Paper - Some Gross Structural and Quantitative Aspects of the Developmental Anatomy of the Human Embryonic, Fetal and Circumnatal Skeleton
| Embryology - 30 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Noback CR. Some gross structural and quantitative aspects of the developmental anatomy of the human embryonic, fetal and circumnatal skeleton. (1943) The Anat. Rec. 87: 29–51.
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
Some Gross Structural and Quantitative Aspects of the Developmental Anatomy of the Human Embryonic, Fetal and Circumnatal Skeleton
- A portion of the thesis submitted to the faculty of the Graduate School of the University of Minnesota in partial fulfillment of the requirements for the degree of Doctor of Philosophy. The author wishes to express sincerest thanks to Dr. Richard E. Scammon for advice and counsel during the entire progress of the problem.
Charles R. Noback
Department of Anatomy, University of Minnesota, Minneapolis
- Now at the University of Georgia, School of Medicine, Augusta, Georgia.
Two Plates (Ten Figures)
Introduction
Researches of the macroscopic human prenatal and circumnatal osseous skeleton are generally concerned with (1) the determination of the normal number and the time of appearance of the ossification centers and their variations, (2) the use of the skeleton as an index for the determination of age, and (3) the developmental anatomy of one or more individual bones. The few published studies interpreting, by direct observation, these phases of skeletal growth have been made on specimens prepared by dissection.[1] In none has the staining and clearing method been used in studying the “in situ” skeleton. Few have presented any general principles of the developmental anatomy of the prenatal and circumnatal human osseous skeleton.
With this in mind, the present work was undertaken, to analyse this “in situ” development by direct observation, to correlate these observations with the literature of the subject, and to find some general principles of the developmental anatomy of the osseous skeleton during these periods. More specific aspects of this problem will be presented later.
On the basis of the techniques used to investigate the developmental anatomy of the prenatal and circumnatal human osseous skeleton, the history of the literature may be divided into two periods; that before 1895, and that since 1895.
The literature before 1895 was based primarily on observations of gross dissected embryos, fetus and newborn infants. Kerkring (1670, 1717), Albinus (1737), and Rambaud et Renault (1864), the outstanding workers of this period, described the prenatal skeleton in much detail, both verbally and pictorially. Their numerous illustrations are in many respects far superior to any other skeletal figures ever published.
The literature after 1895 is based on researches using one of three techniques: The roentgenographic method, which has been extensively employed; the clearing—staining methods which have been used to a much less extent; and the sectioning and reconstruction technique, which has hardly been applied at all.
Dorland and Hubeny (’26), Ruckensteiner (’31) and Koff (’32) present summaries of the roentgenographic literature and its relation to this problem. The use of the clearing and staining methods is found in Mall (’06) on the skeleton up to 100 fetal days; Horand (’08) on the bones of the extremities; Augier (’13, ’31 and later) on the skull bones; Vallois and Cardenat (’26) on the premaxillary and maxillary bones; VVissmer ('27) on the mandible; La Coste (’31) on the occipital bone; Inman (’33) on the skull bones; and Inman and Saunders (’37) on the frontal bone. The sectioning and reconstruction technique has been utilized by Macklin (’21) to interpret the osteology of the 43—mm. CR skull, by Low (’09) for the development of the mandible, and by Hanson (’20) for the early ossification of the clavicle.
The determination of the number of ossification centers that form certain bones, the determination of the time of the appearance of these centers, the developmental anatomy of the individual bones, and the use of the skeleton to determine fetal age form the subjects of practically all papers in the literature of this field. The technique used, the age of the specimens examined, the use of postnatal anomalies to interpret prenatal normalities, and the inaccurate determination of the age of the fetus examined are the basis of most of the controversies in the literature.
Weber (1830), Rambaud et Renault (1864), Bardeen (’10), Bryce (’15), Sammon (’23), Ruckensteiner (’31), Koff ( ’31), De Beer (’37) and Krogman (’41) and the standard biblio graphical references are the major bibliographical sources for the study.
Material and Methods
The series of 40 specimens from the 25-mm. CR stage to birth was distributed as follows: 2 in the second lunar month; 12 in the third; 8 in the fourth; 5 in the fifth; 4 in the sixth; 4 in the seventh; 1 in the eighth; 2 in the ninth; 1 in the tenth lunar month; and 1 at term. Ten late fetal and newborn infant skulls, the ages determined from the formulae computed by Scammon (’37), were dissected and examined.
The specimens were stained and cleared by the following technique:
- Fetus larger than 50 mm. CR are skinned, eviscerated, decerebrated and defatted by dissection.
- Fix specimens in 95% alcohol from 2 weeks to 3 months, depending upon the size of the specimen.
- Bleach in a solution of 9 parts of 95% alcohol to 1 part of hydrogen peroxide.
- Return to 95% alcohol until ready for clearing.
- Clear and stain simultaneously in KOH solution plus alizarin red S (alizarin red S is added to the KOH solution until the liquid is a pale lavender color).
- Two per cent KOH in distilled water for small specimens (up to 150 mm CR).
- five to 10% KOH in distilled water for larger specimens (160 mm. CR up to newborn infant size).
The clearing may take from 3 to 4 days for small specimens to 2 to 3 weeks for newborn infants. Solutions are changed every day or so and more stain is added as it is removed from solution by the bones. If the stain is applied in weak concentration, as here described, there is never any staining of the tissues surrounding the bone and destaining is never necessary.
