Paper - A Human Ovum Nine to Ten Days Old
| Embryology - 28 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Davies F. and Harding HE. A Human ovum nine to ten days old. (1944) BJOG: An International Journal of Obstetrics & Gynaecology 51(3): 225-230.
| Online Editor | |||||
|---|---|---|---|---|---|
| This historic 1944 paper by Davies describes early human development in week 2 (Carnegie stage 5).
Hertig AT. and Rock J. On a normal ovum of approximately 9 to 10 days of age. (1945) Anat. Rec. 91: 281. Hertig AT. and Rock J. On a human blastula recovered from the uterine cavity 4 days after ovulation. (1946) J Gerontol. 1(1): 96-117.
|
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
A Human Ovum Nine to Ten Days Old
By
Francis Davies, M.D., D.Sc., F.R.S.E. And H. E. Harding, M.D.
Departments of Anatomy and Pathology, University of Sheffield
Clinical History
The specimen was obtained from the uterus removed from a married woman, aged 26 years, admitted to hospital for pelvic pain. She had had 2 children, both at term, one in December 1938 and the other in March 1940. She had not had any abortions or miscarriages. At operation on September 9th, 1943, endometriosis of the left ovary was diagnosed and a corpus lutea was seen in the right ovary, but no details of the latter are available. The uterus was placed in 10 per cent formolsaline immediately after removal later a pin-head red spot was found in the thickened endometrium on the anterior wall of the uterus near the entrance of the right uterine tube; the uterus appeared otherwise normal. A block of tissue surrounding the red spot was removed, appropriately treated, and embedded in parafiin wax. Some sections were removed and only one of these mounted before the true value of the specimen was appreciated. The remainder of the paraffin block was then melted, the tissue orientated and re-embedded, and serial sections 9 micron thick were cut, stained with haemalum and eosin (Stain - Haematoxylin Eosin) and photographed. Study of these revealed that between one-third and one-half of the ovum had been removed in the preliminary treatment but that the remaining portion was suflicient in amount and in excellence of preservation to enable a thorough study of all the various embryonic elements to be made. The menstrual and coital history, summarized in the table, was obtained at the time from both husband and wife. The maximum coital age of the specimen is 9-Io days, an age which is also indicated by the histological structure of the ovum itself.
Table
Menstrual, coital and operative data. Menstrual cycle 28 days.
Histological Examination
The endometrium (Fig. 2) has the structure characteristic of the secretory (premenstrual) phase of the cycle. It is 5 mm. thick at the implantation site and slightly thinner (4.5 mm.) elsewhere. Compact, spongy and basal strata are clearly differentiated and the “ physiological oedema ” between the stromal cells is minimal. The endometrial glands are lined by columnar epithelium throughout and contain secretion in the form of an eosinophilic, amorphous coagulum, except in the basal layer where secretion is absent. There is no maternal blood in the glands. Spiral arteries are prominent in the stromal partitions between the glands in the spongy layer, where the glands are tortuous and have the characteristic “saw-tooth” edges. The ovum is embedded solely in the stratum compactum. Definite decidual reaction is not present, but some of the stromal cells adjacent to the trophoblast have a broader halo of perinuclear cytoplasm than ordinary stromal cells (pre-decidual cells).
