Paper - A human embryo with head-process and commencing arch enteric canal
| Embryology - 27 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Thompson P. and Brash JC. A human embryo with head-process and commencing arch enteric canal. (1923) J Anat. 58: 1-20. PMID 17103992
| Online Editor | ||
|---|---|---|
| Peter Thompson worked in embryology at King’s College in the United Kingdom in the early 1900's with J. Ernest Frazer. Note this paper was published in 1923 and our understanding of early embryo development has improved since this historic human study. This embryo has been classified as Carnegie stage 7 in Week 3.
Other papers by Peter Thompson:
Modern Notes:
|
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
A Human Embryo with Head-Process and Commencing Archenteric Canal
By The Late Professor Peter Thompson, M.D.,
University of Birmingham , Fellow of King’s College, London,
And James C. Brash, M.A., M.D., B.Sc.,
Professor of Anatomy, University of Birmingham.
- The clinical history of this embryo was detailed by the late Professor Thompson in his Ingleby Lecture before the University of Birmingham, in October, 1918. The first part of this paper, including the “General description of the Ovum,” he left in MS., and it represents all that he was able to complete for publication before his lamented death.—J. C. B.
Towards the end of the third or beginning of the fourth week, and immediately preceding the formation of the neural groove, the human embryo passes through a phase of development characterised by the presence of certain axial structures, viz. the primitive streak, a head -process canalised by a rudimentary arch enteric canal, and the protochordal plate. Even today but few examples of this stage are on record, and Grosser, who has published an interesting account of a young human ovum which showed these structures with great clearness, claims that previous to 1913—the date of his own publication—a similar stage of development in man had not previously been recorded. In 1918 Ingalls published an account of a human embryo, somewhat less advanced in development, but strikingly like the preceding in all the essentials. A third example was recorded by Strahl in 1916, but up to the present[1] the publication does not appear to have reached this country. Ingalls refers to it very briefly, and states that it shows a very similar stage of development, and adds that no data are given as to the age of the specimen.
The present paper is a further contribution to the subject, and, as far as can be ascertained, these four specimens comprise the material which illustrates a phase of development in the human embryo which falls somewhere between that represented by the ova of Fetzer, Von Herff and Beneke on the one hand, and that represented by the ova of Frassi and Eternod and Graf Spee’s Gle on the other. Estimating the age from the stage of development, Grosser gives 18 days, which is perhaps not far out. This archenteric stage, if such a term may be used for convenience, appears to be quite a transient one, and this may account for the small number of specimens which up to the present have been described.
The stage appears to correspond very closely with that which Wilson and Hill have described in their well-known work on monotreme development as the “post-gastrular stage.” They point out that this stage “includes the development, from the primitive knot, of the so-called ‘head-process’ together with various other phenomena associated with this, either causally or contemporaneously. This phase of development is deserving of special recognition,” they say, “as constituting a new era, for, with its onset, the process of ‘notogenesis’ is initiated, and the proper axis of the future embryo (Minot’s ‘primitive axis’) is laid down.” A full description of the axial structures in our embryo will be given at a later stage. In the first place it will be more convenient to set forth the details of the clinical history, which have been obtained with great care and are practically complete.
Clinical History
The ovum was removed from the uterus of a married lady by Mr Beckwith Whitehouse, F.R.C.S., on account of the serious condition of the patient’s health. It came into my hands on the following day, intact, and enclosed within a capsule of decidua. The following notes taken by Mr Whitehouse accompanied the specimen.
The patient, Mrs X, was seen in consultation on December 3rd, 1917. She was of a neurotic and highly sensitive disposition, and had suffered from nephroptosis, for which she had consulted a surgeon a short time previously with a view to an operation for its relief. Pregnancy, however, had supervened, and this fact, together with the knowledge of the pending operation, produced an exacerbation of the mental symptoms, and suicidal tendencies were exhibited. After full consultation it was decided to explore the cavity of the uterus and terminate pregnancy if such really existed.
The catamenial history had previously been quite regular—menstrual
cycle 28 days. The last period began on October 25, 1917, and ended on
November 1. No menstrual period occurred as expected on November 22.
The husband, who had been away from home, returned on November 2, and
coitus took place on the evening of November 6. Mr X left Birmingham on
the morning of November 7, and no further coitus occurred. (The coitus
previous to that of the evening of November 6 took place some time before
October 23, the day on which the husband left on a business journey for South
Wales, but how long before is uncertain. That no coitus took place between
these two dates, i.e. October 23 and November 6, forces us to the conclusion
that a previous cohabitation may be definitely rejected as a possible factor in
the case.)
Three days later, i.e. on November 9, the patient complained of pains in the breast, and thought that she was pregnant.
On December 3, upon examination under an anaesthetic, the body of the uterus was found to be somewhat globular in shape and Very slightly enlarged. No softening of the cervix or other sign of pregnancy was present. However, taking into consideration the very slight increase in size of the organ, it was thought that a pregnancy might be present, and the uterine cavity was explored at 4.30 p.m.
The cervix was dilated by means of Hegar’s dilators, and a blunt curette was introduced into the cavity of the organ. Presupposing that the ovum was
situated either at the fundus or on the posterior wall, curettage of this area
was performed first. A small ovum was removed intact from the posterior
wall and placed immediately in a 10 per cent. solution of formalin. No chorionic villi were observed, and the ovum appeared as a small disc-shaped vesicle
about half an inch in its greatest diameter. A small quantity of decidual
tissue was also removed by means of the curette from the anterior and lateral
walls of the uterus. The cavity of the organ was then packed with gauze.
From a perusal of the medical history given above,_ and from data supplied
to Mr Whitehouse and myself by the patient and her husband, both of whom
realised the importance of exact statement, it seems certain that fertilisation
must have taken place after and was presumably effected by coitus on the
night November 6-7, 1917. It should be stated that the husband of Mrs X is
a well-educated man occupying a professional position in the commercial life
of Birmingham, and the history detailed above can, in my judgment, be im-
plicitly relied upon.
