Book - The Early Embryology of the Chick 13
| Embryology - 1 Mar 2026 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Patten BM. The Early Embryology of the Chick. (1920) Philadelphia: P. Blakiston's Son and Co.
| Online Editor |
|---|
| This historic 1920 paper by Bradley Patten described the understanding of chicken development. If like me you are interested in development, then these historic embryology textbooks are fascinating in the detail and interpretation of embryology at that given point in time. As with all historic texts, terminology and developmental descriptions may differ from our current understanding. There may also be errors in transcription or interpretation from the original text. Currently only the text has been made available online, figures will be added at a later date. My thanks to the Internet Archive for making the original scanned book available.
By the same author: Patten BM. Developmental defects at the foramen ovale. (1938) Am J Pathol. 14(2):135-162. PMID 19970381 Those interested in historic chicken development should also see the earlier text The Elements of Embryology (1883). Foster M. Balfour FM. Sedgwick A. and Heape W. The Elements of Embryology (1883) Vol. 1. (2nd ed.). London: Macmillan and Co.
Modern Notes |
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
The Development of the Chick During the Third and Fourth Days of Incubation
External Features
Torsion
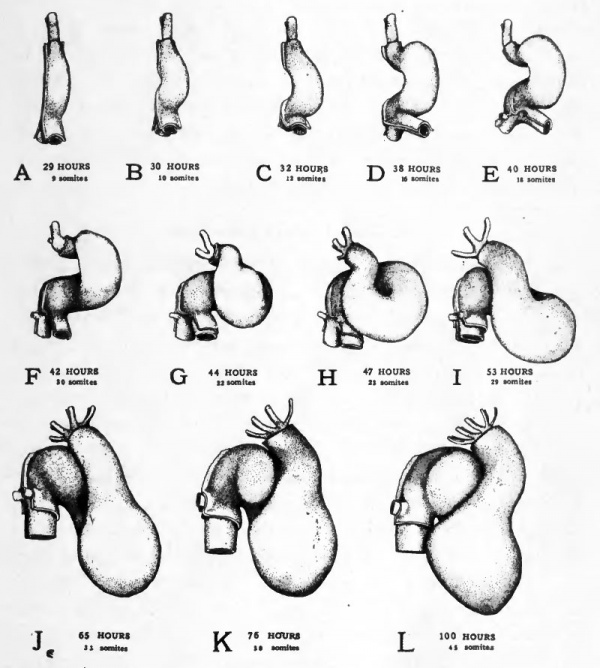
Chicks of three days incubation (Fig. 39) have been affected by torsion throughout their entire length. Torsion is complete well posterior to the level of the heart but the caudal portion of the embryo is not yet completely turned on its side. In four-day chicks the entire body has been turned through 90 degrees and the embryo lies with its left side on the yolk (Fig. 40).
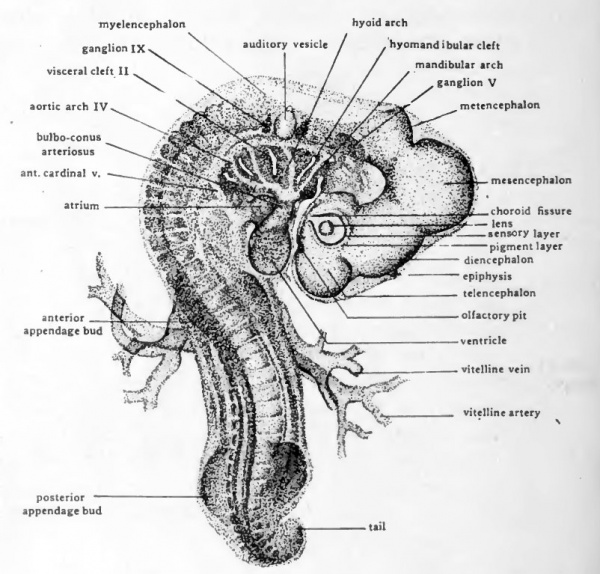
Fig. 39. Dextro-dorsal view ( X 14) of entire chick embryo of 36 somites (about three days incubation).