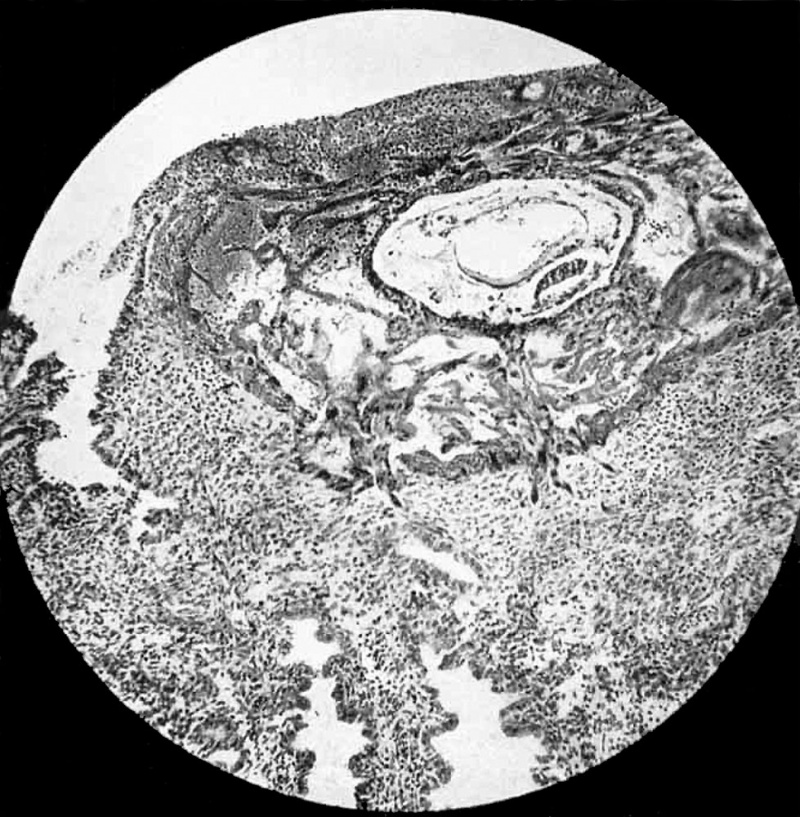
The trophoblast is clearly differentiated into cytotrophoblast and plasmoditrophoblast (Fig. 1); all stages in the formation of the latter from the former are seen and
mitotic figures are numerous in the cytotrophoblast. Solid clumps of cytotrophoblast project from the surface of the chorionic vesicle, but they are not penetrated by the
primitive mesoblast,so thattrue villi are not yet formed. Lacunae of various sizes and stages of formation are present in the plasmoditrophoblast; only those at the abembryonic (superficial) pole of the ovum contain maternal blood, elsewhere they contain only leucocytes. The “ operculum,” which indicates the site of entry of the ovum into the endometrium, consists of a mixture of fibrin and leucocytes permeated by plasmoditrophoblast; no epithelialization has yet taken place to cover the surface of the operculum There is no zone of necrosis of the endometrium adjacent to the trophoblast such as was described in the Teacher-Bryce‘ and the Mollendorff Sch? ova, but the stromal cells adjacent to the trophoblast appear to be undergoing cytolysis as they are encroached upon by the trophoblast, and their nuclei show varying degrees of pale staining.
The chorionic cavity (Fig. 1) is permeated by the primitive mesoblast, which has the form of a loose meshwork consisting partly of large stellate cells and partly of smaller elongated cells; the mesoblast is continuous with the cytotrophoblast superficially and with the exocoelomic (Heuser’s) membrane deeply. The latter membrane
is complete, unlike that in the 11.5 day Hertig-Rock ovum,“ and, together with the endodermal layer of the germ disc, it encloses the exocoelomic vesicle (primitive
yolk-sac). Themembrane consists of flattened and elongated cells; it is continuous with the edge of the endodermal layer of the disc, the transition to the cubical endodermal cells being very sudden. This feature favours the view of Heuser‘ that the membrane is mesoblastic in origin, whereas the endoderm is derived from the
inner cell mass.
Fig 1. Ovum and endometrium. Operculum, consisting of fibrin and leucocytes permeated by plasmoditrophoblast, is seen in upper part of photograph. Fibrinous wisp projects into mouth of gland on left. No epithelium on surface of operculum. Sprouts of trophoblast seen penetrating stratum compactum below ovum. Maternal red-blood corpuscles seen in lucunae of plasmoditrophoblast to left of chorionic vesicle. Primitive mesoblast shown permeating chorionic vesicle between cytotrophoblast. and thin exocoelomic membrane. Of the two cavities inside the chorionic.vesicle, the upper, larger one is the exocoelomic vesicle (primitive yolk sac), with the endodermal plate of cubical cells forming its lower wall. The lower, smaller cavity in the amniotic cavity; in the picture its upper wall is formed by the columnar cells of the ectodermal disc. The endometrium adjacent to the trophoblast shows no zone of necrosis. x 90.