The Age of the Specimen
Before entering upon a description of the embryo and its adnexa, something must be said regarding that most diflicult problem, the age of the specimen. In our case we are in the fortunate position of knowing most of the essential facts, and yet the difficulties of arriving at a trustworthy conclusion within narrow limits are practically insuperable. Without a knowledge of such essential facts, the estimation of age is mere guesswork; with them we shall at least be able to arrive at an approximate estimate.
Moreover, from the standpoint of the history of the case, it is difficult to
see how, under the circumstances, any additional or more reliable data could
have been obtained, which would enable us to calculate the age with complete
confidence. The really vital unknown factor is the day of the menstrual cycle
on which the ovum was set free, and the absence of this knowledge is the barrier which hinders us in working out the problem with any degree of
exactness.
The ovum was obtained thirty-nine days from the beginning of the last
period, thirty-two days from the end of the period, eleven days from the
omitted period, and twenty-seven (nearly) complete days after cohabitation.
Assuming that an ovum was awaiting fertilisation, and allowing twenty-four
hours for its occurrence, the absolute maximum time occupied by develop-
ment was twenty-five or twenty-six days.
The fertilising coitus took place on the thirteenth day of the menstrual
cycle, and sixteen days before the next expected but missed period. These
days may well have been taken up by fertilisation, time consumed in travelling
down the tube, the inhibition of menstruation, and the early stages of imbedding. In that case the ovum would reach the mucous membrane of the uterus towards the end of the menstrual cycle, that is to say, at a period well adapted to its nutritional requirements.
This leaves about twelve days from the time of the imbedding of the ovum
to the termination of pregnancy by operation on December 3. Bryce and
Teacher allowed seven days after implantation for the further growth and
development of the young ovum described by them in 1908, and considering
that our ovum is much more advanced in development, the additional five
days seems a reasonable allowance.
But are the assumptions which we have had to make regarding ovulation
and fertilisation justified in the present case‘? Certain considerations, which
must next be advanced, seem to show that they are not, and that a period of
six or seven days must be deducted if, on the ground of the degree of development, we assign our ovum to its appropriate place in the third week of the
chronological tables of young ova compiled by Bryce and Teacher, Keibel and
Mall, and Grosser. For, as everyone admits, it is necessary, in attempting to
ascertain the age of a young ovum, to consider factors other than those avail-
able in the obstetrical history of the case, and particularly the stage of development reached. Age and stage of development by no means always run parallel.
For example, Keibel found in the pig differences which he regarded as equal
to twenty-four to forty-eight hours’ growth at such an early date as the
fourteenth day of pregnancy. Nevertheless, any marked discrepancy between
the stage of development and the estimated age demands careful scrutiny.
Now the stage of development in the ovum under discussion approximates
to that found in the ova of Grosser and Ingalls, both of which have been estimated to be eighteen or nineteen days old, and is undoubtedly earlier than
that shown by the ova of Frassi, Eternod, Delporte and Graf Spee’s Gle, all of which have been placed at the end of the third week (nineteen to twenty-one days). True, amongst the six cases just noted, two only (Eternod’s and Delporte’s) have a clinical history comparable as regards fullness of detail with our specimen, but if, on the ground of the clinical history, it be maintained that ours is about a week older than the stage of development suggests,
then a place in the fourth week in the chronological tables mentioned above,
in company with I-Iis’s Lg. and BB., would be incongruous.
Further, if the age of our ovum be estimated at eighteen to nineteen days
on the ground of ‘anatomical findings, then fertilisation occurred on the
twentieth to the twenty-second day of the previous menstrual month. Such
a date would harmonise with the observations of Frankel on ovulation, which,
based on one hundred and thirty-three laparotomies, go to show that ovulation
occurs in the second half of the intermenstrual period, i.e. between the eleven
to twenty-six days from the beginning of the last menstrual period, with an
average of eighteen or nineteen days. In other words, ovulation would have
occurred about one week after the fertilising coitus of November 6.
This would call for a sojourn of the spermatozoa in the Fallopian Tube of
at least a week, a period during which it is reasonable to suppose they can
H ead-Process and Commencing Archenteric Canal 5
retain their fertilising power. Triepel has adopted a method of estimating the age of embryos based on the work of Frankel, which consists of subtracting eighteen from the number of days reckoned from the beginning of the last period to the day when the ovum is obtained from the uterus. In our case, 39 — 18 = 21 days, which is quite a good result.
It will now be advantageous if we place here for purpose of comparison
the essential points in the histories of the three ova most closely related to
our own:
Frassi’s Ovum
This ovum was obtained at an operation for total extirpation of the uterus on account of persistent menorrhagia due to metritis, forty-two days from the beginning of the last menstruation. Triepel estimates the age as 42 — 18 = 24 days; Bryce and Teacher estimate the age to be eighteen or nineteen days; Frassi himself describes it as being less than ten days, whilst the age reckoned on the basis of the stage of development is nineteen days. Such a result is a striking commentary on the danger of trying to narrow down too sharply the age of very young ova. ‘
Ingall’s Ovum
This ovum was obtained thirty-four days from the beginning of the last menstrual period, as an abortion. Intercourse took place fourteen days previous to the abortion, and (doubtfully) also two weeks further back. Triepel’s method would give an age of about sixteen days, whilst the age on the basis of development would be nineteen days. Ingalls himself estimates the age at seventeen or eighteen days, but he states that he could not bring himself to look upon this figure with any degree of confidence.
Grosser’s Ovum
(IC.. 13). This ovum is younger than that of Frassi, and according to Triepel’s method the age works out at eighteen or nineteen days. It was obtained as an abortion from a healthy uterus after an operation for removal of an ovarian cyst, thirty-seven days from the beginning of the last menstrual period. Owing to the irregularity of the menstrual cycle in this case, any estimate of age based on Triepel’s method is likely to be misleading.