Flexion
The cranial and cervical flexures which appeared in embryos during the second day have increased so that in three-day and four-day chicks the long axis of the embryo shows nearly right-angled bends in the mid-brain and in the neck region. The mid-body region of thee-day chicks is shghtly concaved dorsally. This is due to the fact that the embryo is still broadly attached to the yolk in that region. By the end of the fourth day the body folds have undercut the embryo so it remains attached to the yolk only by a slender stalk. The yolk-stalk soon becomes elongated allowing the embryo to become first straight in the mid-dorsal region, and then convex dorsally. At the same time the caudal flexure is becoming more pronounced. The progressive increase in the cranial, cervical, dorsal, and caudal flexures results in the bending of the embryo on itself so that its originally straight long-axis becomes C-shaped and its head and tail lie close together (Fig. 40).
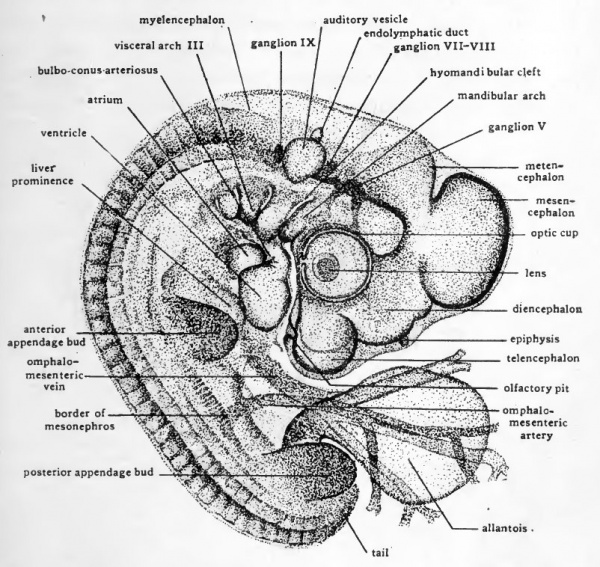
Fig. 40. Dextral view of entire chick embryo of 41 somites (about four days incubation).
The Visceral Arches and Clefts
A fourth visceral cleft has appeared caudal to the three that were already formed in 55hour chicks. The visceral arches are thicker and more conspicuous than in earlier embryos. In lightly stained whole-mounts of a three-day chick it is still possible to make out the aortic arches running through the visceral arches. In a chick of foui days the visceral arches have become so much thickened that it is very difficult to see the vessels traversing them.
The Oral Region
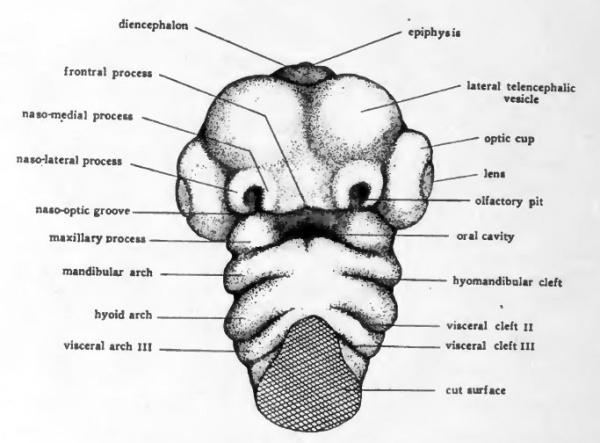
The cervical flexure presses the pharyngeal region and the ventral surface of the head so closely together that it is difficult to make out the topography of the oral region by study of entire embryos. If the head and pharyngeal region are cut from the trunk and viewed from the ventral aspect the relations of the structures about the mouth are well shown (Fig. 41). The mandibular arch forms the caudal boundary of the oral depression. Arising on either side in connection with the mandibular arch are paired elevations, the maxillary processes, which grow mesiad and form the cephalolateral boundaries of the mouth opening. The nasal pits appear as shallow depressions in the ectoderm of the anterior part of the head which overhangs the mouth region. Surrounding each nasal pit is a U-shaped elevation with its limbs directed toward the oral cavity. The lateral limb of the elevation is the naso-lateral process, and the median limb is the naso-medial process. As development proceeds the two naso-medial processes grow toward the mouth and meet the maxillary processes which are growing in from either side. The fusion of the two naso-medial processes with each other in the mid-line, and the fusion of each of them laterally with the maxillary process of its own side gives rise to the upper jaw (maxilla). The fusion in the mid-line of the right and left components of the mandibular arch gives rise to the lower jaw (mandible).
