Paper - A normal human ovum in a stage preceding the primitive streak
| Embryology - 28 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Brewer JI. A normal human ovum in a stage preceding the primitive streak (The Edwards-Jones-Brewer ovum). (1937) Amer. J Anat., 61: 429-481.
| Online Editor | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| This historic 1937 paper by Brewer describes early human development before gastrulation. An embryo age of 15 days could make this specimen Carnegie stage 6 in Week 2. This embryo was initially given the serial number (H1496) and later incorporated into the Carnegie Collection as embryo no. 8819.
Brewer JI. and Fitzgerald JE. Six normal and complete presomite human ova. (1937) Amer. J. Obstet. Gynecol, 34: 210-224.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
A Normal Human Ovum In A Stage Preceding The Primitive Streak. (The Edwards-Jones-Brewer Ovum)
John I. Brewer
Department of Anatomy, The University of Chicago, and Gynecological Service, and Henry Baird Favill Laboratory, St. Luke’s Hospital
Fourteen plates (Thirty figures) A detailed description of the embryo will be published later.
Aided by a grant from the Rockefeller Foundation to The University of Chicago, the Albert B. Kuppenheimer Fund of‘ St. Luke’s Hospital, and a grant from Mrs. Emmons Blaine.
Introduction
The understanding of implantation in mammals depends largely upon histological methods and little progress could be made until the serial section technique was perfected. It was not until 1899 that the first adequate study of a normal human implantation stage appeared (Peters, 1899). Since then J ung’ (’08), Strahl and Beneke (’10), Streeter (’20, ’26), Teacher (’24), Frassi (’07—’O8), Grosser (’27), Von Miillendorff (’21, ’25), Von Spee (1896), and others have advanced this study to a great extent.
It has been my privilege to be associated in the study of six normal and anatomically complete, presomite human ova. Of these the youngest is the Edwards—Jones—BreWer (’36) which is to be reported here. It is listed in the embryological collection of the Department of Anatomy of The University of Chicago as H 1496.
History of the Specimen
Mrs. P., aged 38 years, entered St. Luke’s Hospital on March 19, 1935. She complained of slight pain in the right lower quadrant for 8 months. She had had three normal full—term deliveries, the last in June, 1934. Her menstrual periods began at 13 years of age, occurred regularly, and the flow lasted 4 to 5 days. The last menstrual period began February 15, 1935 and ended February 19, 1935. The period expected on March 15, 1935 failed to appear. No abnormal bleeding was noted by the patient. An accurate coital history could not be obtained. Three weeks prior to operation vaginal examination revealed uterine fibroids and a right ovarian tumor. She Was not subsequently re-examined. This is stressed for the reasons which will be given below. On March 20, 1935 (the thirty—fourth day of the menstrual cycle), a hysterectomy and right oophorectomy were done. There was a dermoid cyst of the right ovary and the congested uterus contained numerous small fibroids. Since an early pregnancy was suspected, the round ligaments were grasped with forceps to support the uterus during the hysterectomy. The uterus was not touched except by the knife during the supra-cervical amputation.
Material and Methods
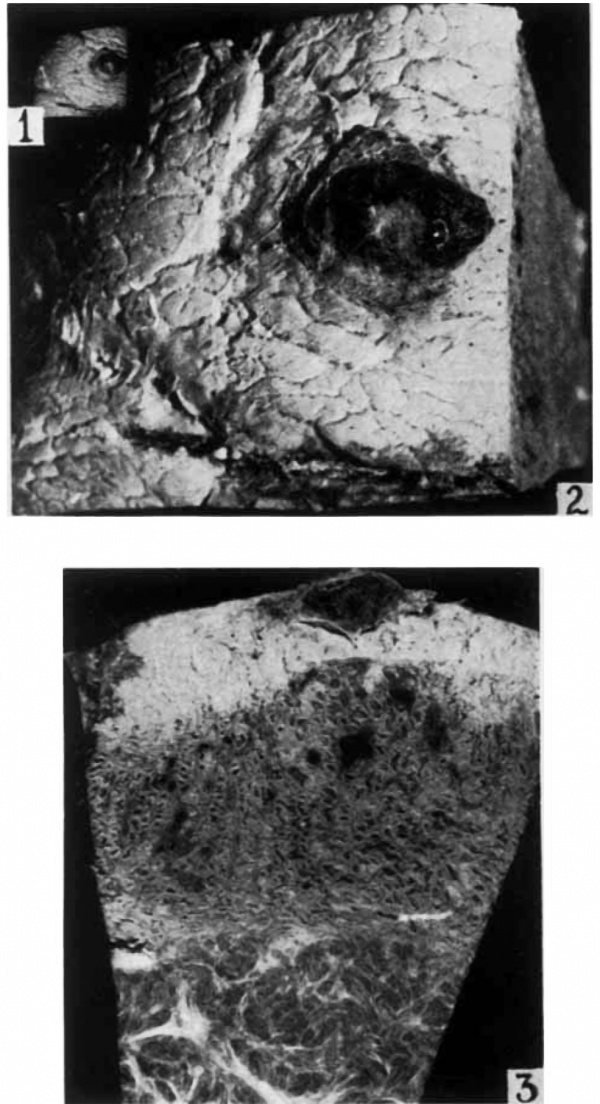
Immediately after removal the uterus was carefully opened longitudinally along its lateral Wall. On the posterior Wall of the endometrium there was a small adherent mass of redblack material distinctly elevated above the surface (figs. 1, 2 and 3). A block of tissue, 12 X 13 X 9 mm. encompassing this mass, was excised and a portion of the myometrium was included. figure 1 shows the actual size of the tissue removed. It was placed in aqueous formol—ehrom—sublimate (Bensley) solution Within 10 minutes after clamping the uterine blood supply so that the fixation is excellent.
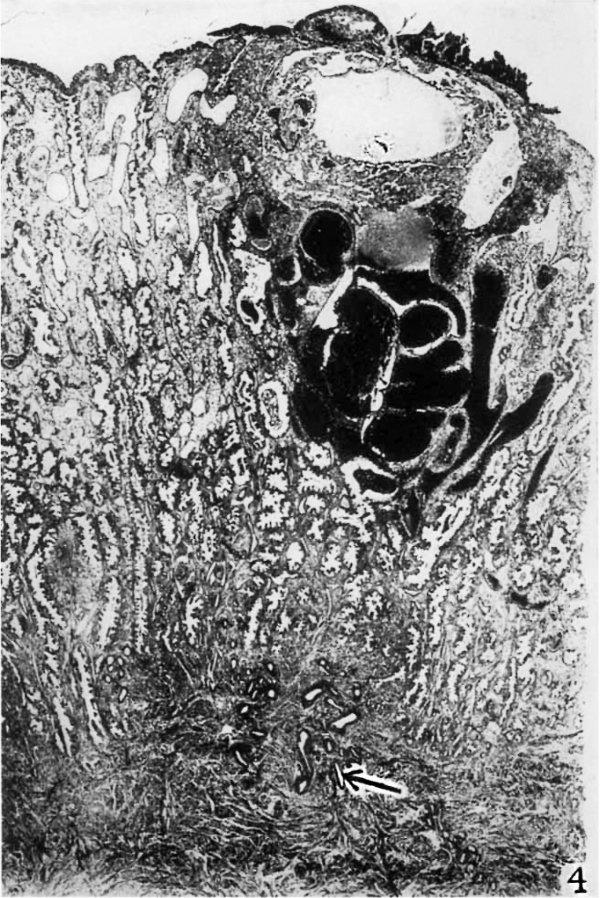
Photographs of the block were taken when in alcohol (figs. 1, 2 and 3). The block was then oriented so that sections were made at right angles to the longest dimension of the chorionic cavity. The long dimension was parallel to the muoosal surface, similar to the Heine—Hofbauer ovum (’11), and contrary to the Jung specimen (’08). It was embedded in paraffin and sections were out and mounted serially, some at 10, 8, 6, 5 and a few at 4 e. No sections were lost. All sections included the entire thickness of the decidua and a portion of the myometrium (fig. 4). The embryonic disc was cut slightly obliquely to the embryonic axis. Sections through the embryonic: anlage were cut at 8 u and stained with hematoxylin and eosin. The remaining sections of the blastocyst were stained -either with Mallory’s connective tissue stain, acid fuchsin and methyl green, Bielschowsky—Maresl1 silver stain, or Best’s earmine.
Description of the Specimen
The dermoid cyst of the right ovary is 9 X 6 X 5 cm. The supracervically amputated uterus containing numerous small fibroids is 7 cm. long, 7 cm. wide and 5 cm. between the horns. The wall of the uterus in the anterior mid—sagittal plane is 33 mm. thick and of this 5 to 6 mm. are gray-red endometrium. The blastocyst, completely embedded on the posterior Wall of the uterus, is not elevated (figs. 1 to 3). Practically all other young ova in vvliic-l1 the location is given are implanted on the posterior Wall, the one exception being the von Möllendorff O. P. ( ’21).