Therefore, from a perusal of these cases and others, one comes to the conclusion that the estimation of the age of young ova, now assigned to the third
week, is a matter of approximation only, and it may be that in the future the
age of some of them may have to be raised several days. For the present we
must not be dogmatic. We shall go further and do more good if we strongly
emphasise the approximate nature of our results. The problem under consideration is not one which can be solved with mathematical exactness, or by
experiment, or by presentation of the unbroken chain of connected evidence.
To sum up, the main points in connection with the age of our ovum are:
- The stage of development, which would indicate an age of eighteen or nineteen days. This estimate must be very uncertain on account of the variability of time taken in travelling down the tube. According to Grosser, the error may, in extreme cases, be as much as four or five days.
- The obstetrical history, which gives a maximum time of development of twenty-five or twenty-six days. If, however, an interval of some days occurred between insemination and ovulation, then this figure is correspondingly reduced, and (1) and (2) are brought more into harmony.
- A slow journey down the tube, which might add several days to an ovum apparently eighteen or nineteen days old. So that if insemination and ovulation coincided and fertilisation took place forthwith, the ovum may be the maximum age, viz. twenty-five or twenty-six days. In this connection it may be pointed out that Grosser estimates the tube journey to be fourteen days at least under normal conditions, and at other times perhaps twenty days.
Between a probable minimum age of eighteen or nineteen days and a maximum age of twenty-five or twenty-six days is a period of seven days—an interval of uncertainty. For the present, therefore, we must assign the ovum to the end of the third or to the early part of the fourth week of development:
General Description of the Ovum
The specimen lay in 10 per cent. formol for two days, and was then transferred to 60 per cent. alcohol, then 70 per cent. and then 80 per cent., when it seemed to be in:a satisfactory condition for examination.
The ovum was enclosed in an envelope of decidua on which no scar could be detected. In shape it was rounded, flattened and lenticular (not unlike an ordinary tabloid, but not quite so flattened), and it measured 13 x 11 x 8 mm. On dissecting away the outer capsule of decidua, which separated quite easily, the chorionic vesicle with well-marked villi was exposed. When completely separated the vesicle measured 10 x 7.5 x 4 mm., so that the decidual capsule was from 1.5 to 2 mm in thickness. On opening the vesicle, which was filled
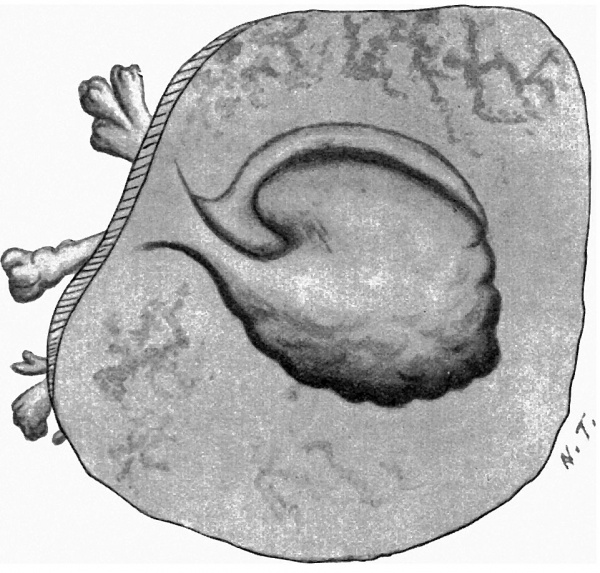
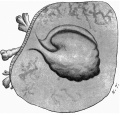
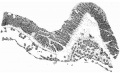
with a light-greyish mucin-like substance--the magma reticulare—a very small elongated papilla, attached by one extremity only, was found, the rest of the papilla projecting into the cavity of the ovum and connected by strands to the magma. The projection measured in spirit and before imbedding in paraffin about 2 mm. in length and about 1 mm. in breadth at its widest part. It must be pointed out that these measurements are probably too great, since one dare not touch the specimen with the dividers in case it should suffer some damage. Much time was spent in trying to obtain a photograph without any satisfactory result, and finally a freehand drawing was made with the assistance of the stereoscopic dissecting microscope at a magnification of 15 diameters (fig. 1). By these means the amnion, yolk-sac and body-stalk were brought clearly into view, together with the prominent caudal projection of the embryo-anlage, all these points being fully confirmed by subsequent examination of the serial sections. The drawing shows in addition the characteristic elevations or knobs on the yolk-sac, representing the angioblast, some villi (which measured 1 to 1.5 mm. in length) projecting from the outer surface of the chorion, and some maternal blood, also on the outer surface of the chorion and visible through the thin chorionic layer.
The projection with a small piece of the chorion to which it was attached was stained in bulk, borax-carmine being used. It Was cut by Professor Carlier, to whom I am much indebted for the trouble he has taken to obtain a good series of sections. Though not coming up to ideal requirements (some of the sections are broken owing to somewhat imperfect preservation), I was able to make a reconstruction in wax which turned out to be exceedingly useful as a means of interpreting certain obscure grooves on the embryo-anlage, and for settling beyond doubt the line of the axial structures. The bulk of the sections are quite satisfactory for study, and this is indeed fortunate, seeing that the combination of circumstances, which brings such a rare specimen, with a really good history, into the hands of an investigator, occurs so seldom. Altogether, reckoning from the cranial end of the embryonic shield to the blind end of the allantois, there are eighty-five sections. As the sections were each 10 microns thick, this gives a length of the shield plus allantois of -85 mm. From the cranial end of the yolk-sac to the attachment of the body-stalk to the chorion, the length estimated for the number of sections is 1-12 mm.
Fig. 1. The “Embryonic Papilla” attached by the body-stalk to the chorion. From a. freehand drawing by Mrs P. Thompson. x about 40.
The Embryo and Adnexa
The embryonic vesicle is similar in general form to that of Graf Spee’s well-known Glaevecke ovum: the main features are shown in the drawing in fig. 1 already described and in the diagrammatic figs. 2 and 3. The embryonic shield, though it is a good deal broader in proportion to its length, more nearly resembles that of the Frassi ovum; but there is no neurenteric canal and the irregular ridges which are present do not appear to bound a neural groove.