Fig. 41. Drawing to show the external appearance of the structures in the ora region of a four-day chick. Ventral aspect.
The Appendage Buds
Both the anterior and posterior appendage-buds have appeared in embryos of three days. They are formed by bud-like outgrowths from somites. The anterior appendages arise opposite somites 17 to 19 inclusive, and the posterior appendages arise opposite somites 26 to 32 inclusive. During the fourth day the appendage buds increase rapidly in size and become elongated but otherwise their appearance and their relationships show little change.
The Allantois
The development of the extra-embryonic membranes has already been considered (Chap. XI) and needs no further discussion here. In order to show the embryos more clearly, the extra-embryonic membranes, except for the allantois, have been removed from the specimens drawn in Figures 39 and 40. The cut edge of the amnion shows at its anterior attachment to the body, opposite the anterior appendage bud and just caudal to the tip of the ventricle. The allantois in the three-day chick is as yet small and is concealed by the posterior appendage buds. In four-day embryos it has undergone rapid enlargement and projects from the umbilical region as a stalked vesicle of considerable size.
The Nervous System
Summary of Development Prior to the Third Day
The earliest indication of the formation of the central nervous system appears in chicks of 16 to 18 hours as a local thickening of the ectoderm which forms the neural plate (Fig. 11). The neural plate then becomes longitudinally folded to form the neural groove (Figs. 14 and 15). By fusion of the margins of the neural folds, first in the cephalic region and later caudally, the neural groove is closed to form a tube and at the same time separated from the body ectoderm. The cephalic portion of the neural tube becomes dilated to form the brain and the remainder of the neural tube gives rise to the spinal cord (Figs. 18 and 21).
In its early stages the brain shows a series of enlargements in its ventral and lateral walls, indicative of its fundamental metameric structure. In the establishment of the three vesicle condition of the brain, the lines of demarcation between prosencephalon, mesencephalon, and rhombencephalon are formed by the exaggeration of certain of the inter-neuromeric constrictions and the obliteration of others (see Chap. IX and Fig. 20). The original neuromeric enlargements persist longest in the rhombencephalon.
The three-vesicle condition of the brain is transitory. By forty hours the division of the rhombencephalon into metencephalon and myelencephalon is clearly indicated (Figs. 20, D and 22). The division of the prosencephalon and the estabhshment of the five-vesicle condition characteristic of the adult brain, does not take place until somewhat later.
In chicks of 55 hours (Figs. 34 and 35) the appearance of the cranial flexure has resulted in the bending of the brain so that the entire prosencephalon is displaced ventrad and then toward the heart. At the same time the head of the embryo has undergone torsion and lies with its left side on the yolk. Although flexion and torsion have thus completely changed the general appearance of the brain as seen in entire embryos, the regions already established in 40-hour chicks are still evident. The prosencephalon has, however, become very noticeably enlarged cephalic to the optic vesicles, and a slight constriction in its dorsal wall indicates the beginning of the demarcation of the telencephalic region from the diencephalic region.