6. When the tissues have reached the proper degree of maceration, are completely cleared and the bones are clearly visible, the skeleton is thoroughly stained. Specimens may then be dehydrated by the addition of increasing concentrations of glycerin. Specimens are stored in pure glycerin.
As alizarin red S is a stain that is specific for calcium salts (Cameron, ’30) and as calcium salt impregnated osseoid tissue is bone, it may be concluded that in specimens properly cleared and stained all prenatal and circumnatal bones will be stained red.
Observations and Discussion
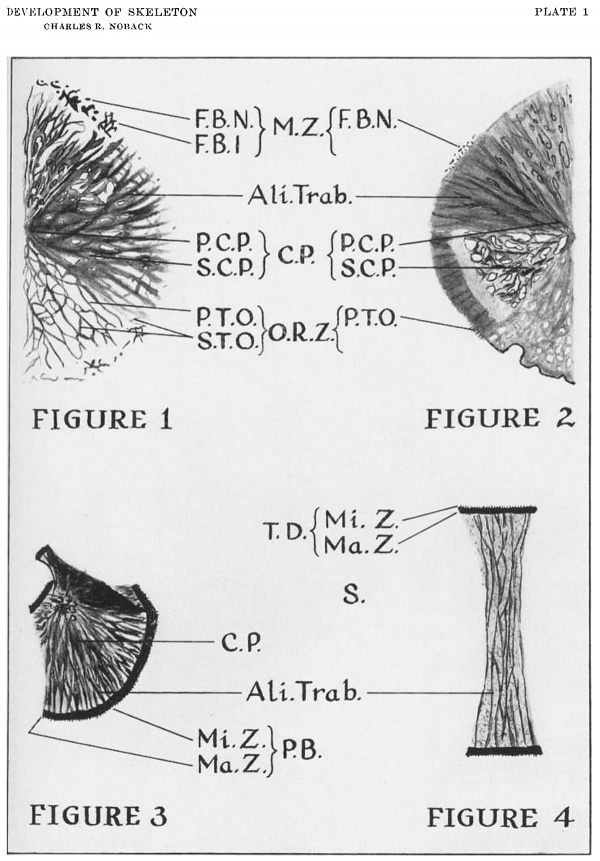
1. Definitions (plate 1)
Primary trabeculae ossei are the meridionally oriented long osseous spicules extending from the corpus of the bone, peripherally, into the unossified connective tissue. Secondary trabeculae ossei are the short osseous spicules which interconnect the primary trabeculae ossei. The open reticular zone is the meshwork of primary and secondary trabeculae ossei. Free bony islands are the small open reticular bony networks composed of short trabeculae ossei. These structures may attain a size of 1 mm. by 5 mm. Free bony nodules are the small dot like masses of bone. These two latter structures are detached from the bone proper. The marginal zone, just peripheral to the open reticular zone, is composed of the free bony nodules and islands. The primary central plate of a developing flat bone is the densely trabecularized thickened portion of the bone which appears as solid bone. The secondary central plate, found in some developing membrane bone (all open reticular and some smooth bordered plate bones) is the weakly trabecularized, thin portion of the bone that is composed of a closely knit meshwork of trabeculae ossei surrounding small unossified areas (in contrast to the elongate unossified areas of the open reticular zone). In open reticular bones, this plate is located between the primary central plate and the open reticular zone. In smooth bordered plate bones, this secondary central plate, if present, is eccentrically located and surrounded completely by primary central plate. In the long bones, the shaft is morphologically equivalent to the central plate.
Each prenatal bone is stained differentially by the alizarin. The intensely stained regions are the alizarin trabeculae ossei, the terminal disks of the epiphyseal end of the diaphyses of the tubular bones, certain portions of the peripheral margins of the flat, irregular cartilage bones, groups of trabeculae called fascicles, and certain thickened regions of certain bones. All of these structures probably represent the sites of recent calcification, as alizarin “reacts readily in Vitro and in vivo with recently deposited calcium phosphate or carbonate, nor mal or pathological, but often fails to stain older deposits.” (Cameron, ’30.)
“Alizarin” trabecular[2] are the alizarinophilic trabeculae ossei and the alizarinophilic trabecular appearing structures on the superficial surfaces of the central plate and shafts. For the purposes of this discussion trabeculae are only those structures, whether true bony trabeculae or not, that by virtue of the selective alizarin aflinity by the bone, form trabecular appearing patterns which stain intensely red. The alizarinophilic trabeculae ossei include the primary and secondary trabeculae ossei and the free bony nodules and islands. In each bone the trabeculae form definite primary and secondary patterns. In the open reticular bones the fine network of the trabeculae of the superficial surface of the central plate is continuous peripherally with the trabeculae of the reticular zone. The trabeculae of the central plate of the smooth bordered plate bones are continuous with the trabeculae ossei of the open reticular zone, when the latter is present. The trabeculae of the peripheral band plate bones extend into the peri pheral bands, while the trabeculae of the shafts of the tubular bones extend into the terminal disks.
2. Four morphological types of developing bones
In the prenatal skeleton cleared and stained by the technique previously described, four morphological types of bones are suggested.
1. Open reticular bordered plate bone (fig. 1). The open reticular bones, which include the membranous calvarial bones (frontal, parietal, squamous portion of the squamosal, interparietal bones and Wing of the alisphenoid, nasal, lacrimal, vomer bones and, when membranous, Kerkring”s ossicle), are composed of primary and secondary central plates bordered by a well developed open reticular zone and, in most cases, by a marginal zone.