There is a well-defined caudal projection or tail-fold and a less well-defined
head—fold. The shield is -68 mm. in length measured from the cranial reflection
of the amnion to the caudal projection, and the estimated length including
the caudal fold is nearly -9 mm. The greatest breadth, which is found a little
in front of the middle of the shield, is also about -9 mm. In association with
the ill-defined head—fold there is apparent evidence of the commencing formation of a foregut, cut separately from the yolk-sac in five sections; but, as there
is in this region some distortion of the vesicle with collapse and infolding of
the amnion and yolk-sac, it remains doubtful whether the foregut appearance is to be attributed to these causes.
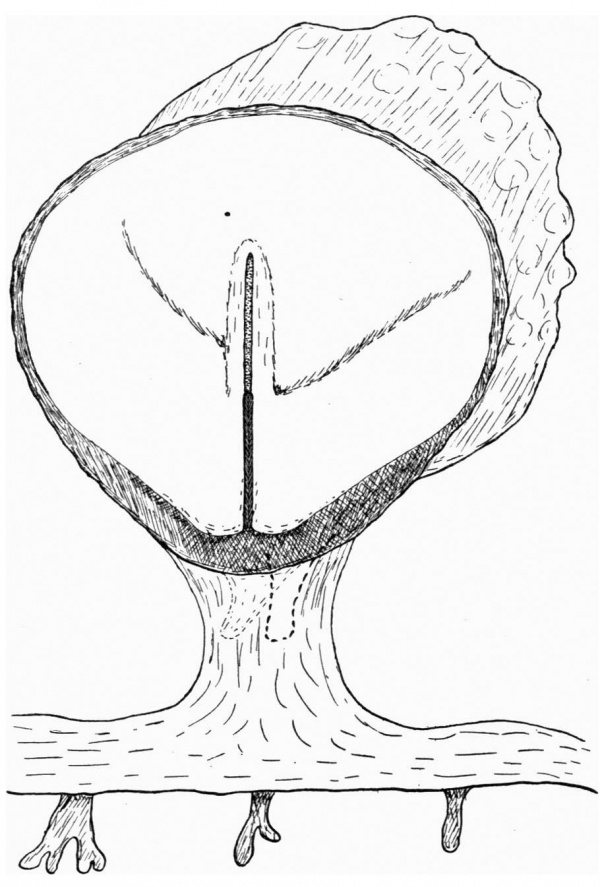
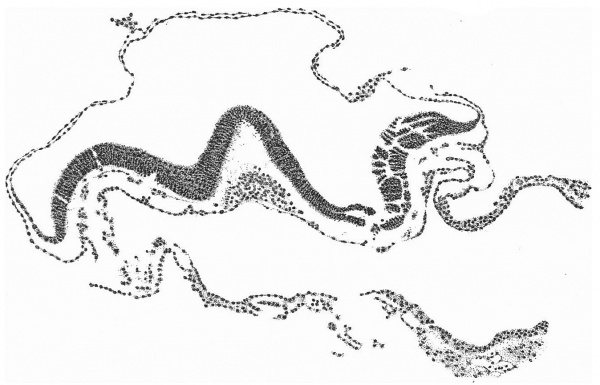
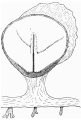
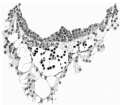
Fig. 2. Diagrammatic reconstruction of the embryonic shield, etc., to show the relation of the primitive streak, the median elevation and the two lateral grooves. The position of the head-process is indicated by a stippled band extending forward from the cranial end of the primitive streak beneath the median elevation. The relation of the amnion and the allantois to the body-stalk is also indicated. Drawn to scale, x about 80.
The irregular ridges whichare present on the shield, somewhat similar to the ridges on the shield of the embryo about the same stage of development described by Ingalls, have provided rather a difficult problem; their relation to the axis of the embryo is not at first sight obvious. It was to elucidate this point that Professor Thomson made the reconstruction to which he refers. I know that he did not look upon the resulting model as completely satisfactory, and there is no doubt that it cannot be taken as an exact representation of the original. This is due to the absence of guide lines, a serious handicap when it is realised that the sections are oblique to both the median plane and to the surface of the shield; in addition there is evident distortion of a number of the sections. The model itself is unfinished, and as it was never intended to be more than an incidental help in interpretation of the appearances of the sections and an aid in settling the question of the middle line, I do not feel justified in reproducing it. I prefer to use it, as was Professor Thompson’s intention, along with a. close study of the sections themselves, in order to reconstruct diagrammatic representations of the surface of the shield and of an ideal median sagittal section of the ovum.
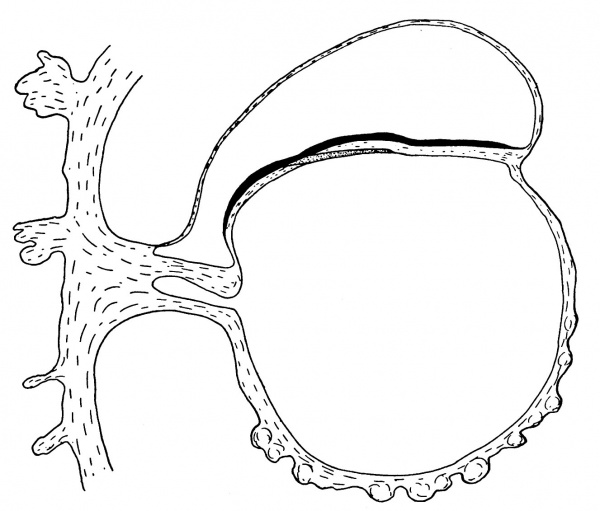
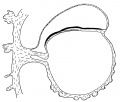
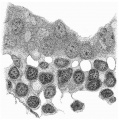
Fig. 3. Diagrammatic median sagittal section; the head-process, etc., as in fig. 2. Drawn to scale, x about 80.