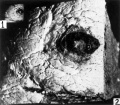
In our specimen the blastocyst is directly beneath the elevated red—black mass noted above (fig. 4.). This mass in the fixed tissue measures 4.2 X 2.7 mm. and projects 0.67 mm. above the surface. Inthe center there is a depressed, circular, more transparent region, 0.95 mm. in diameter (figs. 2, 4, 5 and 12). This represents the site of the point of entrance of the ovum. The measurements of the blastocyst and embryonic structures made from the serial sections are:
- External chorion 3.6 X 3.0 X 1.9 mm
- Internal chorion 1.85 X 1.71 X 1.01 mm
- Embryonic disc 0.208 X 0.188 mm
- Amnion 0.224 X 0.186 X 0.167 mm
- Yolk sac 0.197 X 0.160 X 0.120 mm
- Greatest length of mesodermal villus 0.27 mm
The estimated age of this ovum made by comparison with other ova is 15 days. The blastocyst is completely covered by short villi (fig. 5) which have minute mesodermal cores. The longest is 0.27 mm. They are most numerous on the basal side as compared to the lateral and capsularis regions. The chorionic wall is composed of three layers. The outer multinucleated syncytial layer has a brush border and is of irregular thickness. The inner, single layered Langhans’ cells are cuboidal with scant cytoplasm and definite cell membranes. Many are in mitosis. The cells of the mesoblast are spindleshaped and loosely arranged. There is a condensation of this tissue along the bordering edge of the cavity. Only occasional strands are scattered through the blastocyst cavity. This is similar to the ova of this age group except the Jung specimen (’08) in which the chorionic cavity is filled with mesoblast up to the amnion and yolk sac.
The Langhans’ cells extend out beyond the ends of the villi in cell columns (fig. 6). They extend toward the decidua and also laterally to join similar cells from the neighboring villi. There is, however, no complete trophoblastic shell. These cells to which the term cytotrophoblast has been applied are dividing rapidly by mitotic division, particularly those closest to their source of origin, namely, the Langhans’ cells of the villi (fig. 7). The cytotrophoblastic cells most remote from the villi are enlarged, have unevenly distributed cytoplasm, and irregular nuclei. Hcre_mitotic figures are rare. In the Jones-Brewer I ovum3 lipoids were demonstrated in these cells (Jones and Brewer, ’35).
Many of the cytotrophoblastic cells as they become larger acquire a more deeply staining cytoplasm similar to that of the syncytium. The nuclei are large and typically notched in these cells as in the syncytial cell masses. Neighboring cells fuse to form typical multinucleated syncytium.
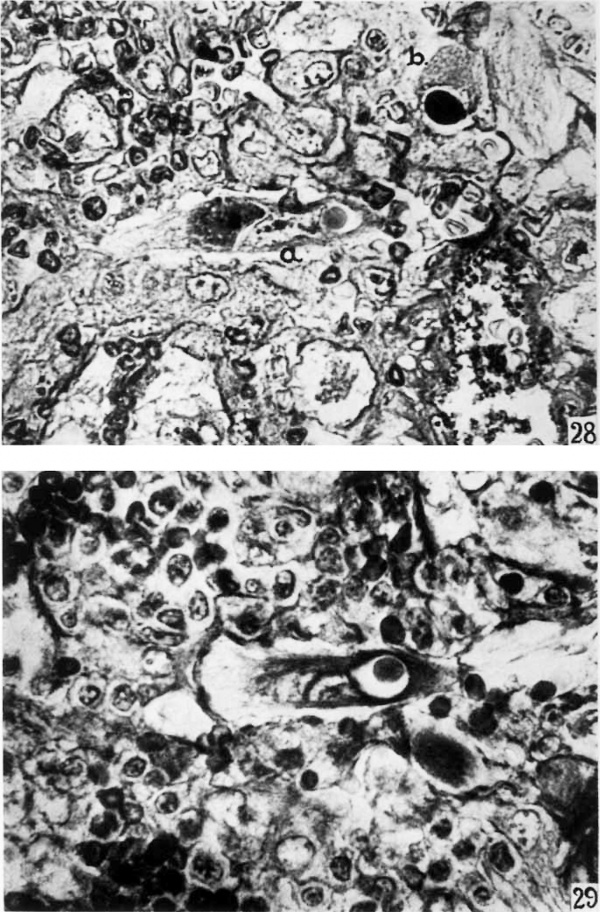
In figure 11, stages in the transition of cytotrophoblast to syncytium are shown. The cell at ‘a’ with a notched nucleus has a more deeply stained cytoplasm than the adjacent cells. The staining quality is identical to the adjacent syncytial. mass. At one place the cell membrane is not recognizable and there is a fusion of the cytoplasm of this cell with that of its neighbor.
The cell at ‘b’ has acquired a distinct brush border similar to that of the large syncytial mass, is slightly larger, and has fewer vacuoles than the adjacent cytotrophoblastic cells. The cytoplasm stains more deeply. The nuclei of the various cells are identical. Transitions to this stage of syncytial development are repeatedly noted. In the Joncs—Brewer I specimen (’35), an identical transformation of eytotrophoblast to syncytiurn was recorded and mitochondrial changes were described. Some of these types demonstrated phagocytic activity. In this study, thus, further evidence is added to the prevalent conception that cytotrophoblast is transformed into syncytium.
The syncytium covering the chorionic wall invests the short villi, except at their tips. It is continuous with some of the masses of syncytium in the implantation cavity. The latter masses are thin, elongated, and joined with one another (fig. 6). This type of syncytium scattered amid the cytotrophoblast is similar to the so-called primary syncytium. It would seem then that the peculiar form is an adaptation to the surrounding medium.
- The Jones-Brewer I ovum (’35) is designated H1459 in the series at The University of Chicago. Its age is definitely placed at 18% days. It has a primitive streak, groove, cloacal membrane, allantois, and beginning neurenteric canal formation. The measurements are:
- External chorion (before fixation) 8 X 7 X 4 mm
- Internal chorion (before fixation) 6 X 5 X 2.5 mm
- Embryonic disc 0,58 x 0,782 mm
- Amnion 0.592 X 0.805 x 0.26 mm
- Yolk sac 0.72 x 0.79 X 0.76 mm
The syncytium of the chorionic wall and that lying free in the implantation site has a brush border. The more deeply penetrating masses do not. The brush border consists of closely grouped filaments, continuous with the protoplasm itself (fig. 11, e). Each filament seems to be composed of granules or short rods arranged end on end. In figure 11 the cell marked ‘b’ has a recently acquired brush border. It is developing also in the cells ‘c’ and ‘d.’
The fetal wandering cells that penetrated into the maternal tissues are similar to those described in the Jones-Brewer I ovum. Frequently they are stretched out and in some instances stain deeply (fig. 8). They correspond to the type that in the later stages invades the decidua in streamer—1ike formation (fig. 5, Jones and Brewer, ’35). A second type is shown in figures 10, 26 and 28. They rather resemble cytotrophoblastic cells and are phagocytie. They are similar to those cells in figure 7, Jones and Brewer (’35). Transitions of cytotrophoblastic cells to form this cell type are noted repeatedly in both specimens. The fetal cells are larger than the adjacent decidual cells and have larger nuclei, both relatively and actually, than the maternal mononuelear phagocytes. That they are fetal cells is further evidenced by the absence of transitional stages from maternal cells.
In the immediate region of the ovum syncytium similar to that in the implantation cavity is noted invading maternal tissues (figs. 22, 23 and 24).
The embryonic anlage consisting of the yolk sac, disc, amnion, and body stalk is situated on the most dependent wall of the blastocyst (fig. 5). The entire structure measures 0.365 X 0.312 X 0.249 mm. Most reported human embryos are attached at this point, or at least on the lower half of the chorion, the principal variant being the Manchester 1285 ovum, described by Florian and Hill (’35), whose body stalk attachment is at the pole of the blastocyst nearest the uterine cavity.
The Operculum
Online Editor - describes the uterine clot region (lid) at the point of implantation.
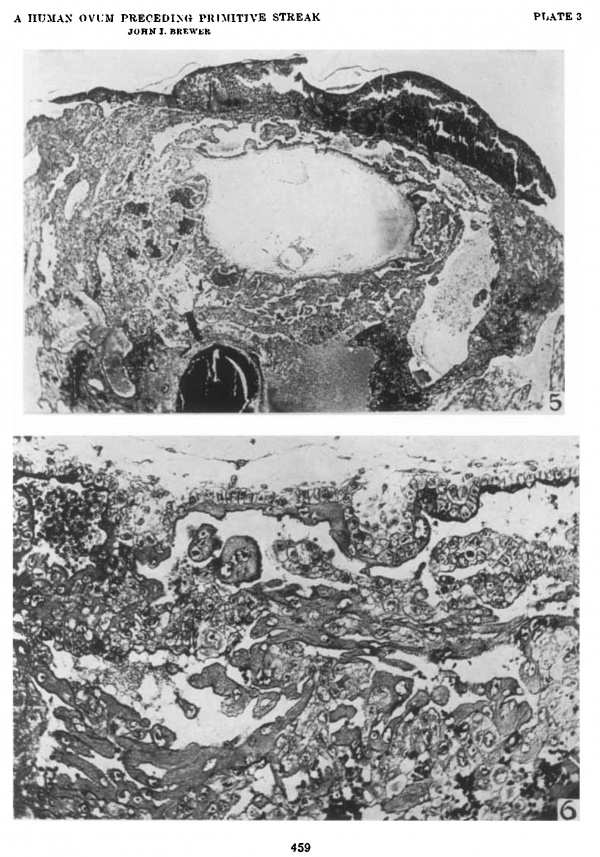
The point of entrance of the ovum is described in the gross specimen as a central depressed region in the elevated blood clot and is pictured in figures 2 and 5. It is 0.95 mm. in diameter. The surface epithelium of the decidua is absent over a slightly larger area than that of the aperture. The opercular tissue is sharply delineated from the surrounding decidua. capsularis (fig. 12). At the base the opercular tissue is inseparably fused with fetal trophoblast.