The development of an open reticular bone proceeds in a definite sequence. Soon after the appearance of its ossification center the bone has a reticular structure of a fine meshwork of primary and secondary trabecular[3] in the form of an open reticulum. A marginal zone of free bony nodules and free bony islands, variable in their size, quantity and location, is found in most open reticular bones soon after the appearance of the center of ossification (figs. 5 and 6, parietal bone). By the third lunar month (fig. 7, parietal bone) the oldest portions of each open reticular bone (in the region of the site of each ossification center) loses its open reticular structure as the unossified spaces of the reticulum are ossified to form primary and secondary central plates containing no dipliie. Peripheral to the central plate is the open reticular zone and peripheral to that, usually, the marginal zone. Thus commencing at the site of original ossification and proceeding peripherally, the bone is progressively less densely ossified. Free bony nodules and free bony islands are associated mainly with the frontal, parietal and interparietal bones along the metopic, coronal and lambdoidal sutures, and with the parietal bone along the squamosal suture. The other open reticular bones rarely have bony islands but they often posses free bony nodules. By the fourth lunar month (fig. 9) the central plate region has enlarged both relatively and absolutely as compared to the other zones, and the open reticular zone is a narrow peripheral band. The marginal zone, if present, is generally restricted to a few bony nodules, and free bony islands are usually absent. At birth (fig. 10) the thickened central plate region forms practically the entire bone. Surrounding the plates are the short peripherally directed ossified spicules of the narrow open reticular zone. A few free bony nodules and an occasional sutural bone (which may represent a modified free bony island) represent the marginal zone.
During prenatal life an open reticular bone has three structural zones: (1) The central diploeless plate of bone which is the body of the bone, (2) the open reticular zone which is peripheral to the central plate, and (3) the marginal zone of free bony nodules and islands which is peripheral to the open reticulum. The central plate (including primary and secondary plates) increases in both absolute and relative area with respect to the open reticulum and marginal zones. The free bony nodules and islands Vary in size from small masses approximately 0.5 mm. in diameter to large ones 1 to 5 mm. in diameter. As fetal age progresses these nodules and islands are incorporated into the bone. The nodules are irregularly distributed on all peripheral borders, but the islands are restricted primarily to the borders, of the coronal, lambdoidal and sagittal sutures. With age the central plate enlarges and the peripheral border and marginal border are reduced in width.
The trabeculae of the frontal bone radiate from a center in the middle of the frontal squama, into the squama; and from a line at the middle third of the supraorbital crest posteriorly into the orbital facies. The center of radiation of the frontal squama rises with age. The trabeculae of the parietal bone radiate from the morphological center of the bone. In each parietal bone ossifying from two centers, the two centers of radiation perist until the two bony entities fuse into one. In the latter stage the parietal bone has one center of radiation obscured by the reticular network of the trabeculae in the center of the bone. From the central portion of the base of the temporal squama, the trabeculae radiate superiorly, anteriorly and posteriorly into the squama. The vomerine trabeculae radiate from a line in the middle third of the junction of the squama and keel anteriorly, superiorly and posteriorly into the squama; and anteriorly, inferiorly and posteriorly into the keel. The trabeculae of the lacrimal and the nasal bone generally form a reticular network. The interparietal trabeculae radiate superiorly and laterally from the central third of the bone’s inferior border into the squama. In Kerkring’s ossicle the trabeculae extend inferiorly from the superior apex to the base of the bone. The trabeculae of the membranous part of the alisphenoid bone radiate distally from the region just anterior to the incisura of the foramen ovale and foramen spinosum, into the wing.
2. Smooth bordered plate bone (fig. 2). These complex trabecular bones, which include the maxilla, the mandible, the zygomatic bone, the palate bone, the inferior border and zygomatic process of the squamosal bone and the tympanic annulus, have two or more trabecular patterns. They possess relatively smooth borders with few, if any, bony nodules and no bony islands; and no long primary trabeculae extending into the surrounding connective tissue. Occasionally a secondary central plate is present. Growth in area is a function of the central plate margins except in those restricted regions of the short primary trabeculae ossei.
The trabecular pattern of the maxilla and mandible is complex. The trabeculae of the external facies of the maxilla radiate (1) from a center in the cuspid region, extendinrv superiorly on the frontal process, postero-laterally toward the infraorbital crest and the postero-lateral facies of the zygomatic process, (2) from a line of the alveolar premolar region, extending superiorly on the zygomatic process and posteriorly on the inferior border of the zygomatic arch and posterior portion of the corpus, and (3) from a center on the medial alveolar border of the premaxilla, extending to the maxillary corpus and to the inferior border of the piriform aperture. Except for the zygoma and its radiations, the trabecular pattern of the internal facies is similar to that of the external facies. The orbital facies trabeculae radiate from the anterior half of the groove of the future infraorbital canal. They extend postero-laterally into the horizontal facies, and supero-medially into the vertical facies. The palatine process trabeculae radiate from a center in the cuspid region and extend anteriorly, medially and posteriorly into the process.
