s ‘H 23’, 24.1.6. Carpus. The lower end of the radius (r.d.) is separated from the metacarpal region (mt.) by two masses of condensed blastemal tissue, which represent the proximal and distal rows of carpal elements (cp.). Between these masses the tissue is rarefied, and not condensed as yet to form typical interzones.
10. 13 mm. Boyd’s ‘H 23’, 40.2.4. Lower limb. The femur is now well defined and the lower end fully chondrified. The hip bone is chondrified and separated from the femur by an interzone. The tibia is well developed and the interzone at the knee has become sharply defined. The quadriceps muscle mass (qu.) surrounds the femoral nerve (f.n.), and the quadriceps tendon (q.t.) is seen passing in front of the knee joint region, quite distinct from it. The hamstring mass (hm.) surrounds the sciatic nerve (s.n.) in a similar way, and is also distinct from the skeletal tissues, being separated by a layer of connective tissue (c.t.). In the foot chondrification has not yet begun. In the tarsal region the elements are represented by dense masses of blastemal tissue (ts.). The metatarsal and phalangeal regions of the blastema (mt. and ph.) are not yet differentiated.
11. 14 mm. Boyd’s ‘ H 8’, 19.2.8. Humero-ulnar joint. The trochlear surface of the humerus is separated from the sigmoid notch of the ulna by a well-defined interzone (i.z.) which follows the shape of the articular surfaces. The olecranon (ol.) and coronoid (cr.) processes of the ulna are now distinct. The interzone, perichondrium and tissues concerned with the tendons near the joint are still intimately blended.
12. 14 mm. Boyd’s ‘H 8’, 19.3.8. Fore-arm and hand. The carpallelements of the proximal and distal rows (cp.) are now chondrified, and are separated from each other and from the radius (rd.) and metacarpals (mt.) by typical interzones. The phalangeal region of the blastema (ph.) is still undifferentiated.
13. 16 mm. Kirk’s ‘Series 1 ’, 22.2.6. Shoulder. The interzone passes peripherally into the dense tissue (d.t.) that will form the capsule, labrum and neighbouring tendons, but these structures are not yet differentiated. Small blood vessels (b.v.) lie outside the perichondrium, forming a network which follows the shape of the cartilages, but none enters the perichondrium itself.
14. 16 mm. Kirk’s ‘Series 1 ’, 23.4.3. Humero-radial joint. Anteriorly and posteriorly the fibrous capsule (f.c.) arches over the joint region, enclosing the synovial mesachyme (s.m.) between its inner surface and the intra-capsular perichondrium (5.1).). Small blood vessels lie within the capsule.
Plate 2
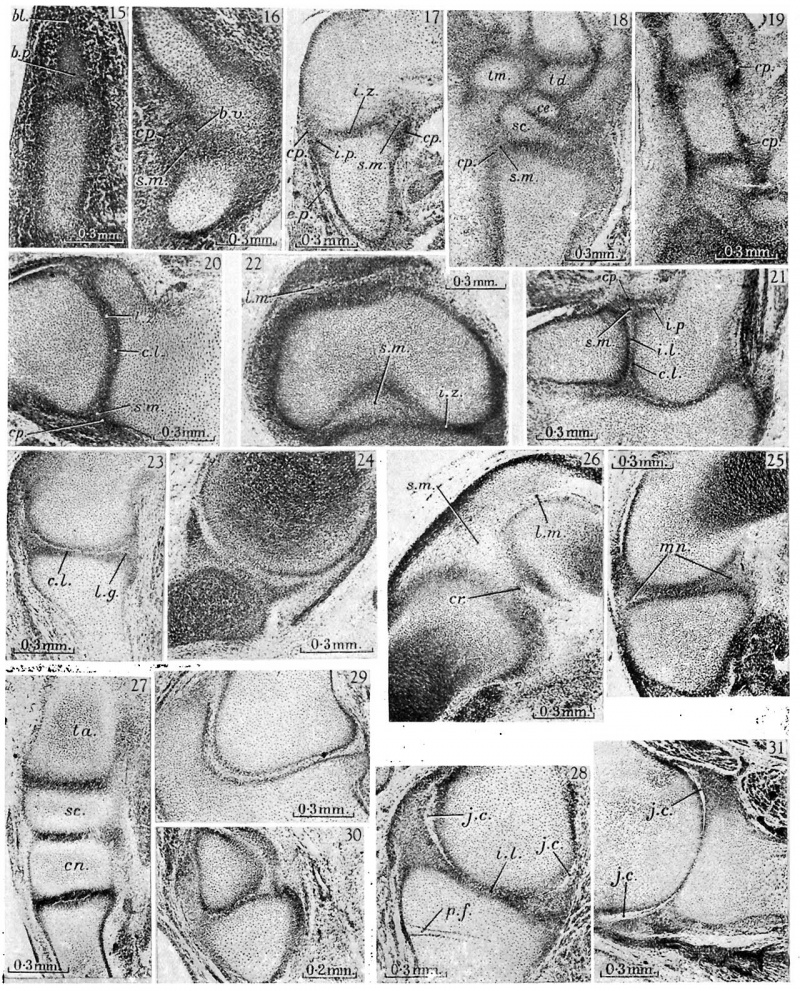