The closing coagulum, as it is called by Bonnet (1899), is similar to that described by Peters (1899). It comprises several tissues. The trophoblast arranged in multinucleated masses or individual large cells is alprominent and constant finding. Some cells are elongated and thin similar to those noted by Teacher (’24). Many of these cells are undergoing regression. The maternal cells dispersed generally through the opercular tissue are fewer in number than the trophoblastic elements but are readily discernible. They are identical to the stromal cells. They are smaller and have absolutely smaller nuclei than any of the fetal cells in this specimen. The nuclei are regular, rounded, and contain relatively more chromatin material. Many cells evidence degenerative changes.
In the lower portion of the plug there is a dense accumulation of fibrinoid. This material stains a dense blue, while the fibrin in the clot appears red in Mal1ory’s connective tissue stain. Teacher (’24) in the Teacher-Bryce II ovum also called attention to the different staining qualities of these materials in the operculum but considered them different stages in fibrin formation. Erythrocytes and a few leucocytes are scattered through the operculum.
The elevated blood coagulum on the surface of the decidua overlies the surface epithelium (fig. 5). This coagulum is made up of erythrocytes and small masses of fibrin and fetal cells. It is firmly adherent about the operculum. The fetal cells in it appear to have penetrated the surface epithelium beneath the clot (fig. 25).
These findings are consist-ent with those of Linzenmeier (’14), Jung ( ’08), Sehlagenhaufer and Verocay (’16) who reported similar findings i11 their specimens, all of which are near the age of the ovum reported here.
The operculum in this specimen is larger and more of an organized structure than that in the Jones—Brewer I specimen which is approximately 3 days older. In still older presomite ova which I have studied the point of entrance is not evident. This agrees with the conclusion drawn by Teacher (’24) that the aperture is rapidly sealed and flattened out, leaving no mark on the capsularis.
Trophoblastic Activity
The exact extent of the activity of the trophoblast is a mooted question. The majority of Writers have described active destruction of maternal tissue by the trophoblast, par-ticularly the vein walls. Many consider the invasion of glands as a chanced happening without significance.
The active invasion and destruction of maternal tissue by fetal trophoblast is evidenced in the study of the reticulum, the maternal blood vessels, the uterine glands, and in the processes of phagocytosis.
The reticulum of the normal Vera in sections prepared by the Bielschowsky—l\Iaresch silver method consists of thin, even, regular strands. The strands branch and anastomose freely, surrounding the stroma cells throughout all portions of the decidua. In. sections stained by Mallory’s connective tissue technique the fibers are colored a deep blue.
In the penetration zone in the silver impregnated sections the reticular strands are widely separated, unevenly arranged about the decidual cells, and frayed (fig. 20). In numerous places the strands are markedly thickened and occasionally are arranged in masses and clumps. Many appear as thickened bundles. They are most abundant at the junction of fetal and maternal tissues. At the margin of the penetration zone Where the maternal tissue abruptly ends, tl1e reticulum is frequently arranged as a border and frayed ends jut into the implantation cavity (fig. 21). There are some irregular strands completely sev-ered from all connections and lying free between the cells of the trophoblast. The occurrence of phagocytized strands within fetal cells will be described later.
In the Jones-Brewer I specimen ( ’35) silver preparations demonstrate similar disruption of the reticulum. In this specimen a gradual transition from the normal reticulum to the scattered, irregular strands nearer the implantation cavity also is noted. At one place there is a dense layer along the border between fetal and maternal tissues. This layer of reticulum appears to act as a barrier to trophoblastic penetration. As noted in the original publication, destruction of maternal tissue behind this layer is at a minimum, while the tissues unprotected by this dense layer evidence extensive disintegration.
Maternal and fetal tissues are more readily distinguished in sections impregnated with silver since no reticulum is de~ veloped in the epithelial fetal tissue (Grosser, ’27). The isolated strands and masses repeatedly noted, both free and phagocytized, are obviously degenerated maternal reticulum. It is thus possible to judge with some degree of accuracy the depth to which maternal tissue has been injured.
The stromal cells in the penetration zone evidence considerable damage, particularly those nearest the fetal tissues. The cells located between the thickened, irregular strands of reticulum are enlarged. Their cell membranes are irregular. The cytoplasm is scant and stains poorly in many instances. The nuclei are uneven and pyknotic. Farther out from the ovum in the penetration zone the normal stromal cells are frequently pushed apart by fetal Wandering cells. In the regions of extensive hemorrhage in the penetration zone, it is occasionally diflicult to identify maternal cells as such. In these two specimens no stromal cells undergoing mitotic division are found in the penetration zone.
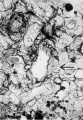
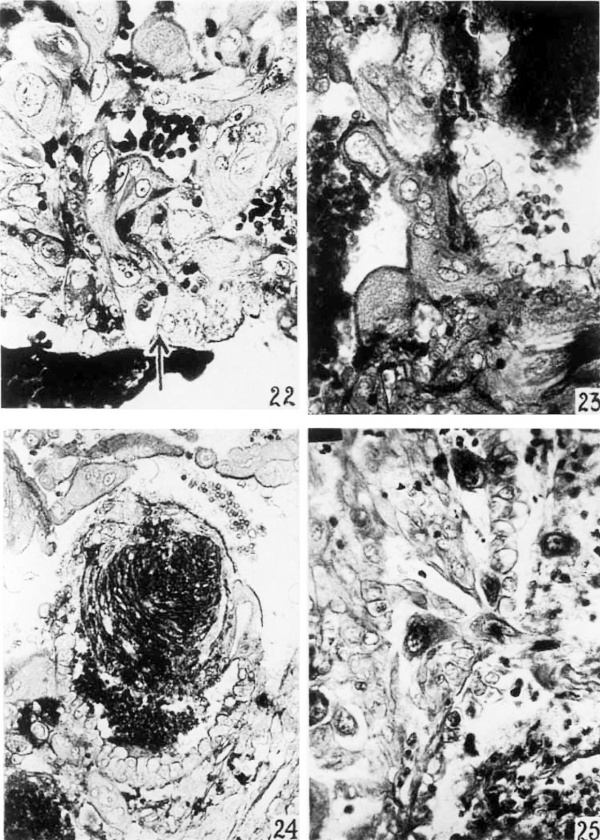
The glands are destroyed and invaded by the trophoblast. All of this maternal tissue necrosis is not, however, the direct result of trophoblastic activity as will be shown later. In figure 22 there are numerous masses of syneytium applied closely to a gland wall in the basal region. At this point there is an extensive disintegration of gland epithelium and a mass of syncytium projects through the gap into the gland lumen. One gland cell has a completely vacuolated cytoplasm and an irregular nucleus. The syncytial mass approximates this cell. In figure 24 a basal gland is demonstrated extending into the implantation cavity. Its wall is replaced in part by syncytium and in the lumen there is an organizing blood clot surrounded by a more recent hemorrhage. Wislocki and Hartman (’29) similarly demonstrated in the macaque a gland opening into the intervillous space. They state it is not common, however. In this specimen it is a frequent finding. figure 23 portrays the destruction of several gland cells with a mass of tropho~ blast in close relation. In the slightly older J ones-Brewer 1 ovum, similar and more extensive destruction of glands occurs.
Beneath the blastocyst numerous glands are widely dilated and filled with blood (fig. 4). This finding has been noted by many authors. Blood enters the gland lumen by means of the destruction of gland epithelium in the penetration zone, through holes made in the gland wall with approximated tro— phoblast as described above, and thirdly, to some extent, perhaps by diapedesis. A direct communication between a capillary and gland lumen is not a common finding. The coagulation of blood in the gland lumen (fig. 24) was noted also by Peters (1899) and Jung (’08) in specimens near the age of the one reported here. From the glands the blood may escape into the uterine lumen. VVhile the destruction of glands in these specimens is extensive, in younger reported specimens, namely, the Miller (Streeter, ’26), the Kleinhans (Gross-er, ’22)., and the Teacher—Bryce I (Bryce and Teacher, ’08), there is little or no necrosis ‘bf gland tissue.
Not only the gland epithelium is destroyed by the trophoblast but also the surface epithelium is occasionally involved. In figure 25 a fetal wandering cell is shown penetrating the surface epithelium. The epithelial cells adjacent to this trophobla.st are degenerating. Peters (1899) described trophoblast directly beneath the surface epithelium and Linzenmeier (’14.) called attention to trophoblast actually penetrating the surface epithelium.
After a careful and prolonged search of the arteries entering the penetration zone, one artery was found whose wall is invaded and destroyed in part by fetal trophoblast (fig. 10). The vessel was not carrying blood as the capillary bed it supplied had been. destroyed. In the Jones-Brewer I specimen there was no demonstrable invasion of arterial structures by fetal cells and there was only this one instance in the EdwardsJ ones-Brewer ovum. The failure to find invasion of arteries is readily explained by the fact that the arteries which lie in the penetration zone have already been altered to simple endothelial tubes, a condition noted in later stages by many observers.
Invasion of capillary walls by trophoblast is an active and extensive process and has been noted in all specimens studied. Fetal cells are found penetrating endothelial walls and lying free within blood vessel lumina.
In figure 9 a large lateral venous space is shown with a portion of its wall destroyed. filling this defect are masses of trophoblast and through the opening maternal blood escapes into the implantation cavity.
The arm of this sinus which anastomoses with the basal sinus has had its wall nearest the fetal tissue entirely destroyed, thus forming an open space in which fetal tissue lies. The other wall is made up of normal endothelial cells. On the ‘opposite lateral margin of the blastocyst there is a similar dilated Venous space whose wall presenting toward the ovum is wanting (fig. 5). The other wall is in some places replaced by fetal trophoblast. Destruction of lateral sinus-es is a common finding in most ova.