From the apex of the angle formed by the symphyso-condyloid fascicle and the marginal fascicle of the mandbile (Wissmer, ’27) trabeculae extend posteriorly. On the inferior margin of the mandible antero-posterior oriented trabeculae extend from the symphysis to the angle. Halfway between the alveolar border and the inferior margin in the cuspid region is a center of radiation from which trabeculae radiate to the symphyseal margin. From a line of radiation extending from the junction of the alveolar border and the coronoid process inferior to the symphyso-condyloid fascicle, the trabeculae radiate proximally on the ramus to the incisura mandibulae. The trabeculae of the splenius are oriented as practically parallel trabeculae approximately parallel to the shallow are formed by the line of the mandibular foramen, the mylohyoid line and the continuation of the mylohyoid line as an arc to the antero-superior end of the symphysis. Exclusive of the splenius the trabecular pattern of the medial facies is the same as that of the lateral facies. Wissmer (’27) has a comprehensive account of the mandibular fascicles.
From a short line of radiation in the middle of .a ridge formed at the junction of the orbital and temporal facies the trabeculae of the zygomatic bone radiate anteriorlv into the orbital process and postero-superiorly into the frontosphenoidal process. An infero—anterior radiation extends from the entire ridge into the maxillary process The small areas between the frontosphenoidal and maxillary processes and the posteroinferior angle of the maxillary process are characterized by the absence of trabeculae. From a center of radiation just anterior to the bases of the pyramidal process at the angle between the horizontal and perpendicular plates of the bone, the palatine bone trabeculae radiate into the two plates and the pyramidal process. The trabeculae of the tympanic annulus and the zygomatic process of the squamosal bone spiral around the long axis of each bone.
3. Peripheral band bone (fig. 3). Peripheral band bones, which include the scapula, ilium, neural arches, supraoccipital, exoccipital, basioccipital, orbitosphenoid, basisphenoid, presphenoid, ethmoid, sternal centers, ischium, pubis, calcaneous, talus and other tarsal and carpal bones, are the flat and irregular cartilage bones whose peripheries have, in whole or in part, a strong affinity for alizarin red 8. The densely stained band-like peripheries, sharply demarcated from the lightly stained body of each bone, have two zones: The major zone of the band, approximating the body of each bone, has a hemogenous intense red color; while the minor zone of the band is a narrow peripheral lightly staining margin of short fine trabeculae perpendicular to the long axis of the zone. These fine trabeculae probably represent both calcified cartilage and bone. The body of each bone has a simple trabecular pattern.
The trabeculae of the orbitosphenoid bone are oriented as a series of curved lines, parallel to each other and to the median borders of the bone. The peripheral band is generally present on all except the median borders. The basisphenoid, presphenoid, lingula and cartilaginous portion of the alisphenoid bones have networks of trabeculae. The peripheral bands of these bones are inconstant except for those at the anterior and posterior borders of the basisphenoid and presphenoid bone. The bands of these two bones resemble the disks of tubular bones.
From a center of radiation at the junction of the external occipital crest and the inferior nuchal line (middle of the superior border of the bone), the trabeculae of the supraoccipital radiate inferiorly and laterally, and the inferior and lateral peripheries of the bone have a peripheral band. The trabecular pattern of the basioccipital and exoccipital bones is a network. Soon after its appearance, the trabeculae of the basioccipital bone radiate from the posterior portion of the middle of the bone extending anteriorly and laterally; the exoccipital trabeculae extend from the jugular process and the exoccipital condyle into the body of the bone. Each border of the basioccipital bone except the posterior has a peripheral band. The anterior borders of the jugular process and the exoccipital condyle and the posterior border of the body of the exoccipital bone have peripheral bands. The petrosal bone has a dense network of fine trabeculae and inconstant peripheral bands generally present in the region of the fossa subarcuata and the hiatus canalis facialis. The trabeculae of the scapula radiate from a center located at the junction of the medial end of the spine and the body of the bone, and they extend into all regions of the bone. The vertebral, glenoid and medial acromial scapular borders possess peripheral bands (fig. 3). Each component of the sternum has a centrally located center from which the trabeculae radiate peripherally (Borovansky, ’31). Each neural arch is characterized by the presence of two sets of parallel trabeculae perpendicular to each other. One row extends supero-inferiorly from the superior to the inferior articular processes, while the other row extends from the distal end of the lamina to the medial end of the pedicle, being interrupted in the middle by the first row. The distal ends of the lamina, pedicle and articular processes have peripheral bands. From a center just anterior to the angle of the greater sciatic notch the trabeculae fan out into the corpus of the ilium. All borders, except the border of the greater sciatic notch, have peripheral bands. The trabeculae of the pupis and the ischium form networks and the ends of the bones may have peripheral bands resembling terminal disks.
4. Tubular bones (fig. 4 ). Tubular bones include all the long bones of the body. They have cartilaginous epiphyses and stain differentially with alizarin red S. The body of the osseous shaft is stained rather homogeneously, but on the surface of the shaft there is a network of subperiosteal trabeculae parallel with the long axis of the shaft. Each end of the shaft is capped by two deeply stained terminal disks. The disk closest to the body of the shaft (major zone) is stained densely and rather homogeneously by alizarin. The narrow disk of the diaphyseal—epiphyseal junction (minor zone) is composed of short fine trabeculae perpendicular to the plane of the disk. It probably represents both calcified cartilage and bone.
Conclusions
The open reticular zones, the marginal zones, the peripheral bands, and the terminal disks are the locations of the sites of proliferative growth. The central plates and the shafts are the regions of bony reconstruction and slow osseous growth. The “alizarin” trabeculae are probably manifest of the sites of recent ossification.