The Surface of the Shield
The reconstruction of the surface of the shield is given in fig. 2, which is drawn carefully to scale. In general shape the dorsal aspect of the shield is oval with its long axis transverse to the long axis of the embryo. The cranial end of the shield is represented by one side of the oval and is bent slightly downwards: the caudal end of the shield appears as a narrowing prolongation of the other side of the oval; it is markedly bent downwards and the projection thus formed is indented in the middle line by the primitive groove. The groove becomes shallower as it is traced forwards to end, a little less than one-third of the distance from the caudal projection to the cranial end of the shield, at the point where the median elevation and the right depression next to be described commence. The whole length of the primitive groove is estimated to be -38 mm.
Immediately in front of the anterior end of the groove there commences a median elevation of the shield in the position usually assigned to Hensen’s knot. This elevation, accentuated by the obliquity of the sections and in addition rendered very prominent by the presence on each side of a depression or groove, extends forwards on the shield for a distance a little greater than that occupied by the primitive groove, and reaches therefore about the junction of the anterior and middle thirds of the shield. On each side of the median elevation is a groove, narrow behind but broadening in front; these grooves are about the same length. The left commences at the middle of the median elevation, extends forwards and to the left and fades away in the cranial half of the shield without quite reaching its edge. The right begins at the hinder end of the median ridge, where it forms a deep depression immediately to the right of the anterior end of the primitive groove, and extends to the lateral edge of the shield about its middle. The interpretation of these ridges depends upon a study of the axial structures in section.
The Axial Structures
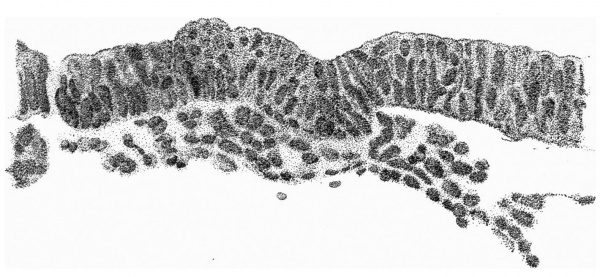
Fig. 4. Transverse section above the middle of the cranial half of the shield. x 95.
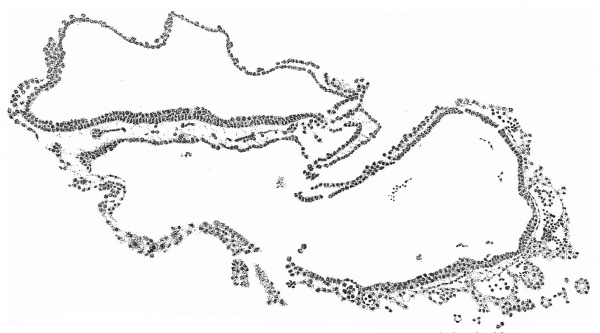
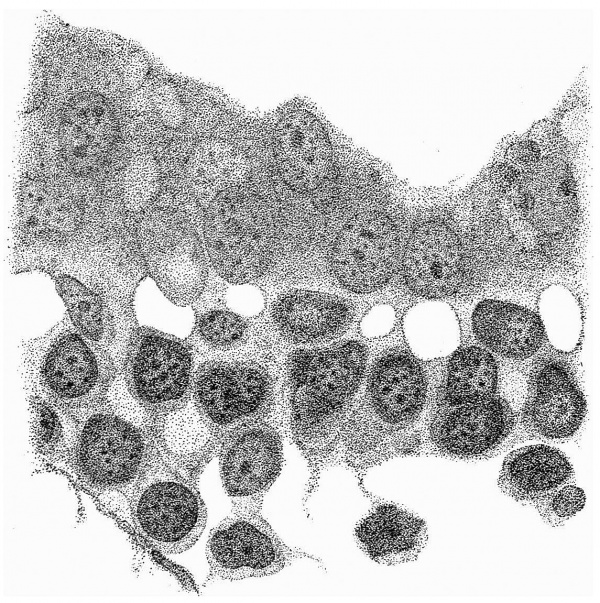
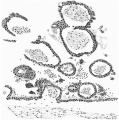
Fig. 4 is from a section a little in front of the middle of the cranial half of the shield. It demonstrates the general structure of the shield and shows that the ectoderm and the entoderm are in this region separated by a small amount of (primary?) mesoderm. There is here no indication of a neural groove. The sections in this region are believed to be practically vertical to the surface of the shield, as is evidenced by the single layer of nuclei in the entoderm and the amniotic ectoderm. The embryonic eetoderm, on the other hand, shows a stratification of its nuclei, two and in some places three deep, and, as the three-layered nuclei are mostly confined to an area on each side of the middle line, it is possible that we see here the commencing formation of the neural plate. Close to the entoderm in the middle line are to be seen a few mesoderm-like cells in the position where the head-process is situated further back. These cells, however, do not constitute a forward continuation of the head-process as the same region of the entoderm is a continuous single layer both in front of and behind this particular section. It is to be noted that there is in fact no evidence in the cranial part of the shield of a “completion plate” continuing the line of the head-process forward. We pass now to the caudal end of the shield.
Fig. 5. Transverse section of the primitive streak region in front of the caudal bend; description in the text. x 385.
Fig. 5 is taken from a section a little in front of the middle of that part of
the primitive streak which appears on the dorsal aspect of the shield, i.e. in
front of the caudal bend. Here is to be observed the continuity of the three layers, but there is by no means complete fusion. The entoderm is somewhat
damaged on the left, but it passes as a continuous thin layer from side to side
closely united to, though quite distinct from, the mesoderm. The ectoderm,
which is relatively independent, is thickened in the middle line where the
nuclei are three and four deep; here also are to be observed the primitive
groove indenting the surface, and, corresponding to this, a ridge-like depression
of the ectoderm towards the entoderm. It is from the anterior end of this ridge that the head-process appears to take origin. Behind the region of this
section the streak becomes progressively thinner until, when a point a little
beyond the caudal bend is reached, it becomes reduced to a single layer of
entoderm and ectoderm in apposition in the region of a cloacal membrane.