Phagocytic Activity of the Trophoblast
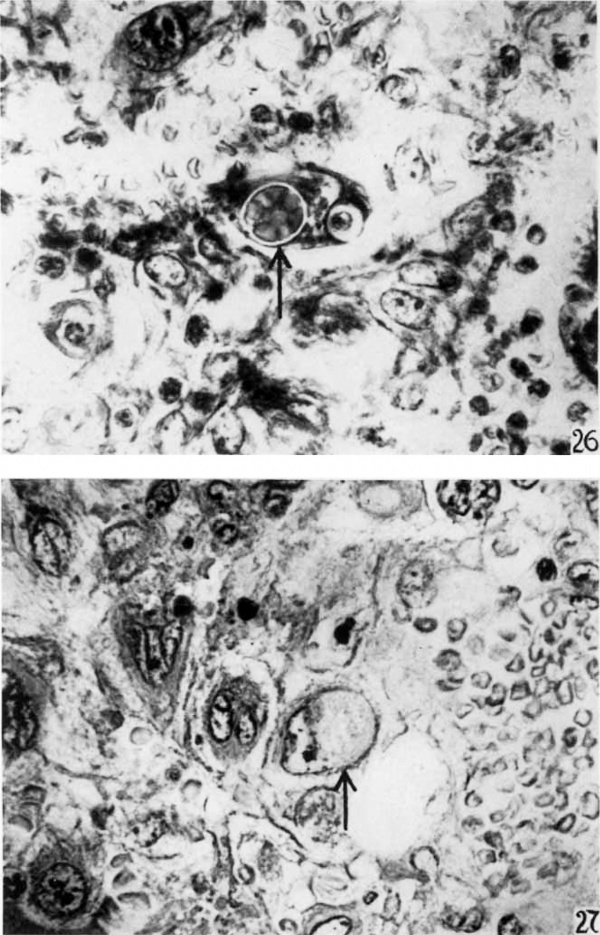
That the fetal trophoblast has the property of phagocytizing maternal tissues has been often described but has not been adequately illustrated. Phagocytic activity is demonstrated by the syncytium and cytotrophoblast in the implantation cavity and by the large trophoblastic wandering cells in the penetration zone. These large wandering cells (fig. 29) with inclusions are fetal and not maternal elements as thought by some. These elements have absolutely as well as relatively larger nuclei than the large mononuelear phagocytes of ma‘ ternal origin. The nuclear membrane is irregularly folded. Also in these cells glycogen is present as demonstrated in the J ones-Brewer I specimen (Jones and Brewer, ’35). The maternal tissues most commonly phagocytized, as noted in these specimens, are red blood cells, leucocytes, lymphocytes, and reticulum. Evidence indicates that most of the material phagocytized has already undergone a certain amount of necrosis. The included material usually appears to lie in a cytoplasmic vacuole. This is not true, however, of the phagecytized reticulum which, in all instances, is in intimate relation with the cytoplasm. A fetal wandering cell is shown in figure 26 with a large mass of red blood cells in its cytoplasm. At the opposite end of the cell there is a phagocytized lymphocyte. The phagoeytized erythrocytes rapidly lose their l1vemo— globin and are recognized only by their sharp regular outline. In stages still more advanced in the disintegration of phagoeytized red blood cells the membranes disappear and leave a more or less granular mass as a cytoplasmic inclusion (fig. 27). In some cells the granular inclusion mass is dense (fig. 28, Li). This becomes, then, a homogeneous mass which stains an intense red with eosin and usually a blue in the Mallory connective tissue stain (fig. 28, 17). Similar inclusions have been noted previously and were thought to be secretory products of the fetal cells. A detailed study of the phases of phagocytosis leads to the conclusion that these uniformly staining inclusions are the end—products of disintegration of the inclusion material. Greenhill ( ’27) has demonstrated the presence of blood pigments in these Wandering cells. Most of the phagocytized leucocytes are in stages of degeneration. Some, however, are normal appearing except for a clumping of the cytoplasm.
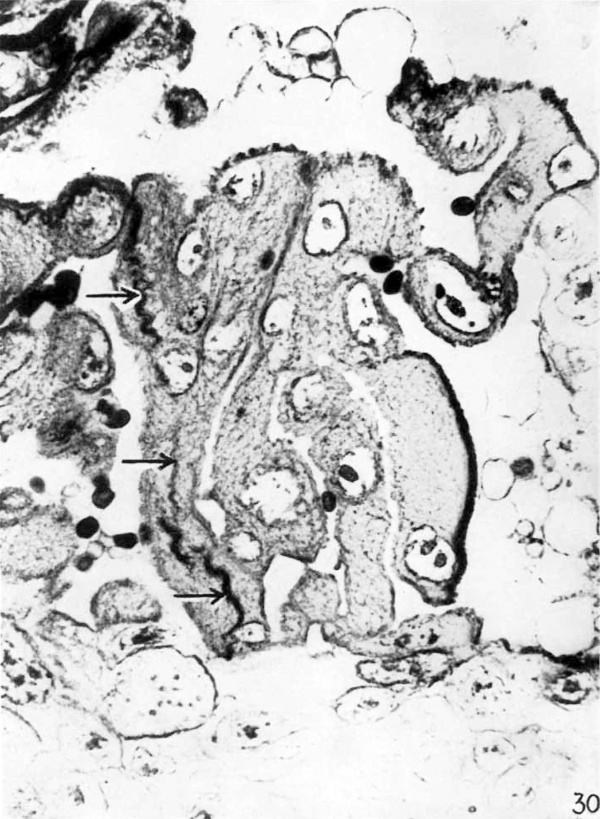
Phagocytized reticulum is readily demonstrated in sections prepared by tl1e Bielschowsky-Maresch silver technique and in sections stained with Mallory’s connective tissue stain. figure 30 portrays a dense collection of strands of reticulum in the cytoplasm of a syncytial mass in theimplantation cavity. \Vith silver stains this material is deeply impregnated. Trophoblastic elements in the penetration zone contain within their cytoplasm strands of reticulum which are directly continuous vvith reticulum of the maternal tissues (figs. 20 and 21).
In figure 11 a group of cytotrophoblastic cells is shown, two of which contain phagocytized material at ‘d.’ The materials phagoeytized are leucocytes in various stages of regression. These inclusions are proved to be intracellular by a study of the adjacent serial sections.
The cells in figure 11, ‘d’ are similar to the cell ‘b’ except that there is only a faint brush border.
Vascular System
A study of the vascular bed is possible in this ovum since a portion of the myometrium and the entire thickness of the endometrium are included in the sections. Such a study is important because of the large amount of blood and hemorrhage about the implantation site. The hemorrhage is not a result of trauma by bimanual examination since the patient was not examined for 3 weeks prior to operation. Trauma during surgical removal of the uterus and resection of the specimen was avoided by holding the uterus only by the round ligaments.
Since the ovum is implanted directly over a spiral artery the possibilities of hemorrhage in this region may consequently be greater than that in the tissues between the regu« larly spaced spiral arteries. In the Peters ovum, which is almost identical, there is a similar amount of blood and hemorrhage (Peters, 1899); Some consider this to indie-ate that the Peters ovum is abnormal. The hemorrhage and vaseularity are normal conditions in the Edwards-Jones-Brewer specimen, however, as a study of the vascular phenomena demonstrates. The Peters ovum may be similar.
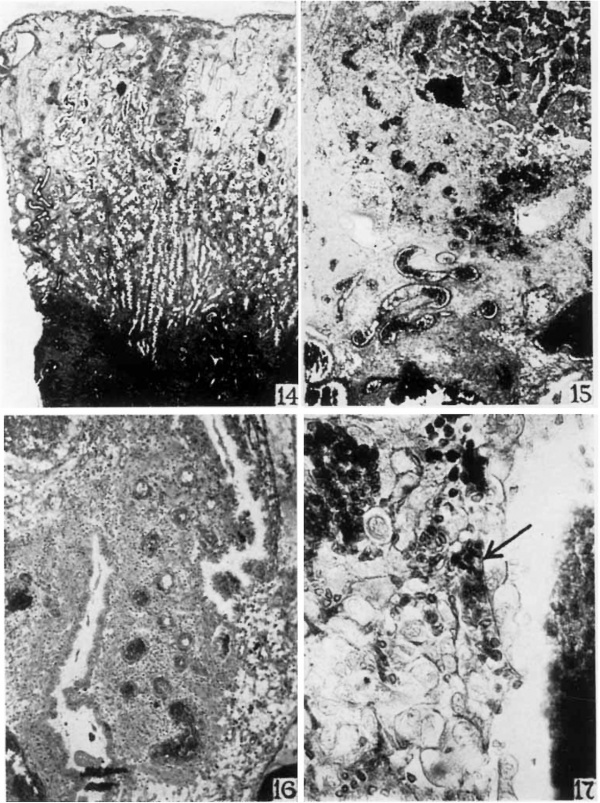
A spiral artery in the deeper half of the vera is shown in figure 14. It is large and tortuous and extends perpendicularly toward the surface. In this deeper portion of the vera there are few branchings. Near the middle of the photomicrograph a spiral artery is shown in the peripheral half of the vera. It is divided into two main branches which extend to the surface epithelium. Just beneath the surface each breaks up into a dense- capillary system. Smaller branchings are numerous in the superficial zone.
The spiral arteries are independent of the endometrial glands. About the spiral arteries in the superficial vera there are accumulations of closely grouped young decidual cells (fig. 14). There are no uterine glands in these accumulations. The superficial half of the vera except the regions about the arteries noted above is edematous (fig. 14).