The Formation of the Telencephalic Vesicles
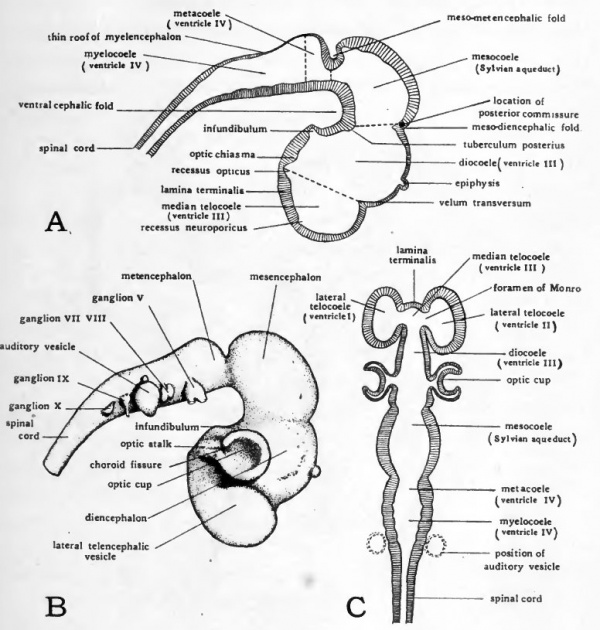
By the end of the third day the antero-lateral walls of the primary forebrain have been evaginated to form a pair of vesicles lying one on either side of the mid-line (Figs. 39, 41, and 42, B). These lateral evaginations are known as the telencephahc vesicles. The openings through which their cavities are continuous with the lumen of the median portion of the brain are later known as the foramina of Monro. The telencephahc division of the brain includes not only the two lateral vesicles but also the median portion of the brain from which they arise. The teloccele has therefore three divisions, a median, broadly confluent posteriorly with the diocoele, and two lateral, connecting with the median through the foramina of Monro (Fig. 42, C).
Before the formation of the telencephalic vesicles the most anterior part of the brain lay in the mid-line, but the rapid growth of the telencephalic vesicles soon carries them anteriorly beyond the median portion of the teloccele. The median anterior wall of the teloccele which formerly was the most anterior part of the brain, and which remains the most anterior part of the brain lying in the mid-line is known as the lamina terminalis (Figs. 42, A, and C, and 43). The telencephalic vesicles become the cerebral hemispheres, and their cavities become the paired lateral ventricles of the adult brain. The hemispheres undergo enormous enlargement in their later development and extend dorsally and posteriorly as well as anteriorly, eventually covering the entire diencephalon and mesencephalon under their posterior lobes.
Fig. 42. Diagrams to show the topography of the brain of a four-day chick.
- A, plan of sagittal section. The arbitrary boundaries between the various brain vesicles (according to von Kupffer) are indicated by broken lines. B, dextral view of a brain which has been dissected free. C, schematic frontal section plan of brain. The flexures of the brain are supposed to have been straightened before the section was cut.
As a matter of convenience in dealing with the morphology of the brain, more or less arbitrary lines of division between the adjacent brain regions are recognized. The division between telencephalon and diencephalon is an imaginary line drawn from the velum transversum to the recessus opticus (Fig. 42, A), Velum trans versum is the name given to the internal ridge formed by the deepening of the dorsal constriction which was first noted in chicks of 55 hours as indicating the impending division of the primary fore-brain (Fig. 35). The recessus opticus is a transverse furrow in the floor of the brain which in the embryo leads on either side into the lumina of the optic stalks.
The Diencephalon
The 'lateral walls of the diencephalon at this stage show little differentiation except ventrally where the optic stalks merge into the walls of the brain. The development of the epiphysis as a median evagination in the roof of the diencephalon has already been mentioned (Chap. Xll). Except for some elongation it does not differ from its condition when first formed in embryos of about 55 hours. The infundibular depression in the floor of the diencephalon has become appreciably deepened and lies in close proximity to Rathke's pocket with which it is destined to fuse in the formation of the hypophysis (Fig. 43). Later in development the lateral walls of the diencephalon become greatly thickened to form the thalami, thus reducing the size and changing the shape of the diocoele, which is known in adult anatomy as the third brain ventricle. The anterior part of the roof of the diencephalon remains thin and by the ingrowth of blood vessels from above is pushed into the third ventricle to form the anterior choroid plexus.
Fig. 43. Diagram of median longitudinal section of four-day chick. Due to a slight bend in the embryo the section is para-sagittal in the mid-dorsal region but for the most part it passes through the embryo in the sagittal plane.
The boundary between the diencephalon and the mesencephalon is an imaginary line drawn from the internal ridge formed by the original dorsal constriction between the primary fore-brain and mid-brain, to the tuberculum posterius (Fig. 42, A). The tuberculum posterius is a rounded elevation in the floor of the brain of importance chiefly because it is regarded as marking the boundary between diencephalon and mesencephalon.