The position of the cloacal membrane is indicated in fig. 3, but, owing to the great obliquity of the sections to the surface in this region, it is hardly possible to determine its exact extent. We now turn to the head-process itself and the relation which it presents to the anterior end of the primitive streak.
Now about the point where the primitive groove, as seen in fig. 5, gives
place to the median ridge already mentioned and seen in the next section
figured, i.e. in the position assigned to Hensen’s knot, there is the appearance
of a downgrowth of a wedge of cells from the ectoderm in direct continuity
with the ridge beneath the primitive groove. This apparent downgrowth is
clearly marked four or five sections behind the most anterior showing the
primitive groove, and is the result of the sectioning of a column of cells which
is found to continue the line of the primitive streak forwards between the
ectoderm and the entoderm. Its cehtre appears at first to be slightly to the
left of the anterior end of the primitive streak, but as it passes forward it lies
directly beneath the median ridge of the shield. It is in continuity behind with
the ectoderm at the anterior end of the streak as stated, and it maintains the
same relation to the mesoderm as is exhibited by the primitive streak itself.
Traced forward it begins at once to separate from the ectoderm and is from
its origin in contact with the entoderm; in the course of eight or nine sections
it is entirely free from the former and closely united to the latter.
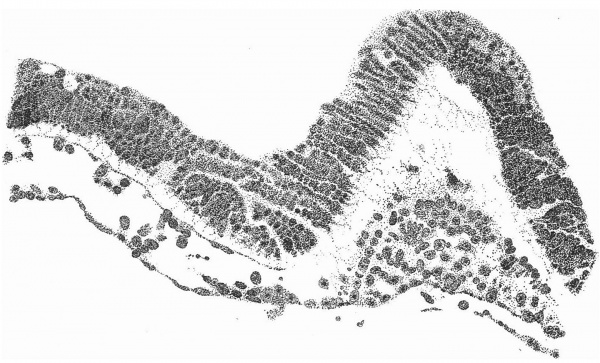
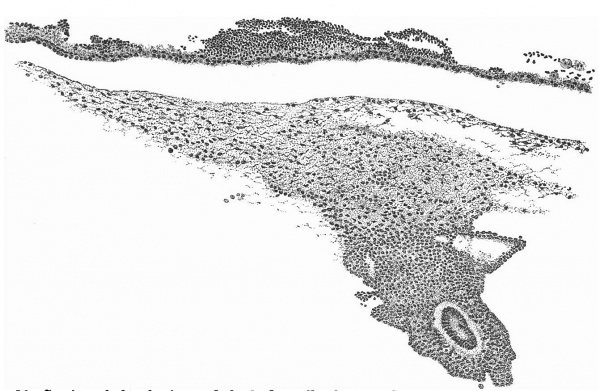
A section at the most favourable point (figs. 6 and 7 ) makes clear the general
relations of the column, which is thus identified as a head-process. The figures
also show that although there is no distinct lumen present yet the dorsal cells
are arranged fanwise as if the lumen were about to appear. The section figured
is situated fifteen sections in front of the origin of the process from the ectoderm of the knot and fourteen sections behind its cranial termination: the
whole length of the head-process is estimated to be nearly -3 mm. In front of
the process the simple condition of the entoderm, as seen in fig. 4, appears at
once, and, as already stated, there is no evidence of the formation of a “completion plate” continuing the line of the process forward. The thickening of
the process is merely replaced by (primary?) mesoderm and the entoderm is
unaffected. These topographical points are summed up in the shield diagram
of fig. 2 and the schematic sagittal section of fig. 3, where the head-process is represented by a stippled band passing forward beneath the median elevation of the ectoderm and in continuity with the entoderm. There can be little doubt, judging from the absence of a definite canal and the manner in which the process ends in front, that we are dealing with an example of the head-process, in course of formation, probably just before the appearance of a lumen and the breaking down of the ventral floor. There is no sign of perforation towards either the ectoderm or the entoderm, so that the stage is without doubt previous to that of the notochordal or archenteric canal and afortiori of the neurenteric canal.
The more highly magnified View of fig. 7 shows very clearly the arrangement of the cells. The distinction between the ventral simple layer (plaque
lécithoentérique of van Beneden) and the dorsal clump with its radial arrangement foreshadowing the canal (plaque notochordale of van Beneden) is indicated both by the actual arrangement of the cells and by the staining reactions.
These figures (6 and 7) also demonstrate that the line of the head-process
corresponds to the line of the central elevation of the shield and that this is
to be considered as indicating the axial line of the embryo. The appearance of
thickening of the ectoderm along this ridge is probably largely due to the
obliquity of the sections, which is also the case with the other two ridges previously mentioned. All three ridges must be considered to be due to a
folding of the surface of the shield, partly due to exuberant growth and partly
artificial.
Fig. 6. Transverse section at the midpoint of the head-process showing the ridges and groove on the shield and the relation of the head-process to the median elevation. x 95.
Fig. 7. Portion of the preceding, x 300, showing the head-process in detail.
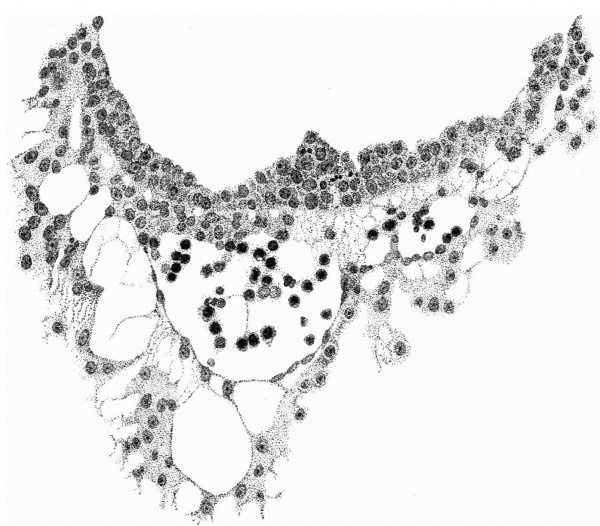
Fig. 8. The wall of the yolk-sac, near the cranial pole, showing blood-island. x 385.