Although one center of ossification usually gives rise to one morphological type of bone, an ossification center may give rise to more than one (morphological) type. For example, the wing of the alisphenoid bone is an open reticular bordered plate bone while the base is a peripheral band bone.
A definitive bone may be formed of two or more of these morphological types of bone. For example, in the definitive occipital bone, the interparietal bone and Kerkring’s ossicle, when membranous, are open reticular bordered plate bones; while the basioccipital, the exoccipital and the supraoccipital bones, and Kerkring’s ossicle, when cartilaginous, are peripheral band plate bones.
The open reticular bordered plate bones and the smooth bordered plate bones are membrane bones, while the peripheral band bones and the tubular bones, except possibly the clavicle (Hanson, ’20) are cartilage bones. There is an intimate relation between the developmental morphology and the tissue origin of the bones appearing prenatally.
Each bone stained by this technique has superficial subperiosteal lines which are stained more deeply than the matrix of the bone. These lines generally radiate from one or more centers in each bone, or in a few cases, the_lines are parallel and do not radiate from a center. A bone with one or two centers of radiation has a simple trabecular pattern. A bone that has more than two centers of radiation is considered as having a compound trabecular pattern. Each bone of the fetal skeleton has a definite trabecular pattern of subperiosteal trabeculae.
3. Periods of Development
Three periods of development in the morphogenesis of the bones of the embryonic, fetal and circumnatal osseous skeleton are suggested. They are:
1. The period of differentiation
This is the time of the appearance of the ossification centers. This time of appearance, subject to variation which during the fetal period is measured in days (Pryor, ’28; Koff, ’32), is influenced by such factors as sex (Pryor ’28; Sawtell ’29; Christie et al. ’41), heritable tendencies (Pryor, ’39), nutrition (Francis, ’40), race (Christie et al. ’41), pathology (Francis, ’39; Gross, ’40), and parity (Adair and Scammon, ’21; Christie et al. ’41). Groups of ossification centers exhibiting relative independence in their time of appearance, may exist in the extremities (Robinow, ’42).
From a survey of the literature, partially confirmed by original observations, the general order of appearance of the ossification centers by region is as follows: (a) diaphyses of the two proximal segments of the extremities, (b) bones of the facial region, (c) and (d) calvarial bones and the thoracic bones (ribs, clavicle, scapula and primary vertebral bones), (e) free vertebral column primary bones, (f) the diaphyseal bones of the hand, (g) and (h) basicranial bones and pelvic bones (with the exception of the ilium that appears early), (i) diaphyseal bones of the foot, (3') sternal bones, and (k) hyoid bones.
2. The period of proliferation
This is the time of the rapid extension of ossification into unossified regions. During this period the linear growth of a bone proceeds at a higher rate of increment than that of the corresponding body segment, and the morphological features characteristic of each bone appear.
For the facial bones, this period extends from the period of differentiation to the 7 0-mm. to 80-mm. CR stage (figs. 5 to 7). Early in the period each facial bone (except the nasal, vomer, and lacrimal bones) assumes the characteristics of the typical smooth bordered plate bone. Their period of ontogeny from the trabecular center of ossification is short as contrasted to the period of ontogeny of the open reticular bordered plate bones from the reticular network to the formation of an extensive central plate late in the period of proliferation. The widths of the broad suture spaces between the facial bones are continually being reduced.
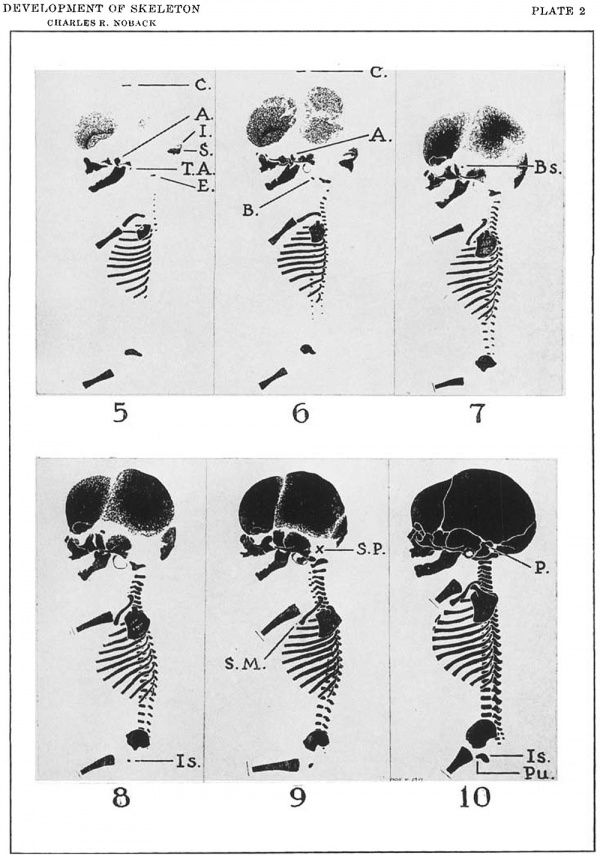
For the membranous calvarial bones, this period ends at about 140-mm. to 160 mm. CR stage (fig. 8). During this interval these rapidly expanding bones grow peripherally by the open reticular zone and the marginal zone. Inman (’33), who analysed quantitatively the growth of the membrane bones during fetal life, states that these bones grow with constant increments until the 140-mm. to 160-mm. CR stage, and subsequently at a constant increment of less magnitude.[4] At the 140-mm. to 160 mm. CR stage, they have assumed a form resembling their definitive form and an area roughly proportional to their definitive area. The reticular zone and the marginal zone are narrow; the dominance of the central plate over the two peripheral zones is definite.