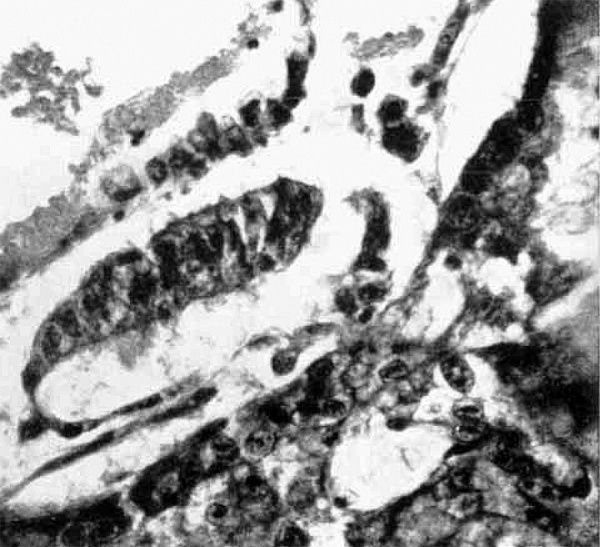
The embryo proper (Fig. 3) consists of a bilaminar disc, comprising a single layer of tall columnar ectoderrnal cells which form the floor of the amniotic cavity, and a single
layer of cubical endodermal cells which form the roof of the exocoelomic vesicle. Mesoderm does not penetrate between these two germ layers. Part of the disc is missing, but assuming it was circular, and such an assumption is warranted by comparison with other early human and with monkey ova, the diameter of the disc would be 0.117 mm.; that of the disc in the 11.5 day Hertig-Rock ovum is 0.138 mm. At the edge of the disc the ectodermal cells suddenly change to the flattened cells comprising the side walls and roof of the amniotic cavity, a feature which supports the views of Ramsey‘ and Heuser and Streeter“ that in primates the ectodermal layer of the disc is formed from the inner cell mass, whereas the amnion is derived from the mesoblast, which in its turn has been delaminated from the cytotrophoblast. In the present specimen the roof of the amnion is deficient in part ; here the cavity is roofed over by cytotrophoblast and transition cells between cytotrophoblast and amniotic cells are present. Definite yolk sac has not yet been formed.
Fig 2. Ovum embedded in stratum compactum of endometrium: the endometrium shows the structure characteristic of the secretory phase. Spiral arteries, cut across several times in the section, are seen between two glands just to the right of the'centre.; x 11.
Fig 3. Part of ovum. The tall columnar cells of the ectodermal disc are seen to continue at the edge of the disc into the flattened cells of the amnion. The lower part (really roof) of the latter is seen to merge into the cytotrophoblast. and transition cells between the two are shown just to the right of the centre of the picture. In the upper part the cubical cells of the endodermal layer of the disc are seen to continue into the flattened cells of the exocoelomic membrane. Eosinophilic deposit is seen in the exocoelomic vesicle (above), in the amniotic cavity. (flow) and in the meshes of the primitive mesoblast (above and to the right). x 430.
Discussion and Comparison with Other Early Human Ova
The smaller dimensions of the chorionic vesicle and of the embryonic disc, and the lesser degree of enlargement of the endometrial sinusoids in the present specimen indicate that it is younger than the II. 5 day Hertig-Rock ovum. More recently, Rock and Hertig’ have published a preliminary report, including a photomicrograph of one section, of an implanted human ovum (Wi-8004) estimated to be 9.5 days old, but a detailed account of its structure and measurements is not given. Inspection of this photograph shows that there are many similarities with the present specimen. The 9. 5 day Rock-Hertig ovum and the present specimen comprise the earliest stages of fully‘ implanted human ova so far discovered. The incomplete Miller ovum“ was tentatively concluded by Streeter’ in 1926 to have an ovulation age of IO to II days. After
- The term "fully implanted," as used in this paper, implies that no part of the ovum is freely exposed to the uterine cavity.
Streeter’s‘° new profile reconstruction of the
Miller ovum in 1939, Hertig and Rock“
noted that it closely resembled their II. 5
day specimen and quote Streeter as now
being of the opinion that the Miller ovum
is slightly older than their specimen.
Streeter“ expressed a the View that the differences in details of the form and in the rate of differentiation and growth between the embryos of man and monkey do not become appreciable until the end of the 2nd month, and that therefore for at least the Ist month or 6 weeks, the ages known for macaque embryos may be transferred to human ‘embryos of corresponding development. The present specimen is similar in its general features to the Io-day macaque embryo described by Heuser and Streeter, but it is more advanced, however, in respect of the development of the amniotic cavity and of the primitive mesoblast. Heuser“’” has stressed the precocious formation of the primitive mesoblast in the monkey»; the present-specimen indicates that mesoblast formation in the human is even more precocious than that in the monkey, and, taken in conjunction with the human ova described by Hertig and Rock, it indicates that there are considerable differences in general organization of the human embryo between the ages of 9.5 and 12.5 days and that of macaque embryos of corresponding ages. Heuser and Streeter‘ illustrate variations in the state of development of the various elements in macaque embryos of the same age; likewise the present specimen shows some differences of detail from the 9. 5-day Rock-Hertig ovum in that it is more deeply implanted, surface epithelialization has not yet occurred, the trophoblast on the abembryonic side is thicker, the exocoelomic cavity is larger and the endoderrn consists for the main part of only a single layer of cells.