In figure 4 there is a spiral artery just beneath the implantation site. It is divided into two main branches in the myometrium just below the endometrium. Each branch enters the vera and extends upward toward the surface. Unlike the vessels in the vera. these arteries do not reach the surface but extend only to the penetration zone. In figure 15 one of these main branches is shown to enter the penetration zone. It curves outward about the blastocyst. This portion of the vessel is dilated and filled with blood (fig. 15). That portion of the vessel deeper in the spongy zone is of small caliber and is empty, indicating a vascular spasm (fig. 4). Similar evidence of spasm is noted repeatedly near the ovum.
The portion of the artery in the penetration zone (fig. 15) is void of muscular coat. The coats of most of the arteries are lost when the vessels enter this zone. The vessel then ends blindly as an endothelial tube in the penetration zone (figs. 15 and 18). A few of the arterioles retain their muscle coats but terminate in the same region (fig. 19).
About the ends of these capillaries and small terminal arterioles in the regions Where the endothelial wall is ruptured there is extravasation of blood (figs. 15, 18 and 19). In some instances a rupture of the vessel wall cannot be demonstrated although there are adjacent accumulations of extravaseular blood.
Evidence of vasoconstriction is had in the deeper endometrial region as noted above. figure 16, however, shows the vasoconstrietion phenomenon in an isolated branch of a. spiral artery near the implantation site. One branch has a narrow, empty lumen while the other is dilated and filled with blood. Definite evidence of arterial spasm such as this is noted at various levels in the endometrium.
The venous spaces in the Vera (fig. 14) are dilated only in the superficial zone. Some are empty and others contain blood. In the region of the ovum the venous spaces are much larger and are more markedly distended with blood in comparison with the sinuses in the Vera (figs. 4 and 5). The sinuses nearest the ovum frequently fuse with one another and form larger blood filled spaces (fig. 4). Many of the sinuses, particularly those at the base of the ovum, are growing as evidenced by mitotic division of the endothelial cells. One sinus contains seven endothelial cells in mitosis in five of the consecutive histological sections.
The huge lateral sinus shown in figure 9 communicates directly with the large basal sinus (fig. 4). The opposite lateral sinus (fig. 5) also is connected with the basal sinus. The large basal sinus is filled with blood and is similar to the basal sinuses described in most early ova. The veins leading away
from the sinuses are straight and narrow. Their lumina are filled with blood (fig. 4).
Comparison of the Vascular Phenomena to those of Menstruation
A survey of the Vascular phenomena in the Edwards-Jones-Brewer ovum demonstrates that they are fundamentally similar to those of menstruation in the human and in the macaqu-e. The phenomena characteristic of menstruation are vasoconstriction and Vasodilatation of the long spiral arteries, superficial venous engorgement, disruption of the superficial capillary system, extravasation, a11d ischemic necrosis of the superficial endometrial tissue. Bartelmez (’37) has summarized and correlated these phenomena.
The anatomical arrangement of the vessels of the Vera, their extension to the epithelial surface, the formation of a dense superficial capillary system, and the dilatation and engorgement of the venous sinuses are identical to the described vascular system in the pseudopregnant endometrium of an ovulatory cycle (Lahm, ’26; Bartelmez, ’31 and ’33; Daron, ’36; Markee, ’33).
The vascular changes in the region of the ovum differ slightly in that they resemble more closely those associated with beginning menstruation in an ovulatory cycle.
The variations between the vascular phenomena in the Vera and in the region of the ovum are similar to the variations noted by Bartelmez (’33), Daron (’36) and Markee (’36) in different portions of the same uterus at the onset of menstruation. This similarity of reaction in the non-pregnant and the pregnant uterus suggests that there is a close relationship between the phenomena noted in the two.
In the region of the ovum the Vascular system is similar to that in the endometrium at the onset of menstruation and differs from that in the Vera in the following ways. The spiral arteries do not reach the surface epithelium. The arteries are shown to lose their coats some distance beneath the surface and end in a capillary system in the penetration zone (fig. 15). The arteries do not reach the surface because of this alteration to capillaries. Bartelmez (’33) demonstrated an identical loss of muscular coats of the arteries during menstruation and Peters (1899) described the same phenomenon in an early implantation. Bartelmez (’33) indicated that it was an ischemic necrosis resulting from vasoconstriction. In this ovum a similar conclusion is tenable.
The second point is that the vessels in the region of the ovum are in spasm. This spasm or vasoconstriction of the spiral arteries noted in the deeper portions of the pregnant endometrium is identical to that. described as being characteristic of menstruation by Lahm ( ’26), B-artelmez (’33), Daron (’36) and Markee (’36). Vasoconstriction, however, occurs at all levels as shown in figures 15 and 16, which is identical to the findings of Daron (’36) and Markee (’36). The occurrence of spasm in some arteries, and even in a few isolated branches of an artery, as described in this ovum is similar to the desqriptions by Bartelmez (’33) and Daron (’36) of the individual variability of the vessel response.
The phenomenon of vasoconstriction while identical to that noted in menstruation is not as extensive or as intense. It seems adequate, however, to explain the necrosis in the superficial zones in the same manner as Lahm (’26), Bartelmez ( ’33), Daron ( ’36), and others account for the ischemic. necrosis i11 menstruation. The fact that ischemic necrosis and vasoconstriction are so intimately associated and are so restricted in occurrence in this pregnant uterus supports this contention.
The third point is that in the region of the ovum there is a venous congestion and stasis. The extensive character of this is shown in figures 4 and 9. As compared with other ova, it is considerably greater with the exception of the Peters specimen (1899). It is, however, not unlike that described for menstruation. The cause of the congestion is believed to be directly due to spasm of the spiral arteries since the congestion in this ovum is noted only in that region where vascular spasm is profound.
The superficial venous. system during the development of the endometrium in the menstrual cycle becomes increasingly large simultaneously with increased vascular spasm. The development of such sinuses continues to increase in the region of the implanted ovum (fig. 4). Not only do the individual sinuses become larger here but also the lateral walls of adjacent sinuses fuse to form larger blood spaces (fig. 4). The large basal sinus characteristically found in all early implantations communicates freely with the large sinuses noted above. In this ovum the sinusaformations are so large and extensive that the ovum to a large extent li-es free in these blood filled spaces. These facts indicate that the development of the sinuses is an essential part of the implantation phenomenon. In this manner not only blood is available for the implanted ovum but also there is abundant opportunity for the trophoblast to extend centrifically with little interference through fluid filled spaces. This latter fact is consistent with the rapid growth and extension of the fetal tissue.
The fourth point is that there is extensive extravasation about the implantation site. This extravasation, which is intimately associated with venous congestion, is identical to that which occurs near the onset of menstruation. The extravasated blood is mingled with stromal cells identically to that described by Bartelmez ( ’33) in menstruation.
In menstruation -extravasation occurs as a result of a disruption of the superficial capillaries. The endothelium undergoes an ischemic necrosis according to Lahm (’26) and Bartelmez ( ’33). I11 this pregnancy there is a similar disruption of the capillary system in the region of the ovum with marked extravasation. Extravasation occurs not only as a result of a necrosis of the endothelial cells but also by diapedesis in a manner identical to that described by Markee (’33,) in menstruation and by Teacher (’24) in a human implantation. In menstruation the endothelial necrosis has been shown to be the result of Vasoeonstriction. A similar explanation is suggested in this ovum since there is multiple evidence of vascular spasm in the region of the disrupted capillary system.
This view is in contradistinction to the previous contention that all the disrupted capillaries are destroyed by the trophoblast, either by direct apposition of the tissues, or by distant action of ferments.
The necrosis of the endothelium in many instances in this specimen, however, is more logically ascribed to the vascular phenomena noted. The fact that identical necrosis occurs in menstruation enha.nces this conclusion. The works of Long and Evans (’20), Evans ( ’28) and Kreihbel (’35) support this view. They demonstrated that extravasation identical to that described above occurred in deeiduomas in the absence of pregnancy.
The purpose of the extravasated blood was described by Hubrecht in 1889 who stated that it was needed for nourishment for the early ovum. Hartman (’32), Bartelmez (’33), and others have repeated this statement. The distinct regional occurrence of the phenomenon in this ovum is evidence in favor of such a purpose of extravasation in the human.
Another point and one closely associated with a disruption of the capillary system and extravasation is the extensive necrosis of the maternal stromal and gland cells. I11 the Edwards—Jones—Brewer ovum a hole in the side wall of a gland is demonstrated through Which maternal blood enters the gland lumen. This hole has been produced in the absence of adjacent trophoblast. Similar pictures were described in the J ones—Brewer I ovum (Jones and Brewer, ’35) and they also have been described by numerous other authors. The holes are the result of disintegration of the gland cells as evidenced by the irregular and poorly staining cells. The means by which this necrosis is produced has been thought to be due to a substance secreted by the syncytium which has the power to destroy tissue. On the other hand, the theory of a deficient vascular supply as elaborated above is quite possibly a factor in such necrosis.
In favor of this are the facts that the necrosis is found only in the regions Where vascular spasm is _evidenced, and that similar necrosis occurs in menstruation in the absence of pregnancy. Additional evidence described above supports this View since the maternal tissue is demonstrated to have undergone degenerative changes prior to attack and phagecytosis by the trophoblast.
The Circulation in tiie Region of the Ovum
Vasoconstriction of the spiral arteries has been shown to control the bleeding in menstruation. The circulation in the region of this young implanted ovum is controlled in a similar manner. The extensive disintegration of the capillary system about the ovum, the degeneration of the arterial coats, and the evidence of ischemic necrosis of the gland and stromal cells indicate that there is a reduced arterial flow in this region. Similar evidence in menstruation indicated to Bartelmez (’33) and Daron ( ’36) that there was no typical arterial circulation in the vessels involved. The engorgement in the superficial venousspaces near the implanted ovum is also indicative of a diminished and slowed up circulation.