The Amnion
The amniotic ectoderm consists of a single layer of flattened cells continuous with the peripheral two-celled embryonic ectoderm and backed by an equally thin mesodermic covering. The amniotic cavity is a good deal distorted in many of the sections, but there is no doubt that there is a greater depth of this space at the cranial than at the caudal end of the shield. In the region of the caudal bend the amnion appears to be stretched over the shield, and as this diminution of the cavity corresponds with the presence of the grooves on the shield, the impression that these grooves may be due to an exuberant growth of the shield is somewhat strengthened. Traced back- wards the amnion is found to bend in conformity with the caudal bend of the shield itself, and, with a slight convexity towards the deep depression between the shield and the body-stalk, it passes directly on to the dorsal aspect of the latter. It covers the stalk for about half its extent, and, as a result of the bend mentioned, the last few sections of the amnion appear as a narrow space lying on the left side of its dorsal aspect (figs. 3 and 10). There is no evidence of the presence of an amniotic duct, the amniotic ectoderm remaining histologically the same at the extremity of this small diverticulum as elsewhere, with no extension towards the chorion.
The Entodermic Cavity
The median sagittal diagram of fig. 3 indicates clearly the conditions present. In five sections at the cranial end the lumen of the intraembryonic portion is cut separately from the yolk-sac cavity, but a doubt, accentuated by the obliquity of the sections, remains whether this is in reality foregut or due to a distortion of the wall of the yolk-sac. There appears, however, to be some thickening of the mesoderm, with.a doubtful coelomic rudiment, in the angle between. The allantoic diverticulum extends from the dorsal part of the caudal wall of the yolk-sac into the body-stalk, which it traverses for rather more than half its length. The total length of the allantois is estimated to be -14 mm. There is no hindgut.
The Mesoderm
A thin layer of (primary?) mesoderm—mostly one cell thick—extends throughout the shield, while secondary mesoderm is in process of active formation in the primitive streak and is in continuity with the head-process in front, with the somatopleuric and splanchnopleuric layers, and with the chorionic mesoderm along the body-stalk. The chorionic mesoderm forms a continuous layer lining the chorionic ectoderm and extending into the primary villi.
Angiogenesis
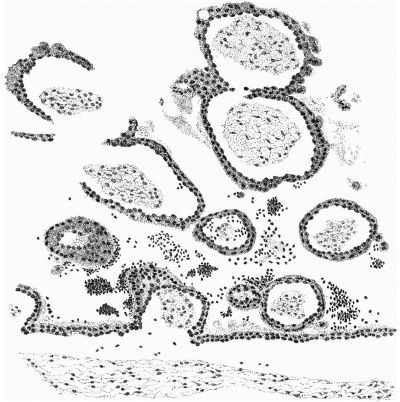
The distribution of the angioblastic tissue is, with doubtful exceptions in the body-stalk, confined to the wall of the yolk-sac. The evident blood-islands in this situation have already been noted in the general description of the ovum, and a detailed examination of the sections reveals that they are distributed thickly on the whole of the ventral aspect of the sac and extend on to both its cranial and caudal extremities. On the whole it appears that the islands in the cranial half of the sac are in a more advanced state of development. It is not proposed to give a detailed histological description of the appearances presented by these blood-islands, but attention may be directed to certain important features which are to be seen in the illustrations given.
It will be observed from fig. 4 that the entoderm for about the dorsal half of the wall of the yolk-sac consists of a single layer of cells, whereas in the ventral half it is thickened so that there are two, three and in some places four and even five nuclei. It may be thought that this appearance is due to distortion and consequent obliquity of the sections, but a. careful examination of all the sections reveals that this is the condition throughout the extent of the yolk-sac. Associated with this difference it is found that, whereas in the dorsal half of the yolk-sac the overlying mesoderm is separated from the entoderm by a distinct space and is clearly independent of it, in the ventral area there is no such clear separation between the two. Here also the mesoderm is thickened and the blood-islands occur. The separation of the yolk-sac into two areas, a dorsal and a ventral characterised by the differences in the entoderm mentioned, was described by Minot, and in Streeter’s account of a human embryo (Mateer) of the presomite period the same separation of the mesoderm from the entoderm in the dorsal half of the sac is noted. In that embryo, however, which, in a younger stage than the present specimen, exhibits a much less differentiated condition of the yolk-sac blood-anlage, it is the ventral part of the mesoderm which is thinner and “fused tightly with the entoderm.” The significance of these observations must lie in their relation to the formation of the blood vessels and the blood cells.
If we now examine the blood islands themselves we find that there are all intermediate stages present between the extremes of small clumps of cells lying between apparently simple entodermic and mesodermic layers and completely formed vessels with uninterrupted endothelial walls containing the developing blood cells. The three main stages are illustrated in fig. 4a, where from left to right are to be seen (1) clumps of cells between entoderm and mesoderm each single-layered, (2) partially formed vessels with endothelial wall unformed towards the thickened entoderm, and (3) a fully formed vessel. The middle of these stages is illustrated on a larger scale in fig. 8, taken from a different section nearer the cranial pole of the yolk-sac. This section shows the’ formation of the endothelial wall on the side of the island away from the entoderm and the characteristic relation of the blood cells to the entoderm, from which they appear to hang in a cluster into the lumen of the developing vessel. In fig. 9, on a still larger scale, is shown the portion of the entoderm with which these cells are apparently continuous. It will be observed from this figure that the cells from which the clump in the lumen apparently springs are differentiated from the cells next the cavity of the sac by a number of points. There is the same absence of cell outlines throughout, but the cytoplasm surrounding the nuclei in the deeper layer is more clearly differentiated, the out- lines of the nuclei are less regular, the nuclei are more deeply stained, and the layer as a whole is separated from that next the cavity by a row of spaces. It is clear that these cells are to be separated from the overlying entoderm, and that between the two layers will be formed the completion of the endothelial wall. Although the appearances here described are at first sight suggestive of the origin of the blood cells from the entoderm, yet there is no unequivocal evidence to warrant a conclusion on this diflicult question.