The exocoelomic‘ (Heuser’s) membrane, unlike that in the 11.5-day Hertig-Rock ovum, is complete and, with the endodermal
disc, encloses the exocoelomic coelom. The
definitive yolk sac has not yet developed.
The precise method of development of the
latter has not been witnessed either in the
human or in the closely graded series of
macaque embryos studied by Heuser and
Streeter.° Even in the youngest human ova
in which a true yolk sac may be considered
to be present, namely the 13-day Torpin
ovum (Krafka"), the 13- to I4-day Linzenmeier ovum ,“’ the I3- to 14-day Yale ovum,‘
and the 13- to 14-day Peters ovum,“ the sac
is already complete. In the macaque,
Heuser‘ observed that, once the cells of
the primitive endodermal plate begin to
multiply rapidly (after the IIth day), they
separate into layers to form the yolk sac,
“ which makes a belated appearance within
a few hours,” and is completely developed
and segregated from the exocoelomic vesicle
by the I2-day stage. As the true yolk
sac has not yet developed in the I2. 5-day
Hertig-Rock human ovum,” in which the
exocoelomic vesicle resembles that in the
present specimen, and as the conditions in
the 13- to 14-day Yale ovum resemble those
in the I 3-day macaque and suggest that the
development of the yolk sac in the human
is similar to that in the monkey, it appears
that a closely graded series of human embryos round about the 12th and 13th day
need to be studied in order to solve the
problem of the precise manner of formation
of the human yolk sac. ' ’
Of 12 early human ova described by Rock and Hertig,’ 5 deemed to be abnormal were
implanted in the anterior wall of the uterus
and 7 considered to be normal were embedded in the posterior wall. These authors
state that this relation may be spurious;
this expectation is realized by the fact that
the present specimen, which shows no abnormal features, is implanted in the anterior
wall. The fact that in the present case the
corpus luteum was situated in the right ovary would not appear to be causally related to the fact that the site of implantation
of the ovum was near the entrance of the
right uterine tube, in view of the findings
of Rock and Hertig that in their 12 specimens the proximity of the implantation site
to one or other uterine tube bore no constant
relation to the ovary containing the active
corpusluteum. As these authors remark,
it seems that the free blastocyst is passively
moved about within the uterine cavity until
it is able to attach itself to the endometrium.
Following the estimation of the probable age of the ovum from its histological features, ovulation in the present instance was
deduced to have occurred about the 9th day
of the cycle of 28 days. In these circumstances the resultant corpus luteum would,
by its progesterone (and oestrogen) secretion, begin to influence the endometrium
to induce the secretory phase 5 days earlier
than would have been the case if ovulation had occurred on the I4th day. It
is noteworthy that the histological structure
of the endometrium on the 20th day of the
cycle in the present case is closely similar to
that of the endometrium on the 24th or 25th
day of a 28-day cycle in a number of specimens examined by the present authors, and
it is certainly much more advanced in the
secretory phase than many 20-day specimens examined from women with 28-day
cycles; in these cases ovulation probably
occurred about the 14th day. Thus, in the
absence of knowledge of the ovulation time,
the stage of development of the endometrium in a case of a very early pregnancy
cannot be a reliable guide to the age of the
embryo.
In normal circumstances there is a balanced physiological interrelation between
the trophoblast and the endometrium, a
normal endometrium, sensitized for the
reception of an ovum by the right degree of
synergic activity of progesterone and oestrogen, allowing the appropriate degree of proliferative activity of a normal trophoblast. Alteration of any of these factors
beyond a certain limit will result in a
departure from this normal relationship
between maternal and embryonic structures, and it is in respect of this upset
of balance that early histological changes
are to be sought which in icate the inability of the physiological relationship
to continue and the existence of a state
of affairs likely to lead to abortion.
For example, the Teacher-Bryce‘ and the
Mollendorff Sch.’ ova are both surrounded
by a well-marked zone of necrosis of the
endometrium ; consequently the belief was
held for some time that implantation of the
ovum was normally accomplished by ne
crosis of the maternal tissues. That such is
not the case is indicated by the absence of
necrosis of the stroma immediately adjoining the trophoblast in the present specimen
as well as in others, and the necrosis in
these two ova is undoubtedly to be correlated with the fact that both specimens are
abortions. In the Barnes ovum (Hamilton
etal”) the pathological oedema of the endometrium may be the causative factor underlying the superficial nidation of the ovum
and may represent one of the very early
changes in the histological structure of the
endometrium which may ultimately lead to
abortion. Ramsey,‘ in a specimen obtained
at autopsy from a woman poisoned by an
abortifacient, found that the trophoblast
was devoid of cytotrophoblast and consisted solely of tightly packed plasmoditrophoblast with crowded nuclei. As Ramsey
remarks, in a normal specimen a nice
balance is maintained between the cytotrophoblast, plasmoditrophoblast and primitive mesoblast, and, as the last two are
derived from the first, there should be an
abundant supply of vigorous cytotrophoblast in the early stage of development.