The fact that no arteries communicate with the implantation cavity further indicates a reduced arterial flow. This is true for most ova described. Thevessels that do communicate are shown to be either dilated venous structures or capillaries. The intervillous space in the normal young pregnancies studied in situ is usually not filled with blood.
These facts imply that the flow of blood into the implantation cavity is necessarily passive, consisting principally of a gradual seeping of blood into that cavity. Thus, it is concluded that there is no real, active circulation in the implantation cavity at this period of development.
‘Placental Sign’ Bleeding
Closely associated with extravasation and implantation is the so-called ‘placental sign’ bleeding of Long and Evans (’20). They demonstrated in the rat. that during implantation there was an extravasation of blood, some of which reached the uterine cavity and appeared as gross bleeding externally. They termed this the ‘placental sign.’ Hartman (’32) demonstrated the ‘placental sign’ in the macaque. In the human demonstration of the ‘placental sign’ has been wanting because of an inadequate number of young implantations. Furthermore, the recognition of this sign in the human may have been hindered by the microscopic character of the bleeding, or the sealing up of tlie uterus by the plug of cervical mucus.
The extravasation of blood limited to the region about this young implanted ovum is identical to that described by Wislocki and Hartman ( ’29) and associated with ‘placental sign’ bleeding in the macaque. Hartman (’32) described blood accumulations in the uterine glands and stated that the blood reached the lumen of the uterus by Way of the gland months. In all human pregnancies reported there is accumulation of blood in the uterine glands. The large amount of blood in the glands of the EdWards—.Iones—BreWer specimen is shown in figure 4. In this specimen blood enters the gland lumen principally by means of a necrosis of the gland Walls. Occasiong ally capillaries open directly into the gland lumen.
Figure 5 shows the blood clot projecting into the uterine lumen. Its character indicates that there has been some bleeding into the uterine lumen some time before the removal of the specimen on the thirty—fourth day of the cycle but that just prior to removal there was no active bleeding. This is consistent with the findings of Hartman (’32) in the macaque in which he stated that the ‘placental sign’ bleeding appeared 29.2 days from the onset of the last flow. Thus, the time element for the appearance of the ‘sign’ is consistent with that described by Hartman in the macaque.
The ‘placental sign’ bleeding although closely associated with implantation is purely a maternal phenomenon and is fundamentally similar to the vascular phenomena described for menstruation by Lahm (’26), Bartelmez (’33), Markee (’36), Daron (’36) and others, and for implantation as described in the Edwards-Jones—BreWer specimen. This is conclusively proved by the works of Evans (’28) who described all of" the phenomena of extravasation and ‘placental sign’ bleeding in the absence of pregnancy in rats. Krehbiel (’35) added to this by producing deciduomas in the macaque and demonstrating the ‘placental sign’ bleeding associated with extravasation in the absence of pregnancy.
The time of occurrence and the similarity of the findings at the implantation site in this human pregnancy to those associated with the ‘placental sign’ bleeding in the macaque and rat indicate the probability of its occurrence in man.
Summary
- This embedded, normal, and complete human ovum with an estimated age of 15 days was obtained by hysterectomy on the thirty-fourth day of the menstrual cycle (figs. 2, 3, 4 and 5).
- A slab of the uterine wall including; the implantation site was placed into formol-chrom-sublimate (Bensley) within 10 minutes after clamping the uterine blood supply. The fixation is excellent.
- The entire block of tissue including the blastocyst, surrounding decidua, and myometrium was cut and mounted serially. The embryonic disc was cut slightly obliquely to the embryonic axis (fig. 5).
- The measurements were made on the prepared slides and model.
- The point of entrance is closed by fetal trophoblast, degenerating maternal cells, and blood (figs. 12 and 13).
- Syncytium is formed from cytotrophoblast (fig. 11).
- The trophoblast invades and destroys maternal tissues (figs. 8, 9, 10, 21, 22, 23, 24: and 25).
- The fetal wandering cells demonstrate phagocytie activity (figs. 20, 21, 26, 27, 28 and 29).
- The large amount of blood about the blastocyst and the clot on the surface are normal features in this ovum (fig. 4).
- The vascular phenomena of vasoconstriction and vasodilatation, superficial ischemic necrosis, superficial venous congestion, extravasation, and reduced arterial flow in the region of the ovum are similar to those described for menstruation.
- Some of the superficial necrosis is the result of these vascular phenomena as described for menstruation i11 contradistinction to the View that the trophoblast or its ferments produces all such necrosis.
- These vascular phenomena serve to prepare and maintain a site suitable for implantation and growth of the young ovum.
- There is no active circulation in the implantation site at this period of development.
- The ‘placental sign’ bleeding probably occurs in Homo.
I wish to express. my appreciation to Dr. G. VV. Bartelmez for his supervision of the work of this report and to Mr. Robert Bensley for the pliotograpliy.
Literature Cited
BARTELMEZ, G. VV. 1931 The human uterine mucous incnibrane during menstruation. Am. J. Obst. and Gynec., vol. 21, pp. 623-643.
1933 Histological studies on the menstruating mucous membrane of the human uterus. Oontrib. to E1nbryol., no. 142. (Reprinted from Pub. no. 443 of Carnegie Inst. of VVash., pp. 141-186.)
1937 Menstruation. Physiol. Rev., vol. 17, pp. 28-72.
BONNET, R. 1899 fiber Einbryotrophe. Deutseh Med. VVoehensehr., Bd. 25, S. 733-735.
BRYCE, T. 11., AND J. H. TEACHER 1908 Contributions to the study of the early development and embedding of the human ovum. James Maelehose and Sons, Glasgow.
DARON, G. H. 1936 The arterial pattern of the tuniea mucosa of the uterus in Maeaeus rhesus. Am. J. Anat., vol. 58, pp. 349-397.
EDWARDS, E. A., H. 0. JONES AND J. I. BREWER. 1936 A normal human ovum in a stage preceding the primitive streak. Am. J. Obst. and Gynec., vol. 31, pp. 672-674.
EVANS, H. M. 1928 Spontaneous deciduoniata in pseudopregnancy. Am. J. Physiol., vol. 85, pp. 149-153.
FLORIAN, J., AND J. P. HILL 1935 An early human embryo (Mancliester collection no. 1285) with capsular attachment of the connecting stalk. J. Anat., vol. 69, pp. 399-411.
FRASSI, L. 1907-1908 VVeit-ere Ergebnisse des Sturliunis cines jungen rnenselr lichen Eies in situ. Arch, f. Mikros. Anat. u. Entwickelungs., Bd. 71, 667-695.
GREENHILL, J. P. 1927a A human ovum approxiniately 19 days old. Surg. Gynee. Obst., pp. 493-501.
- 1927b A young huinan ovum in situ. Am. J. Anat., vol. 40, pp. 315—353.
GnosSF.e,. OTTO 19:22 Zur Kenntnis der Trophoblastschale bei jungen n1ensch lichen Eiern. Ztsehr. f. Anat. 1.1. Entwiekelungs, Rd. 66, S. 178-198.
- 1927 Friihentwicklung, Eihautbildung und Placentation des Meir sehen und der SE—iugetiere. Deutsch. Frauenlieilkunde, Geburtshilfe, Gynérkologie und Xaehbargebiete in Einzeldarstelluiigen. Verlag von J. F. Bergniann, Munehen.
HARTMAN, C. G. 1932 Studies i.n the reproduction of the monkey Maeaeus (Pithecus) rhesus, with special reference to menstruation and pregnancy. Carnegie lnst. Wash. Pub. no. 43-.3, Contrib. to Embryol. no. 134, vol. 23, pp. 1-161.
HEINE AND HOFBAUER 1911 Beitrag zur friihcsten Eicntwicklung. Ztsohr. f. Geburtseh., Bd. 68, S. 665-688.
HUBRECHT 1889 The hedgehog ovum. Quart. J. Micr. Sc., vol. 30, p. 283.
JONES, H. 0. and J. I. BREWER 1935 A normal human ovum. in the primitive streak stage (approximately 18.5 days). Surg., Gyneo. and Obst., vol. 60, pp. 657-666.
JUNG, P. 1908 Beitriige zur friiheten Eieinbettung beim menschliehen Weibe. S. Karger, Berlin.
KREHBIEL, R. II. 1935 Cytological studie of the docidual reaction in the rat during early pregnancy and in the production of deeiduornas. Anat. Rcc., vol. 61, p. 30. (Abstract)
LAHM, W. 1926 Zur Morphologie und Biologie des Menstruationsvorganges in der Uterusschleimhaut. Zentralbl. f. Gyniik., S. 2699-2704.
LINZENMEIER., G. 1914 Ein junges menschliehes Ei in situ. Arch. f. Gyn‘2lk., Bd. 102, S. 1-17.
LONG, J. A., AND H. M. EVANS 1920 A characteristic sign of pregnancy in the rat detectable from the thirteenth to the sixteenth day. Anat. Rec., vol. ‘18, p. 249. .
MARKEE, J. E. 1933 Menstruation in ocular en(10n‘ietria1'tralisplnnts. (A.bstract.) Anat. Rec., vol. 55, Supplement, p. 66.
- 1936 Menstruation in intra-ocular endometrial transplants in Macaca. mulatta. (Abstract) Anat. Rec., vol. 64, Supplement 110. 3, p. 32.
VON MÖLLENDoRFF, WILH. 1921 Uber oinen jungen operativ gewonnenen menschlichen Keim (Ei OP). Ztschr. f. Anat. u. Entwieke1ungs., Bd. 62, S." 406-432.