The Mesencephalon
The mesencephalon as yet shows no specializations, beyond a thickening of its walls. The dorsal and lateral walls of the mesencephalon later increase rapidly in thickness and become the optic lobes (copora quadrigemina) of the adult brain. The optic lobes should not be confused with the optic vesicles arising from the diencephalon of the embryo. They are entirely different structures. The floor of the mesencephalon also becomes greatly thickened and is known in the adult as the crura cerebri. It serves as the main pathway of the fiber tracts which connect the cerebral hemispheres with the posterior part of the brain and the spinal cord. The originally capacious mesocoele is thus reduced by the thickenmg of the walls about it to a narrow canal (Aqueduct of Sylvius).
The Metencephalon
The boundary between the mesencephalon and metencephalon is indicated by the original interneuromeric constriction which separated them at the time of their establishment (Cf. Figs. 20 and 42). The caudal boundary of the metencephalon is not definitely defined. It is regarded as being located approximately at the point where the brain roof changes from the thickened condition characteristic of the metencephalon to the thin condition characteristic of the myelencephalon. The metencephalon shows practically no differentiation in four-day chicks. Later in development there is ventrally and laterally an extensive ingrowth of fiber tracts giving rise to the pons and to the cerebellar peduncles of the adult metencephalon. The roof of the metencephalon undergoes extensive enlargement and becomes the cerebellum of the adult brain.
The Myelencephalon
In the myelencephalon the dorsal wall has become greatly reduced in thickness indicative of its final fate as the thin roof of the medulla. Like the roof of the diencephalon, the roof of the myelencephalon later receives a rich supply of small blood vessels by which it is pushed into the myelocoele to form the posterior choroid plexus (choroid plexus of the fourth ventricle). The ventral and lateral walls of the myelencephalon become the floor and side-walls of the medulla of the adult brain.
The Ganglia of the Cranial Nerves
In the brain region, cells derived from the cephalic portion of the neural crest have become aggregated to form ganglia. The largest and the most clearly defined of the gangha present in four-day chicks is the Gasserian ganghon of the fifth (trigeminal) cranial nerve (Fig. 42, B). It hes ventro-laterally, opposite the most anterior neuromere of the myelencephalon. From its cells sensory nerve fibers grow mesiad into the brain and distad to the face and mouth region. In four-day chicks the beginning of the ophthalmic division of the fifth nerve extends from the ganglion toward the eye, and the beginning of the mandibulo-m axillary division is growing toward the angle of the mouth (Fig. 40). Immediately cephalic to the auditory vesicle is a mass of neural crest cells which is the primordium of the gangha of the seventh and eighth nerves. The separation of this double primordium to form the geniculate ganglion of the seventh nerve and the acoustic gangUon of the eighth nerve begins during the fourth day. Posterior to the auditory vesicle the ganglion of the ninth nerve can be clearly seen even in whole-mounts (Fig. 40). The gangha of the tenth (vagus) nerves can be recognized in pections of chicks at the end of the fourth day but are difficult to make out in whole-mounts.
The Spinal Cord
The spinal cord region of the neural tube when first estabhshed, exhibits a lumen which is elhptical in cross section. As development progresses the lateral walls of the cord become greatly thickened in contrast with the dorsal and ventral walls which remain thin. In this process the lumen (central canal) becomes compressed laterally until it appears in cross section as little more than a vertical slit. The thin dorsal wall of the tube is known as the roof plate; the thin ventral wall as the floor plate; and the thickened side walls as the lateral plates.
The Spinal Nerve Roots
During the fourth day the establishment of the spinal nerve roots has begun. The growth of nerve fibers from the neuroblasts can only be traced with the aid of special methods of staining. The more general steps in the development of the roots of the spinal nerves can, however, be followed in sections prepared by the ordinary methods.