fig. 15. 16mm. Kirk’s ‘Series I’, 23.4.5. Metacarpo-phalangeal region. The metacarpal (mt.) is well chondrified and proportionately large, the basal phalanx (b. 10.) has now chondrified, but beyond this phalanx the blastema (bl.) is undiiferentiated. The joint region is surrounded by mesenchymal tissue in which the capsule has not yet appeared.
16. 16 mm. Kirk’s ‘Series I’, 41.1.6. Knee. The capsule (cp.) is well developed posteriorly and cuts off an abundant synovial mesenchyme (s.m.) with its included blood vessels (b.v.). 17. 19 mm. Appleton’s ‘H 19’, 4.3.6. Humero-radial joint. The homogeneous interzone (i.z.), intra,- and'extra-capsular perichondrium (i. p. and e.p.), synovial mesenchyme (.s.m.) and fibrous capsule (01).) take the classical form. _
18. 19 mm. Appleton’s ‘H 19’, 3.3.5. Carpus. The carpal elements, the trapezium (tm.), trapezoid (td.), scaphoid (sc.) and centrale (ce.), are separated from each other and from the radius and metacarpals by interzones of the typical form. The capsule of the wrist joint (cp.) can be distinguished enclosing the synovial mesenchyme (s.m.).
19. 19 mm. Appleton’s ‘H 19’, 3.2.3. Carpo-metacarpal and metacarpo phalangeal joints of the thumb. The fibrous capsules (cp.) are dense in these joints, for the tissue destined to form the collateral ligaments is not yet differentiated from them. The bulging curves of the capsule of the metacarpo-phalangeal joint are very clearly shown, uncomplicated by associated condensed structures.
20. 21 mm. Kirk’s ‘Series 8’, 78.1.2. Shoulder. A loose zone (l.z.) separating two dense chondrogenous layers (c.l.) can barely be distinguished in the interzone. The capsule (cp.) is now distinct and encloses a scanty synovial mesenchyme (s.m.).
21. 21 mm. Kirk’s ‘Series 8’, 90.1.3. Elbow. The three-layered interzone with its chondrogenous layers (c.l.) and loose intermediate layer (i.l.) is shown fully developed between humerus and radius and partly developed between humerus and 11lna. The chondrogenous layers are continued into the intra-capsular perichondrium (i.p.) and the intermediate layer into the synovial mesenchyme (s.m.) which lies within the capsule (cp.).
22. 21 mm. Kirk’s ‘Series 8’, 85.1.3. Knee. The condyles of the femur are separated from the tibia by homogeneous interzones (i.z.), and the intercondylar space is filled by synovial mesenchyme (s.m.). The menisci have not yet appeared. Between the femur and the quadriceps tendon is a layer of loose synovial mesenchyme (l.m.) where the joint cavity will appear at a later stage.
23. 23 mm. Appleton’s ‘H 23’, 8.2.2. Shoulder. A typical three-layered interzone has appeared at this stage. The chondrogenous layer (c.l.) covers the glenoid surface of the scapula and is continued into the labrum glenoidale (l.g.).