Such has been shown to be the case in the present specimen.
Summary
- The structure of a previllous human ovum estimated to be 9 to I0 days old is described. With the 9.5 day Rock-Hertig ovum, it constitutes the earliest stage of the fully implanted human ovum so far discovered.
- The specimen indicates that primitive mesoblast formation is even more precocious in its development in the human than in other primates and that, in contrast to the claim of Streeter, differences are found in the structural details of early human and macaque ova of corresponding ages.
- The findings favour the view that whereas the ectodermal and endodermal layers of the germ disc are derived from the formative cell mass, the amnion and exocoelomic membrane are mesoblastic in origin.
- Comparison with other ova suggests that the yolk sac develops rapidly round about the 12th and 13th day in the human.
- Reference is made to early pathological changes in both embryonic and endometrial elements which may lead to abortion.
- Ovulation is deduced to have occurred about the 9th day of the cycle; the endometrium is as far advanced in the secretory phase on the 20th day in the present case as on the 24th or 25th day in a number of specimens. in which ovulation probably occurred about the 14th day.
Author's Addendum
Since this. paper was written, a more extended account of the ovum Wi—8004 has been published (Hertig, A. T. and Rock. J., Amer. Journ. Obstet. and Gynecol., 1944, Vol. 47, pp. 149-184). Its authors now state that the ovum may be slightly younger than 9.5 days and that it is “ approximately two-thirds implanted.” This further description also shows that some of the embryonic structures are in a slightly earlier stage of development than the Davies—Harding ovum.
References
1. Bryce, T. H. and J. H. Teacher. " Contributions to the study of the early development of the human ovum." Glasgow, 1908, p. 7.
2. Moillendorff, W. v. Ztschr. f. Anat. u. Ervtwicklungsgesch., 1921, lxii, 352.
3. Hertig AT. and Rock J. Two human ova of the pre-villous stage, having an ovulation age of about eleven and twelve days respectively. (1941) Carnegie Instn. Wash. Publ. 525, Contrib. Embryol., 29: 127-156.
4. Heuser, C. H. Co-operation in Research, Cameg. Inst. Wash., 1938, Pub. 5oI, p. 383.
5. Ramsey, E. M.. Corutrib. Embryol. Carnage. Inst. Wash., 1938, xxvii, clxi, 67.
6. Heuser, C. H. and G. L. Streeter. Contrib. Embryol. Cameg. Inst. Wash., 1941, xxix, No. 181, I5.
7. Rock, J. and A. T. Hertig. Amer. Jouen. Obstet. and Gynecol., 1942, xliv, 973 (Corrections, Ibid, 1943, xlv, 356.)
8. Miller, J. W. Berl. klin. Wohnschr., 1913, xix, 1
9. Streeter GL. The "Miller" ovum—the youngest normal human embryo thus far known. (1926) Carnegie Instn. Wash. Publ 363, Contrib. Embryol., 18: 31-48.
10. Streeter, G. L. Anat. Rec., 1939, lxxii, suppl. 2, p. 75.
11. Streeter, G. L. Annual Report Director Dept. Embryology, Carnegie Institution for 1932-33» P- 4.
12. Heuser CH. The chimpanzee ovum in the early stages of implantation (about 10.5 days). (1940) J Morphol. : 155- .
13. Krafka J. The Torpin ovum, a presomite human embryo. (1941) Contrib. Embryol., Carnegie Inst. Wash. Publ. 525, 29: 167-193.
14. Linzenmeier, G. Arch. 1‘. Gyna'kol., 1914, cii, I.
15. Peters, H. “ Ueber die Einbettung des menschlichen Eies und das friiheste bisher bekannte menschliche Placentationstadium.” Leipzig and Vienna, 1899.
16. Hamilton WJ. Barnes J. and Dodds GH. Phases of maturation, fertilization and early development in man. (1943) J. Obstet. Gynaecol, Brit. Emp., 50: 241-245.
Cite this page: Hill, M.A. (2024, April 28) Embryology Paper - A Human Ovum Nine to Ten Days Old. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Paper_-_A_Human_Ovum_Nine_to_Ten_Days_Old
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G