- 1925 Das mensehliche E1 W0 (lfring). Implantation, Verschluss der Implantationsiiffnung und Keimesentwicklung beim Menschen vor Bildung dos Primitivestreifens. Ztschr. f. Anat. u. Entwie.kelungs., Bd. 76, S. 1.6-4.2.
PETERS, II. 1899 Einbettung des menschlichen Eies und das friiheste bisher bekannte n1.ensch.lic.he Plaeentationsstadiuni. Franz Deuticke, Leipsig uud Wien. _
SCHLAGENHAUFER AND Vsnoonr 1916 Ein jungcs mensehliches Ei. A1-eh. f. Gyn'ail<'., Bd. 105, S. 151-168.
von SPEE, G. 1896 Neuc Beobachtungen iibor sehr friihe Entwieklungsstufen des menschlichen Eies. Arch. f. Anat., S. 1-28.
STRAHL, H., AND R. BENEKE 1910 Ein junges mensehliches Ei. Bergmann, Weisbaden.
STREETER, G. L. 1920 A human embryo (Mateer) of the presomite period. Oontrib. to Embryol., no. 43 (extract from Pub. no. 272 of Carnegie Inst. of Wash., pp. 389-424). ‘
- 1926 The ‘Miller’ ovum—the youngest normal human embryo thus far known. Contrib. to Embryol., no. 92 (extract from Pub. no. 363 of Carnegie Inst. of Wash., pp. 31-48).
TEACHER, J. II. 1924 On the implantation of the human ovum and the early devempmcnt of the trophoblast. J. Obst. and Gyncc. Brit. Emp., vol. 31, pp. 166-217.
WISLOCKI, G. B., AND C. G. IIART1-IAN 1929 On the placentation of :4, 1nac:-xque (Macacus rhesus) with observations on the origin of the blood constituting the placental sign. Johns Hopkins Hosp. BuI1., V01. 44, pp. 165486.
Plates
Plate 1
1 The photograph shows the actual size of the block of tissue removed.
2 This photograph of the excised block of tissue shows on the surface a dark, elevated, hemorrhagic region with a lighter center. The latter represents the point of entrance of the ovum. X 6.
3 This photograph of the cut surface of the decidua, and myometrium shows the character of the early pregnancy glands. In the basal glands there is blood. X 6.
Plate 2
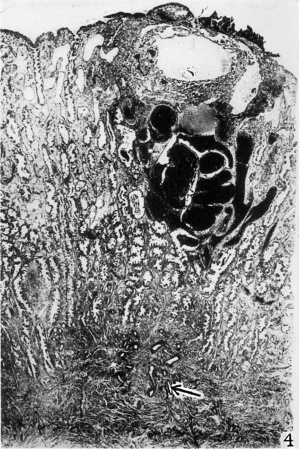
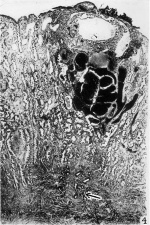
4 This photomicrograph of one of the serial sections shows the embedded. blastocyst directly beneath the surface hemorrhage noted in figures 2 and 3 About the blastocyst there is a large basal sinus and a large lateral sinus. The basal glands are dilated and filled with blood. The glands throughout the remainder of the section are tortuous, large, and contain secretion. The implantation site is situated directly above a spiral artery which is seen in they myometrium near its origin. The superficiail venous sinuses are dilated. Section 24-1-1. X 16.7.
Plate 3
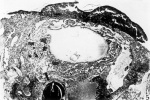
5 The blastocyst is entirely surrounded by short villi. The villi are most numerous on the basal side. The embryonic anlage is attached to the most dependent pole. The amnion is larger than the yolk sac. The embryonic plate is a simple, flat structure without distinctive surface markings. There is a condensation of cells in the region of the body stalk. The large basal and lateral. sinuses surround the blastocyst almost completely. The coagulum on the surface overlies the surface epithelium. It consists of blood and some fetal and maternal cells. The depression in the central portion is the point of entrance. Section 23-1-4. x 27.5.
6 This portion of the chorionic wall shows two short villi. with mesodermal cores. The Langhans cells extend out beyond the entl of the villus and join with those from the neighboring villi. The syncytiiun resembles the so-called primary syncytiunl. Maternal blood is found in the spaces between the fetal tissues. X 180.8.
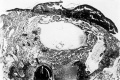
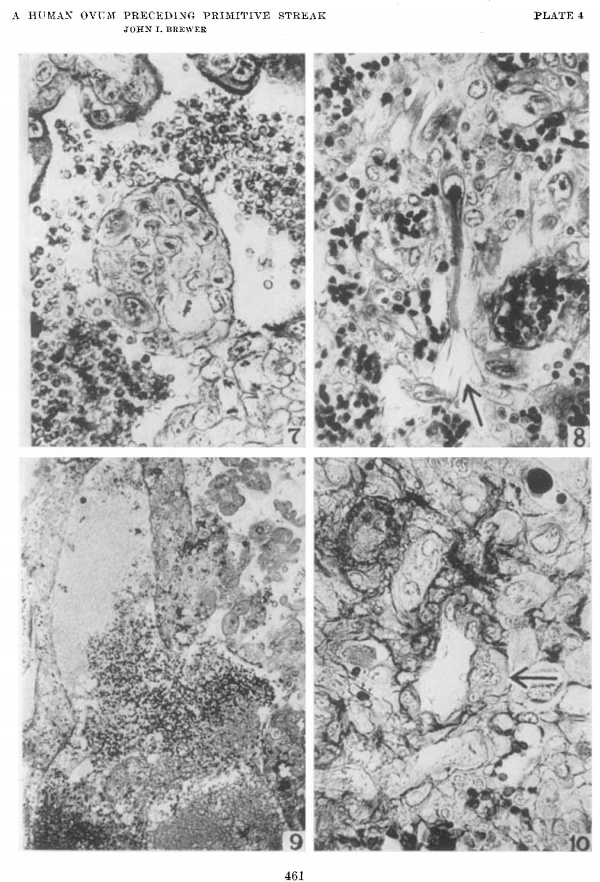
Plate 4
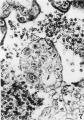
7 The cytotrophoblastic cells are dividing rapidly by mitotic division x 395.
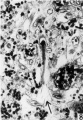
8 An elongated fetal cell is shown amid maternal tissue in the penetration zone. X 395.
9 The lateral sinus shown has a hole in its wall toward the blastocyst, and fetal trophoblast fills the gap. X 90.
10 A spiral arteriole with a syncytial cell in its wall is shown near its termination in the penetration zone. A rare finding. X 395.
Plate 5
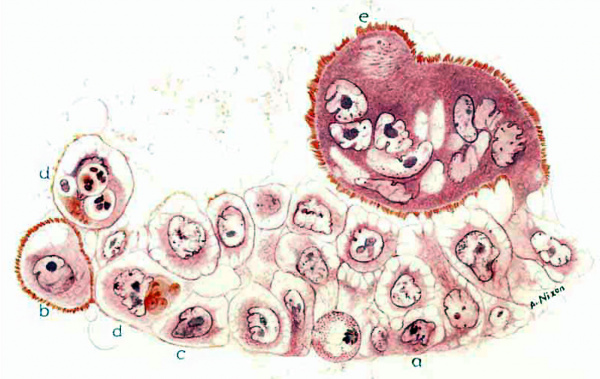
11 This drawing of an group of cytotrophoblastic cells demonstrate stages in the transition of this tissue to syncytium. At ‘a’ the cytoplasin stains similarly to that of syncytium and there is a loss of cell membrane with fusion with the cytoplasm of the adjacent cell. The cell ‘c’ has acquired 21 faint brush border. While the cell ‘b’ is still a distinct cytotrophoblastic cell, it is larger and has a well-developed brush border. The syncytial mass ‘e’ represents the end process of the forination of the multinucleated syncytium with a well-formed brush border.
The cells ‘d’ demonstrate phagocytic activity similar to that of other trophoblastic elements noted in figures 26, 27, 28, 29 and 30.
Plate 6
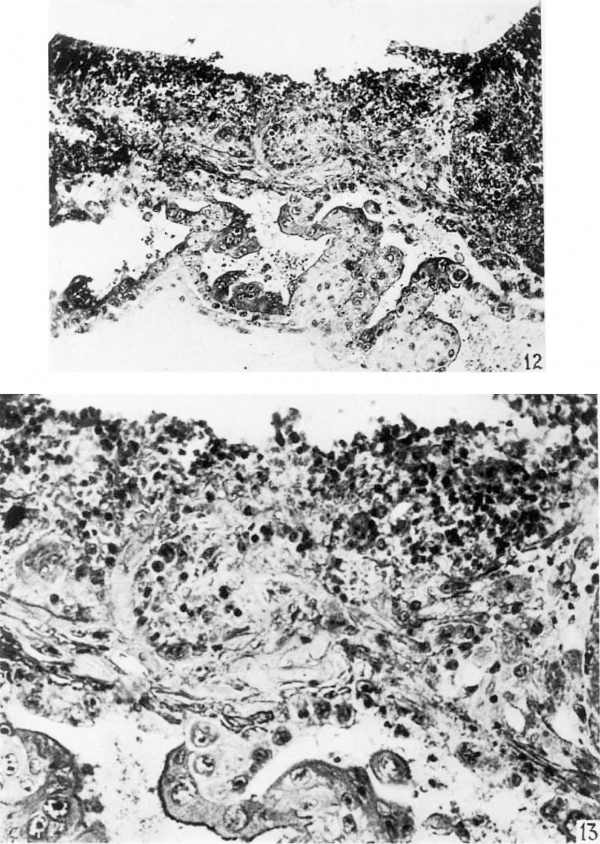
12 The main portion of the operculum with one lateral margin is shown. At this margin the opercular tissue is sharply differentiated from the maternal tissue and the surface coagulum. The chorlonic villi are adherent to the base of the operculum. X 142.