24. 24 mm. London Hospital ‘mx’ (Rutherford), 120.12. Shoulder. The labrum glenoidale is very distinct and is continuous with the chondrogenous layer of the interzone.
25. 26 mm. Boyd’s ‘H 11’, 75.2.1. Knee, condylar region. The interzone between the condyle of the femur and tibia is in the early three-layered stage. The menisci (mn.) are indicated by faint condensation of the mesenchyme.
26. 26 mm. Boyd’s ‘H 11’, 74.1.3. Knee, intercondylar region. In the abundant synovial mesenchyme (s.m.), which contains numerous small blood vessels, is the posterior cruciate ligament (cr.). The patella is separated from the femur by a layer of loose synovial mesenchyme (l.m.). p
27. 26 mm. Boyd’s ‘H 11 ’, 75.2.1. Tarsus. The talus (ta.), scaphoid (.90.), first cuneiform (cn.) and metatarsal are separated by homogeneous interzones.
28. 29 mm. Appleton’s ‘H 29’, 10.2.2. ‘Shoulder. Liquefaction of the synovial mesenchyme has begun at the peripheryof the joint, so that the position of the joint cavity (j.c.) is clearly defined, but the cavity is still partly filled with cells. The intermediate layer of the interzone (z'.l.) appears collapsed, and its flattened cells are clumped, but this condition is probably an artifact caused by swelling of the cartilage. A pressure fold ( p. f.) indicates swelling of the cartilage at some stage in the preparation. .
29. 29 mm. Appleton’s ‘H 29’, 26.2.1. Humero-ulnar joint. The three layers of the interzone are sharply defined and in the intermediate layer the cells have become flattened and arranged parallel to the articular surfaces. separated by a synovial cavity which still contains some scattered strands of cells (s.c.).
30. 29 mm. Appleton’s ‘H 29’, 28.2.5. Fourth metacarpo-phalangeal joint. The interzone is in the early three-layered stage. .
31. 30 mm. Appleton’s ‘H 30’, 12.2.7. Shoulder. In the peripheral parts of the joint the synovial mesenchyme is now liquefied to form the joint cavity (j.c.). More centrally the scapula and humerus appear to be in continuity, but this is probably due to an artifact, as in fig. 28.
Plate 3
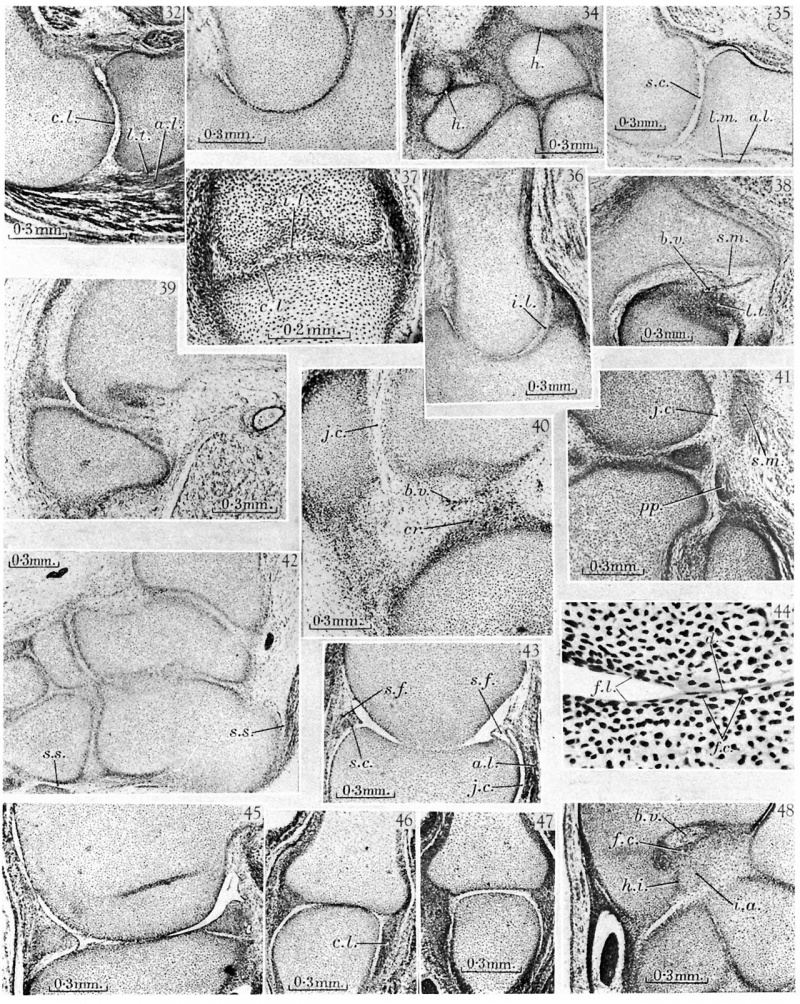
32. 30 mm. Appleton’s ‘H 30’, 5.8. Humero-radial joint. The middle layer of the interzone is liquefying and a small joint cavity has appeared anteriorly. The chondrogenic layers of the interzone ('c.l.) are still recognizable. The surface of the synovial mesenchyme is ragged. Loose tissue (l.t.) separates the annular ligament (a.l.) from the head of the radius.