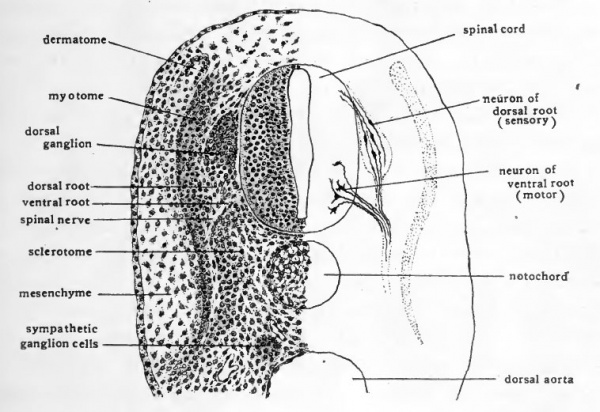
Fig. 44. Drawing to show the structure and relations of a spinal ganglion and the roots of a spinal nerve.
- The left half of the drawing represents structures as they appear after treatment by the usual nuclear staining method. The right half of the section shows schematically the nerve cells and the fibers growing out from them as they may be demonstrated by the Golgi method. (Nerve cells and fibers after Ramon y Cahal.)
In the adult each spinal nerve is connected with the cord by two roots, a dorsal root which is sensory in function and a ventral root, which is motor in function. Lateral to the cord the dorsal and ventral roots unite. The spinal ganglion (dorsal root ganglion) is located on the dorsal root between the spinal cord and the point where dorsal and ventral roots unite. Distal to the union of dorsal and ventral roots is a branch, the ramus communicans, which extends ventrad to a ganglion of the sympathetic nerve cord.
When first formed from the neural crest cells, the spinal ganghon has no connection with the cord (Fig. 37). The dorsal root is established by the growth of nerve fibers from cells of the spinal ganglion mesiad into the dorsal part of the lateral plate of the cord. At the same time fibers grow distad from these cells to form the peripheral part of the nerve (Fig. 44). The fibers which arise from the dorsal root ganglion conduct sensory impulses toward the cord.
Coincident with the estabUshment of the dorsal root, the ventral root is formed by fibers which grow out from cells located in the ventral part of the lateral plate of the cord (Fig. 44). The fibers which thus arise from cells in the cord and pass out through the ventral root, conduct motor impulses from the brain and cord to the muscles with which they are associated peripherally.
The sympathetic ganglia arise from cells of the neural crest which migrate ventrally and form cellular masses lying on either side of the mid-line at the level of the dorsal aorta. By the end of the fourth day these cells constitute a pair of cords in which enlargements can be made out opposite the spinal ganglia. These enlargements are the primary sympathetic ganglia. Each sympathetic ganglion is connected with the corresponding spinal nerve by a cellular cord which is the primordium of the ramus communicans. The sympathetic ganglia later receive both sensory and motor fibers from the spinal nerve roots by way of the rami communicantes, and from nerve cells in the sympathetic gangha, fibers extend to the viscera.
The Sense Organs
The Eye
The primary optic vesicles arise in chicks of about 30 hours as dilations in the lateral wall of the prosencephalon (Figs. 19 and 23). At first the optic vesicles open broadly into the brain, but later constrictions develop which narrow their attachment to the form of a stalk (Fig. 22). In chicks of 55 hours the primary optic vesicles are in vagina ted to form the double-walled secondary optic vesicles or optic cups. The invagination takes place in such a way that the ventral wall of the cup is incomplete, the gap in it being known as the choroid fissure (Figs. 35 and 36, B).
The lens arises as a thickening of the superficial ectoderm which becomes depressed to form a vesicular invagination extending into the optic cup (Fig. 36, B).
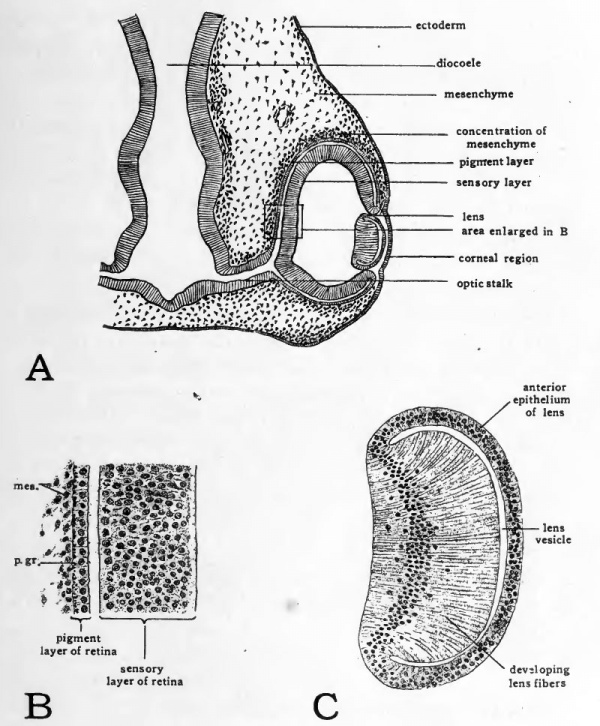
Fig. 45. Drawings to show structure of the eye of a four-day chick.