The principles of the prenatal growth of the cartilage bones including both the peripheral band plate bones and the tubular bones are summarized by Inman (’33). “The ossification centers apearing in the primordial chordocranium grow with constant increments in relation to sitting height. Throughout the body, ossification centers arising in cartilage appear to grow by constant increments; this occurs in the ossification centers for the femur, humerus, tibia, radius, ulna, metacarpals, metatarsals and the bodies of the vertebrae,” . . . “The rates of growth of endochondral bones are also the rates of proliferation or activity of the epiphyseal ends of the bones (if one accepts the no interstitial bone growth hypothesis). Each group of cartilage cells contributing to the formation of a particular bone has a constant rate of proliferation when compared not with time but to the size of the fetus.” Hesdofier and Scammon ( ’28) and Robb and Clarke ( ’35) demonstrate the rectilinear relation of the growth of the femur, humerus, radius, ulna, fibula and tibia to other linear dimentions during prenatal life. Objective data of the quantitative growth of the other postcranial cartilage bones during prenatal life have not, to this author’s knowledge, been presented. However, if Inman’s (’33) suggestion of the constant rate of proliferation of the cartilage cells when compared to the size of the fetus is accepted for all cartilage bones during the fetal period, these bones may be classified as growing in a retilinear relation to the body length during fetal life. The cartilage bones, except the ribs and the clavicle, grow by constant increments and do not reach definitive proportions in relation to their body segments before birth. This is qualitatively visible. Brodie’s (’41) data suggests that the interval between the two periods for the basi-cranial bones may occur at 1-1; years of age. However, the power curves of the postnatal growth of the bones and the body segments may obscure the determination of the stage between these two periods.
A comparison of the cartilaginous supraoccipital bone and the membranous interparietal bone in the figures in plate 2 gives a graphic illustration of the relatively rapid growth of the calvarial bones as compared to the relatively slow growth of the cartilage bones.
3. The period of construction
This is the time of the osseous reorganization of each bone, and of linear osseous growth at a rate of increment approximately equal to that of the body segment with which it is associated. As previously noted, the period of proliferation ends and the period of construction begins approximately at 70 mm. to 80 mm. OR in the facial bones, clavicle and ribs (figs. 7 to 10 incl.) ; approximately at 140 mm. to 160 mm. OR in the membranous calvarial bones; and after birth in the cartilage bones. It is characterized by an abrupt decrease in the growth increment of the bones, with a concomitant formation of a bony border about the peripheral edges of the bone (Inman, ’33); and the assumption, by the bones, of an area or length proportional to their definitive area or length (figs. 9 and 10).
Summary
- On the basis of the techniques used to investigate the development of the ‘embryonic, fetal and circumnatal skeleton, the history of the literature may be divided into two periods: (a) before 1895, and (b) after 1895.
- By clearing in KOH and staining with alizarin red S simultaneously, overmaceration and overstaining of human embryos and fetus can be prevented more readily than in previously published techniques.
- Structures associated with the developing bones are defined. These include the definitions of primary and secondary trabeculae ossei, open reticular zone, free bony islands, free bony islands, free bony nodules, marginal zone, primary and secondary central plates and the structures resulting from the differential staining affinity of “alizarin” to bone.
- On the basis of the gross structure of the developing prenatal bones cleared by KOH and stained by “alizarin” four morphological types of bones are described: (a) open reticular bordered plate bones, (b) smooth bordered plate bones, (c) peripheral band bones and (d) tubular bones. The relation of the terms noted in (3) to these four types of bones and a brief description of the alizarin trabecular pattern in the prenatal bones are presented.
- Three developmental periods in the morphogenesis of the bones during prenatal life are postulated: (a) the period of differentiation or the time of the appearance of the ossification centers; (b) the period of proliferation or the time of relatively rapid osseous growth (growth of each bone at a rate greater than its corresponding body segment); (c) the period of construction or the time of relatively slow osseous growth (growth of each bone at a rate approximately equal to its corresponding body segment).
- ↑ The roentgenographic studies are indirect observations.
- ↑ Unless otherwise specified, hereafter “trabeculae” mean “alizarin” trabecular.
- ↑ Not “alizarin” trabecular.
- ↑ During the 140-mm. to 160-mm. CR stage to birth period (period of construction) the dimensions of the calvarial bones measured by Inman (’33) are identical to some of the external dimensions measured by Scammon and Calkins (’29). The diflerence between the dimensions meaured by these workers during the period before the 140-mm. to 160-mm. CR stage (period of proliferation) is that Inman did not measure the unossified regions between the bones and the soft tissue over them while Scammon and Calkins did.
Whether the growth curves of the bone with respect to the external body dimensions are interpreted as rectilinear curve or as power curves does not effect the concept of the developmental periods. During the period of proliferation the curve of the various external dimensions of the body to body length and the curve of the dimensions of the bones to body length converge toward each other until the end of the period. During the period of construction these two sets of curves are roughly parallel to each other. At the stage between the two period these two sets of curves, if straight lines, will intersect to form an angle or, these curves, if curved lines, will exhibit their greatest curvature.