33. 30 mm. Appleton’s ‘H 30’, 10.3. Humero-ulnar joint. This section is from the same series as that shown in fig. 32, and probably had a three-layered interzone, but as a result of pressure the interzone appears as a single dense layer of cells.
34. 30 mm. Appleton’s ‘H 30’. Carpal region. Most of the interzones are three layered in structure. The homogeneous appearance of some of the interzones (h.) is probably due to artifact.
35. West’s 34 mm. embryo, 12.2.4. Humero-radial joint. The humerus and radius are Between the head of the radius and the annular ligament (a.l.) is a layer of loose mesenchyme (l.m.) which will later break down to form a part of the joint cavity.
36. West’s 34 mm. embryo, 10.1.4. Humero-ulnar joint. Near the coronoid process the interzone has a typical three-layered structure, with a well-developed intermediate layer (i.l.). More posteriorly the intermediate layer appears as if compressed, an appearance probably due to artifact. . ' 37. West’s 34 mm. embryo, 10.1.3. Proximal inter-phalangeal joint of the fourth digit of the hand. The interzone is in a typical three-layered stage, with distinct chondrogenic layers (c.l.), and a loose intermediate layer (i.l.). This stage of the interzone has not usually been recognized in the inter-phalangeal joint.
38. West’s 34 mm. embryo, 20.1.5. Hip-joint. The head of the femur is seen in acetabulum, The ligamentum teres (l.t.) lies in a mass of synovial mesenchyme (s.m.) accompanied by conspicuous blood vessels (b.v.).
39. West’s 34 mm. embryo, 9.2.4. Knee. A small joint cavity has developed between the meniscus and the condyle of the femur, but the meniscus is still attached to the tibia by synovial mesenchyme.
40. West’s 34 mm. embryo, 6.2.5. Knee. A joint cavity (j.c.) is forming between the patella and femur. The anterior cruciate ligament (cr.) lies in a mass of synovial mesenchyme well supplied with blood vessels (b.v.). _
41. West’s 34 mm. embryo, 4.1.6. Knee. The tendon of the popliteus (pp) is seen in its characteristic position above the head of the fibula and outside the meniscus. The sesamoid in the lateral head of the gastrocnemius (am) is in the pro-cartilaginous stage. A small joint cavity (j.c.) is liquefying between the sesamoid and the femoral condyle.
42. West’s 34 mm. embryo, 9.1.4. Ankle. The interzones of the ankle and intertarsal joints are all in the three-layered stage. The synovial sheaths (s.s.) of the peroneus longus and Achilles’ tendon are already developed.
43. 45 mm. Appleton’s ‘H 45’, 4.2. Humero-radial joint. The humerus and radius appear joined, but this is probably an artifact. The joint cavity (j.c.) between the annular ligament (a.l.) and the head of the radius is well developed. Synovial folds (8. f.) project into the joint» and are covered by smooth strata of synovial cells (s.c.).