- A, diagram to show topography of eye region; B, drawing to show cellular organization of the pigment and sensory layers of the retina. Abbreviations: mes,, mesenchymal cell; p.gr., pigment granule; C, drawing to show cellular organization of the lens.
In chicks of four days the choroid fissure has become narrowed by the growth of the walls of the optic cup on either side of it (Figs. 40 and 42, B). The orifice of the optic cup becomes narrowed by convergence of its margins toward the lens (Fig. 45, A). Meanwhile the lens has become freed from the superficial ectoderm and forms a completely closed vesicle. Sections of the lens at this stage show that the cells constituting that part of its wall which lies toward the center of the optic cup are becoming elongated to form the lens fibers (Fig. 45, C).
At this stage we can identify the beginning of most of the structures of the adult eye. The thickened internal layer of the optic cup will give rise to the sensory layer of the retina (Fig. 45, B). Fibers arise from nerve cells in the retina and grow along the groove in the ventral surface of the optic stalk toward the brain to form the optic nerve. The external layer of the optic cup gives rise to the pigment layer of the retina. Mesenchyme cells can be seen aggregating about the outside of the optic cup. From these the sclera and choroid coat are derived. Some of the mesenchyme makes its way into the optic cup through the choroid fissure and gives rise to the cellular elements of the vitreous body. The complex ciliary apparatus of the adult eye is derived from the margins of the optic cup adjacent to the lens. The corneal and conjunctival epithelium arise from the superficial ectoderm overlying the eye. Mesenchyme cells which make their way between the lens and the corneal epithelium give rise to the substantia propria of the cornea.
The Ear
Of the structures taking part in the formation of the ear, the first to appear is the auditory placode. The auditory placode is recognizable in 36-hour chicks as a thickened plate of ectoderm. Almost as soon as it appears the placode sinks below the level of the surrounding ectoderm to form the floor of the auditory pit (Fig. 22). By constriction of its opening to the surface the epithelium of the auditory pit becomes separated from the ectoderm of the head and comes to lie close to the lateral wall of the myelencephalon (Fig. 36, yl). A tubular stalk, the endolymphatic duct, remains for a time adherent to the superficial ectoderm, marking the location of the original invagination (Fig. 40).
The degree of development reached by the ear primordium in four-day chicks gives little indication of the nature of the later processes by which the ear is formed. The auditory vesicle by a very complex series of changes will give rise to the entire epithelial portion of the internal ear mechanism. Nerve fibers arising from the acoustic ganglion grow into the brain proximally and to the internal ear distally establishing nerve connections between them. There is at this stage no indication of the differentiation of the external auditory meatus. The dorsal and inner portion of the hyomandibular cleft which gives rise to the eustachian tube and to the middle ear chamber has not yet become associated with the auditory vesicle.
The Olfactory Organs
The olfactory organs are represented in three-day and four-day chicks by a pair of depressions in the ectoderm of the head. These so-called olfactory pits are located ventral to the telencephalic vesicles and just anterior to the mouth (Figs. 40 and 41). By growth of the processes which surround them, the olfactory pits become greatly deepened. The epithelium lining the pits eventually comes to lie at the extreme upper part of the nasal chambers and constitutes the olfactory epithelium. Nerve fibers grow from these cells to the telencephahc lobes of the brain to form the olfactory nerves.