Literature Cited
ADAIR, F. L., AND R. E. SCAMMON 1921 A study of the ossification centers of the wrist, knee and ankle at birth with particular reference to the physical development and maturity of the newborn. Am J. Obst. and Gynec., vol. 2, pp. 35-60.
ALBINUS, B. S. 1737 Icones ossium foetus humani accedit osteogeniae historia. Leidae, Batavorum, 162 pp. and 16 pl.
AUGIER, M. 1913 Recheehes sur l’os frontal de 1’homme. 151 pp., Paris.
- 1931a Squelette cephalique. (In: Poirier, P. et Charpy, A., eds., “Traite d’anatomic humaine. 2, vol. 1, pp. 89-630.) Masson et Cie., Paris.
- 1931 b Quelque problemes relatif an developpement due crane. Arch. d’a.nat., d ’hist. et d’embryo1., vol. 13, pp. 33-66.
BARDEEN, C. R. 1910 The development of the skeleton and of the connective tissues. (In: Keibel, F. and Mall, F., eds., “Manual of Human Embryology.” 1, pp. 292-453.) J. B. Lippincott, Philadelphia and London.
DEBEER, G. R. 1937 The development of the vertebrate skull. xviii and 552 pp. + 142 pl., Clarendon Press, Oxford.
BOROVANSKY, L. 1931 Osifikase hrudni kosti u jeji rust u clovekn. After Rozpravy II. Tridy Ceske Akadamie, V01. 40, pp. 1-30.
BRODIE, A. G. 1941 On the growth pattern of the human head from third month to the eighth year of life. Am. J. Anat., vol. 68, pp. 209-262.
BRYCE, T. H. 1915 Ostcology. (In Schafer, E., Symington, J. and Bryce, T., eds., “Quain’s Elements of Anatomy.” vol. 4, pt. I, pp. 1-216.) Longmans, Green and Co., London and New York.
CAMERON, F. R. 1930 The staining of calcium. J. Path. and Bact., vol. 33, pp. 929-955.
CHRISTIE, A. V., E. C. DUNHAM, R. M. JENss AND A. L. DIPPEL 1941 Development of center for cuboid bone in newborn infants. Am. J. Dis. Child., vol. 61, pp. 471-482.
DORLAND, W., AND M. HUBENY 1926 The X-ray in embryology and obstetrics. xv + 420 pp., Bruce Publishing Co., St. Paul.
FAWCETT, E. 1911 The development of the human maxilla, vomer and paraseptal eartilages. J. Anut. and Pl1ysiol., vol. 45, pp. 817-830.
FRANCIS, C. 1939 Factors influencing appearance of centers of ossification during early childhood. Am. J. Dis. Child., vol. 57, pp. 817-830.
- 1940 Factors influencing appearance of centers of ossification during early childhood. Am. J. Dis. Child., vol. 59, pp. 1006-1602.
GROSS, R. D. 1940 Neoplasms producing endocrine disturbances in childhood. Am. J. Dis. Child, vol. 59, pp. 579-628.
HANSON, F. B. 1920 The history of the earliest stages in the human clavicle. Anat. Rcc., vol. 19, pp. 309-325.
Ilr.snoRrr1«;R, 1\l., AND R. E. SCAMMON 1928 Growth of long bones of human fetus as illustrated by the tibia. Proc. Soc. Exper. Biol. and Med., vol. 25, pp. 638-641.
HORAND, R. 1908 Vision du squelette d’un corps diuphanise per la methods do Sehultze. Rev. d’orthop., Paris, vol. 9, pp. 535-543.
INMAN, V. 1933 Observations on the growth and development of the human fetal cranium. A thesis submitted to the faculty of the graduate school of the University of California in partial fulfillment of the requirements for the degree Doctor of Philosophy.
INMAN, V., AND J. SAUNDERS 1937 The ossification of the human frontal hone. J. Anat., vol. 71, pp. 383-394.
KERKRING, T. 1670 Osteogznia foetnum. Amsterdam. x + 280 pp. + 8 pl. (Cited by Raxnbaud et Renault, 1864).
- 1717 Osteogenia foetuum. Leiden.
Korr, R. 1932 Norms of ossification of the bones of the extremeties. A thesis submitted to the faculty of the graduate school of the University of Minnesota. in partial fulfillment of the requirements for the degree of Master of Science. 124 pp. -1- 14 pl.
KROGMAN, W. 1941 A bibliography of human morphology 1914-193.‘), xxxi + 385 pp. The University of Chicago Press, Chicago.
LACOSTE, A. 1931 Sur le developpement do l’ecai1le occipitale otudiee comparative chez lc mouton et chez l’ho1mne. Arch. d ’anat., d ’hist., et d’embry., vol. 13, pp. 1-47.
Low, A. 1905 Further observations on the ossification of the human lower jaw. J. Anat. and Physiol., vol. 44, pp. 83-94.
MALL, F. P. 1906 On ossification centers in human embryos less than one hundred days old. Am. J. Anat., vol. 5, pp. 433-458.
Macklin CC. the skull of a human fetus of 43 millimeters greatest length. (1921) Contrib. Embryol., Carnegie Inst. Wash. Publ., 48, 10:59-102.