13 A high power photomicrograph of the operculum demonstrates an adherent villus. The opernulum is composed of numerous syncytial cells, cytotrophoblastic cells, maternal endometrial cells, and maternal blood. X 358.4.
Plate 7
14 This photomicrograph of the decidua. Vera shows the enlarged and tortuous glands filled with secretion. The superficial venous sinuses are dilated. The spiral arteries extend to the surface of the vera. The superficial half of the Vera is edematous. Surrounding the spiral arteries the deeidual tissues are more dense. x 54.1.
15 The distal portion of the spiral artery entering the penetration zone is filled with blood. The proximal portion (not shown in the photograph) contains no blood and is constricted. The artery ends as an endothelial tube in the penetration zone. See figures 18 and 19. X 32.4.
16 A spiral artery in the lateral penetration zone divides into two branches. One branch is filled with blood and the other is constricted and empty. X 68.
17 The gland epithelium with syncytial elements adjacent is destroyed in places. A capillary has been disrupted and its free end projects into the gland lumen. The latter is filled with blood. X 395.
Plate 8
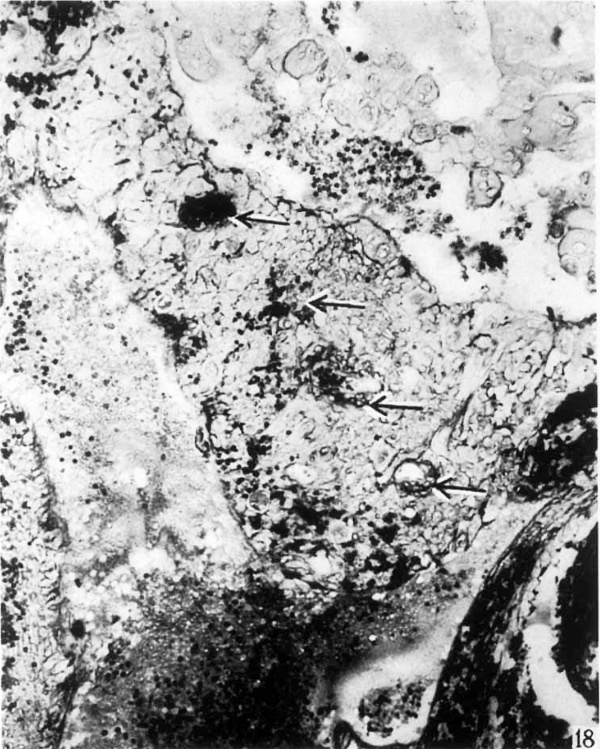
18 A spiral artery with hemorrhage about it is shown terminating in the penetration zone. X 176.
Plate 9
19 Surrounding the end of this spiral artery in the penetration zone there is a marked extravasation of blood. X 176.
Plate 10
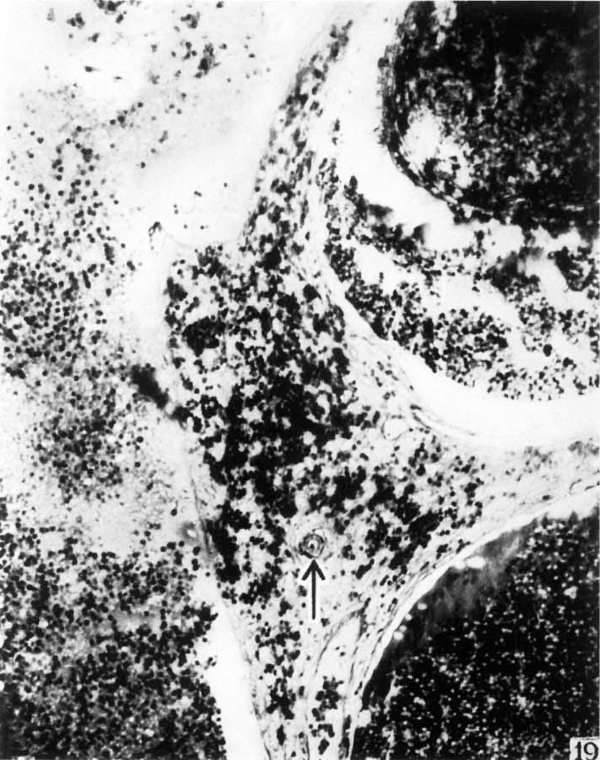
20 The reticulum in the maternal tissue surrminding the multinucleated synrnytial cell mass in the center of the picture is irregularly arranged. In many places the strands are clumped, frayed, and sharply broken off. The syncytial mass has phagocytized particles of reticulzir strands. At one point there is a, phagocytized strand continuous with the reticulum about the syncytial mass. Bielschowsky-Maresch technique. X 790.
21 This photomicrograph shows the junction of the maternal and fetal tissues. The edge of the maternal tissue is indicated by the termination of the reticular f~'ramework. The reticulum is frayed and irregular. Numerous strands project freely into the implantation cavity. There is clumping and irregula1'ity of pattern of the reticular frmliework. The syncytial cell mass has phagocytized some reticular strands and continuity between the reticulum and these strands is demonstrated. Bielschowsky-Maresch teclmique. X 790.
Plate 11
22 This is a photomicrograph of a portion of one wall of an endometrial gland in the penetration zone. The gland lumen is partly filled with blood that is deeply stained. The epithelial cells all show evidences of degeneration, while those near the middle of tho figure have been almost completely destroyed. The cytoplasm which remains is clumped about the nucleus. Approximating this cell is fetal trophololast. Adjacent to the gland wall there is a large mass of trophohlast, some of which has penetrated that structure. X 395.
23 Surrounding this portion of an endometrial gland in the penetration zone there is a syncytial cell mass. This mass has af brush border. The endometrial gland cells are markedly degenerated and in places entirely wanting. The gland lumen contains blood. X 395.
24 This endometrial gland projects partly into the irnplantation cavity. That portion in the cavity has been almost completely destroyed and surroundcd by syncytium. In. the lumen of the gland there is an organizing blood clot as well as some fresh hemorrhage. The brush border of the syncytium appears dark. X 202.
25 The surface epithelium of the decidua is shown with the overlying coagulum (soc figs. 2 and 5). The coagulum consists of maternal blood cells, some degenerated maternal stromal cells, and fetal trophoblast. Two fetal cells are shown in the process of penetrating the surface epithelium. The epithelium about this point is necrotic. X 393.
Plate 12
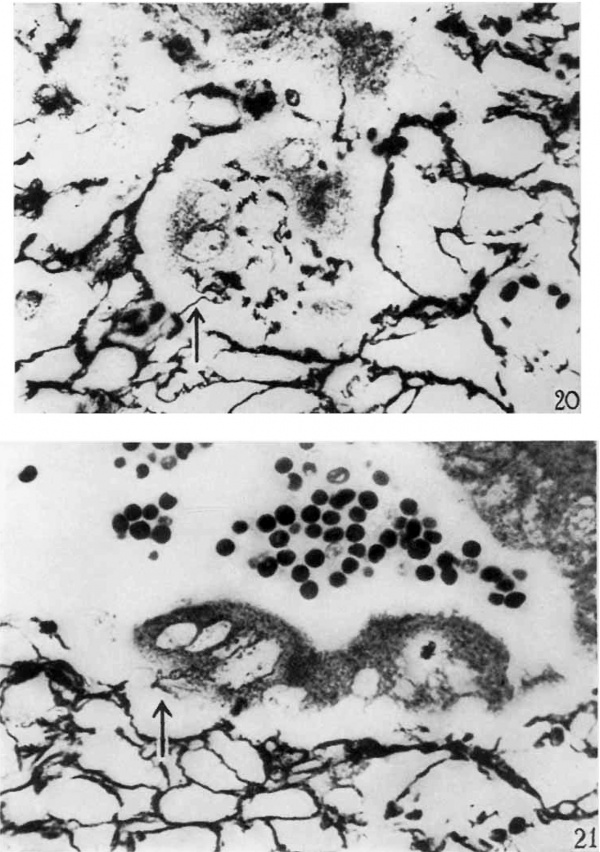
26 In the center of the figure there is a trophoblastic cell with a, phagocytized group of red blood cells. About these erythrocytes there is a vacuolization. At the opposite end of the cell there is a phagocytized lymphocyte with a similar vacuolization surrounding it. X 790,.
27 Near the center of the photomicrograph there is a fetal trophoblastic cell with a mass of faintly stained granular phagocytized material. X 790.
Plate 13
28 The fetal trophoblastic cell ‘a’ contains a more deeply stained granular inclusion material. At the other end of this cell there is a lymphocyte surrounded by a vacuole. The fetal cell ‘b’ contains a deeply stained homogenous inclusion mass surrounded by vacuole. X 790.
29 About the lymphoyte which has been phagocytized by a trophoblastic cell there is 3. large vacuole. X 790.
Plate 14
30 This multinucleated syncytial mass has in its cytoplasm a thick bundle of reticular fibers arraaiged in a wavy pattern. There is no vacuolization about this phagocytized reticulum. The syncytial mass has a brush border which stains deeply. X 790.
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
Cite this page: Hill, M.A. (2024, April 28) Embryology Paper - A normal human ovum in a stage preceding the primitive streak. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Paper_-_A_normal_human_ovum_in_a_stage_preceding_the_primitive_streak
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G