It should be noted that there are great variations in the distribution of the vessels in embryos about this stage of development. The contrast, for example, between this ovum and Streeter’s Mateer specimen is very marked. In the latter, blood Vessels are present in all parts of the chorion and in many of the villi, while the differentiation of the vessels on the yolk—sac is restricted to the caudo-ventral half and has not advanced so far as in the Whitehouse specimen.
Fig. 9. Portion of the preceding (the left upper corner of the island) to show the arrangement of the cells on the yolk-sac side of the island. x 1750.
The Coelom
There is no indication of an intraembryonic coelom with the exception of a doubtful space in the mesoderm between the cranial wall of the yolk-sac and the doubtful foregut. Elsewhere the intraembryonic mesoderm is almost entirely one cell thick. There is no special remark to make regarding the exocoelom, beyond calling attention to the statement in the general description that there was a considerable quantity of magma present, to which the embryonic papilla was connected by strands.
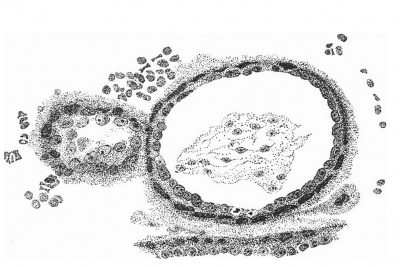
The Body-Stalk
The structure of the body-stalk is illustrated in fig. 10, in which is to be seen the allantois in the centre of the lower part of the stalk and, on the side, a section of the amniotic diverticulum which passes along the stalk for a short distance. It will be noted that the mesoderm immediately surrounding the allantois is more compact than that which is connected with the chorion.
Fig. 10. Section of the chorion and the body-stalk, showing the allantois tlie centre and the amniotic diverticulum on the side of the stalk. x 95.
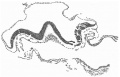
The Chorion and Villi
Figs. 11 and 12 are added, without detailed comment, to demonstrate the structure of the chorionic wall and the villi. It may be noted that the distribution of the villi is very irregular, that the cyto- and plasmodi-trophoblast layers are everywhere very distinct, and that. the cores of the villi consist exclusively of undifferentiated mesoblast, in most cases shrunken away from the walls in preparation. There is no sign anywhere of vascularisation.
Summary
- There is an excellent clinical history, by means of which the age of the ovum can be placed between a minimum of eighteen or nineteen and a maximum of twenty-five or twenty-six days.
- The head-process is in course of formation, and the stage of development is probably just before the appearance of the archenteric canal and the breaking down of the lecithoenteric plate.
- The amnion is stretched over the caudal end of the shield and is continned as a short diverticulum on the dorsal and left side of the body-stalk. There is no evidence of an amniotic duct.
- There is a doubtful foregut, no hindgut, and an allantoic diverticulum of the yolk-sac extending about half-way along the stalk.
- Blood and blood vessel formation is restricted to the ventral wall of the yolk-sac and is slightly more advanced towards the cranial end.
- There is no intraembryonic coelom.
In conclusion I have to thank Mrs Thompson for her ready assent to the proposal that the work begun by her late husband should be completed, “and for her kindness in placing at my disposal his unfinished MS. and notes. Also I have to ‘acknowledge my indebtedness to Professor Barclay-Smith for his advice and to Professor Arthur Robinson for his kindness in looking over the material at my disposal and f& helpful suggestions.
- ↑ This was written in l920.—J. C. B.
References
- VAN BENEDEN. “Sur la presence chez l’homme d’un canal archentérique.” Anal. Anzeig. xv. 349-356. 1899.
- Bryce TH. and Teacher JH. Contributions To The Study Of The Early Development And Imbedding Of The Human Ovum 1. An Early Ovum Imbedded In The Decidua. (1908) James Maclehose and Sons. Glasgow.
- FEKNKEL. “ Das zeitliche Verhalten Von Ovulation und Menstruation.” Zentralbl. f. Gyndkol. No. 46, p. 1591. 1911.
- FRASSI. “Ueber ein junges menschliehes Ei in situ.” Archiv f. milcr. Anat. u. Entwiclc. Lxx. 492-505. 1907.
- Gnossnn. “Ein menschlicher Embryo mit Chordakanal.” Amt. Hefte, XLVII. 649-686. 1913.
- - “Altersbestimmung junger mensehliehen Embryonen; Ovula.tions- und Menstruationstermin.” Amt. Anzeig. XLVII. 264-283. 1914.
- Ingalls NW. A human embryo before the appearance of the myotomes. (1918) Contrib. Embryol., Carnegie Inst. Wash. No.23 Publ. 227, 7:111-134.
- MINOT. “The origin of the Angioblast and the development of the Blood.” Keibel F. and Mall FP. Manual of Human Embryology II. (1912) J. B. Lippincott Company, Philadelphia.
- STRAHL. “Ueber einen jungen menschlichen Embryo, nebst Bemerkungen zu C. Rabl’s Gastrulationstheorie.” Anat. Hefte, LIV. 115-146. 1916.
- Streeter GL. A human embryo (Mateer) of the pre-somite period. (1920) Contrib. Embryol., Carnegie Inst. Wash. Publ. 272, 9: 389-424.
- TRIEPEL. “Altersbestimmung bei menschlichen Embryonen.” Anat. Anzeig. XLVI. 385-398. 1914.
- WILSON and HILL. “ Observations on the Development of Ornithorhynchus.” Phil. Trans., B, vol. 199, p. 61. 1908.
Figures
Cite this page: Hill, M.A. (2024, April 27) Embryology Paper - A human embryo with head-process and commencing arch enteric canal. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Paper_-_A_human_embryo_with_head-process_and_commencing_arch_enteric_canal
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G