44. 45 mm. Appleton’s ‘H 45’, 4.2. Humero-radial joint. High-power view of the articular surfaces from the section illustrated in fig. 43. The surfaces are covered by fibrillar layers (f.l.), which unite where the surfaces join. Flat cells (f.c.) lie in and near these layers, and some cellular debris (d.) can be detected.
45. 45 mm. Appleton’s ‘H 45’, 26.1. Knee-joint. The menisci are free from the femur but still joined to the tibia by loose synovial mesenchyme.
46. 45 mm. Appleton’s ‘H 45’, 4.2. Metacarpo-phalangeal joint of the third digit of the hand. The cartilages appear joined. The collateral ligaments (c.l.) are seen on either side.
47. 45 mm. Appleton’s ‘H 45 ’, 10.6. Metacarpo-phalangeal joint of the fifth digit of the hand. In this section from the same series as fig. 46, the cartilages appear separate.
48. 49 mm. Appleton’s ‘H 49’, 33.1. Wrist. The fibro-cartilage (f.c.) of the wrist is found as a condensation of the synovial mesenchyme, with blood vessels (b.v.) nearby. A cartilage, the ‘intermedium antibrachii’ (i.a.), is found at this stage of development separated, from the styloid process of the ulna by a typical homogeneous interzone (h.z'.).
Plate 4
fig. 49. Hughes’s 60 mm. embryo, 7.3.8. Intercarpal joints between capitate and trapezoid. The interosseous (51.) and dorsal (d.l.) ligaments enclose between them a mass of synovial mesenchyme containing blood vessels (b.'v.).
50. Hughes’s 60 mm. embryo, 3.3.7. Inferior radio-ulnar joint. The joint cavity is spreading by liquefaction of the synovial mesenchyme (s.m.). The synovial surface is ragged, and strands of cells stretch into the cavity. Some of the cells are degenerate in appearance (d.c.).
fig. 51. 66 mm. Appleton’s ‘H 66’, 23.1. Shoulder and acromio-clavicular joint. The synovial surfaces are smooth and the humeral head is more curved than the glenoid surface. The walls of the subgacromial bursa (s.b.) have a. structure similar to those of the joint cavity. The clavicle and glenoid surface are covered with layers of articular fibro-cartilage (f.c.).
52. 66 mm. Appleton’s ‘H 66’, 24.3. Shoulder. The recess of the joint cavity showing the synovial stratum (s.s.), and the vascular sub-synovial tissue. * - ~
53. 125 mm. Appleton’s ‘H 125’, 18.1. Proximal inter-phalangeal joint of the third digit of the hand, illustrating the formation of a synovial fold (8. f.). The ragged synovial surface (r.s.) indicates that the joint cavity is still extending by liquefaction of the synovial mesenchyme.
54. 190 mm. Appleton’s ‘H 190’, 2.3. Humero-radial joint. General view showing the incongruity of the articular surfaces, the synovial folds and synovial villi. .
55. 190 mm. Appleton’s ‘ H 190’, 2.3. Same section. The articular surfaces of the cartilages show distinct fibrillar layers (f.l.), and the structure of the cartilage has not attained its adult pattern.
56. 190 mm. Appleton’s ‘H 190’, 2.3. Same section. High-power view of a synovial villus, showing also the synovial stratum (s.s.), a poorly developed sub-synovial stratum (s.b.), and a typical transition zone_ (tr.) between the articular cartilage and perichondrial tissues.
57. Hughes’s 20 day rat. The radial collateral ligament (lg.) shows twisting of its fibres and a structural similarity to the tendons of origin of the extensor muscles (t.m.).
58. Hughes’s 20 day rat. Humero-radial joint. General view showing the apparent union of the cartilages, and the structure of the fibrous capsule (cp.).
59. Hughes’s 20 day rat. Metacarpo-phalangeal joint of second digit. The cartilages are apparently joined, but in the region of union the fibrillar layer (f.l.) is conspicuous.
Cite this page: Hill, M.A. (2026, March 1) Embryology Paper - The development of joints. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Paper_-_The_development_of_joints
- © Dr Mark Hill 2026, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G