MACKLIN, C. 1921 The skull of at human fetus of 43 mm. greatest length. (Contributions to Embryology, no. 48, vol. 10, pp. 57-103) Washington, D. (3., Carnegie Institution of Washington, Pub. no. 273.
OBATA, R. 1912 Die Knochenkerne des fetalen mensehlichen Reckens. Ztschft. f. Gcburt-sh. u. G_Vnilk., vol. 22-, pp. 533-574.
PRYOR, J. 1928 Differences in ossification of the male and female skeleton. J. Anat., vol. 62, pp. 499-506.
1939 Normal va.‘r.ia.tion of bones due to genetic factors. Heredity, vol. 30, pp. 249-255.
RAMBAUD, A., ET 0. RENAULT 1864 01-igine et developpement des os. 271 pp. Atlas 28 pls., Paris.
Roan, R., AND J. CLARKE 1934 Growth of bone shafts in the human fetus. Proc. Soc. Expcr. Biol. and Med., vol. 31, pp. 633-636.
ROBINOW, M. 1942 The appearance of ossification centers. Grroupings obtained from factor analysis. Am. J. Dis. Child., vol. 64, pp. 229-236.
RUCKENSTEINER, E. 1931 Die normale Entwicklung (les Knochensystems im Riintgenbild. Radiologische Praktika, vol. 16, pp. 1-79.
SAWTELL, R. 1929 Ossifieation and growth of children from one to eight years of age. Am. J. Dis. Child., vol. 37, pp. 61-—67.
SCAMMON, R. E. 1923 A summary of the anatomy of the infant and child. (In: Abt, I. A., ed., “Pediatrics.” vol. 1, pp. 267-444) W. B. Saunders 00., Philadelphia and London.
- 1937 Two simple nomogruphs for et-imating the age and some of the external dimensions of the human fetus. Anat. Rec., vol. 68, pp. 221-225.
SCAMMON, R. E., AND L. CALK1Ns 1929 The development and growth of the external dimensions of the human body in the fetal period. xxiii + 267 pp. The University of Minnesota Press, Minneapolis.
VALLOIS, H., ET E. CARDENAT 1926 Le developpement due premaxillairc chez l’l1omme. Arch. de biol., Paris, vol. 36, pp. 361-425.
WEBER, E. 1830 I-Ii1debrandt’s I-laiidbucli der Anatomie dos Menschen. vol. 2. Stuttgart.
WISSMER, A. 1927 Le developpement ct Porganization statiqne de la mandible foetale ehez l’l1omme. Archiv, d’anat., d’l1ist, et d’en1bryo]., vol. 7, pp. 335-426.
Explanation of Figure
Plate 1
Diagrams illustrating the four morphological types of human prenatal bones. The degree of intensity of the black in the figures approximates the degree of intensity of the red in the p'renat21l bones when stained with “alizarin red S” (degree of affinity of the dye for the bone).
1. Open reticular bordered plate bone (composite figure).
2. Smooth bordered plate bone (composite figure).
3. Peripheral band bone (scapula).
4. Tubular bone.
Legend: Ali. Tralx, “alizarin” trabeculae; C.P., central plate; F.B.I., free bony island; F.B.N., free bony nodule; M.Z., marginal zone; Ma. Z., major zone; Mi. Z., minor zone; P.C.P., primary central plate; P.B., peripheral band; P.T.O., primary trabeculae ossei; O.R.Z., open reticular zone; S., shaft; S.C.P., secondary central plate; S.T.O., secondary trabeculae ossei; T.D., terminal disk.
Plate 2
Side View drawings of six stages in the development of the human prenatal and cireumnntnl ossoous skeleton. C71-own-riunp lengths are held constant in all figures.
Note: (1) the maturation of the facial bones, ribs nnd clavicle in the stage depicted in figure 3, (2) maturation of the calvarial bones in the stage depicted by figure 5, (3) the central plate, the open reticular zone and the marginal zone of the calvarial bones, and (4) the relatively slow growth of most of the cartilage bones as exemplified by the supruoeciptal bone and the 1'eln.tive1y rapid growth of the membrane bones as exemplified by the interparietal bone.
| Figure | CR Length | CH Length | Approximate Age |
|---|---|---|---|
| Figure 5 | 38 mm. | 64 days | |
| Figure 6 | 58 mm. | 75 mm. | 78 days |
| Figure 7 | 88 mm. | 125 mm. | 3.25 lunar months |
| Figure 8 | 105 mm. | 156 mm. | 3.5 lunar months |
| Figure 9 | 139 mm. | 205 mm. | 4.25 lunar months |
| Figure 10 | 330 mm. | 479 mm. | 10 lunar months |
Legend: A., alisphenoid bone; B., basioeoipital bone; Bs., hasisphenoid bone; 0., crown; E., exoccipital bone; I., interparietal bone; Is., ischinm; P., petrosum; Pu., pubis; S., supraoceipital bone; S.M., sternal center of the manubrium; S.P., site of the pertosum; T.A., tympanic annulus.
Cite this page: Hill, M.A. (2024, April 30) Embryology Paper - Some Gross Structural and Quantitative Aspects of the Developmental Anatomy of the Human Embryonic, Fetal and Circumnatal Skeleton. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Paper_-_Some_Gross_Structural_and_Quantitative_Aspects_of_the_Developmental_Anatomy_of_the_Human_Embryonic,_Fetal_and_Circumnatal_Skeleton
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G