The Digestive and Respiratory Systems
Summary of Development Prior to the Third Day
The primary entoderm which gives rise to the epithelial lining of the digestive and respiratory systems and their associated glands becomes established as a separate layer before the egg is laid. In its early relationships the entoderm is a sheet-like layer of cells lying between the ectoderm and the yolk and attached peripherally to the yolk (Fig. 7). The primitive gut is the cavity bounded dorsally by the entoderm and ventrally by the yolk (Fig. 31, A).
Only the part of the entoderm which lies within the embryonal area is involved in the formation of the enteric tract. The peripheral portion of the entoderm goes into the formation of the yolk-sac. There is at first no definite line of demarcation between the entoderm destined to be incorporated into the body of the embryo and that which remains extra-embryonic in its associations. The foldings which appear later separating the body of the embryo from the yolk, establish for the first time the boundaries between intra-embryonic and extra-embryonic entoderm (Figs. 30 and 32).
The first part of the gut to acquire a complete entodermic lining is the fore-gut. Its floor is formed by the caudally progressing concrescence of the entoderm which takes place as the subcephalic and lateral body folds undercut the cephalic part of the embryo (Figs. i6 and ^i, B). At a considerably later stage the hind-gut is formed by the progress of the subcaudad fold (Figs. 35 and 31, C). Between the fore-gut and the hind-gut, the mid-gut remains open to the yolk ventrally. As the embryo is more completely separated from the yolk the fore-gut and hind-gut increase in extent at the expense of the mid-gut. By the fourth day of incubation the mid-gut is reduced to the region where the yolk stalk opens into the enteric tract (Figs. 31, Z) and 43).
The Establishment of the Oral Opening
When first established the gut ends as a blind pocket both cephalcally and caudally. The mouth opening does not appear until the third day, the cloacal opening is not established until much later in incubation. In embryos of 55 hours the processes leading toward the establishment of the oral opening are clearly indicated. A mid-ventral evagination of the pharynx is established immediately cephalic to the mandibular arch (Fig. 35). Opposite this out-pocketing of the pharynx, and growing in to meet it, the stomodeal depression is formed. The thin membrane formed by the meeting of the pharyngeal entoderm with the stomodeal ectoderm is known as the oral plate. The communication of the fore-gut with the outside is finally established by the breaking through of the oral plate.
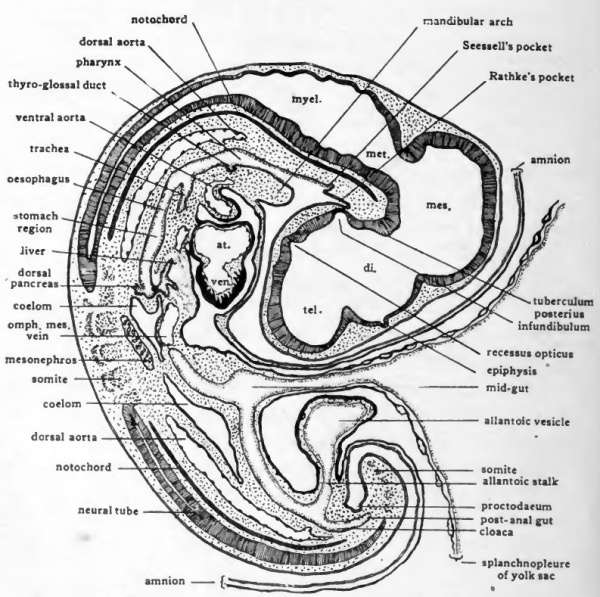
The formation of the mouth opening in the manner described does not take place at the extreme anterior end of the fore-gut. A small gut pocket extends cephalic to the mouth. This socalled pre-oral gut rapidly becomes less conspicuous after the breaking through of the oral plate. The small depression which in older embryos marks its location is known as Seessell's pocket (Fig. 43). Even this small depression eventually disappears altogether. Its importance lies wholly in the fact that it indicates for some time the place at which ectoderm and entoderm originally became continuous in the formation of the oral opening.
The Pharyngeal Derivatives
Several structures arise in the pharyngeal region which do not become parts of the digestive system. Nevertheless the origin of their epithelial portions from fore-gut entoderm and their early association with this part of the gut tract makes it convenient to take them up in connection with the digestive system.
The thyroid gland arises as a median diverticulum from the floor .of the pharynx which makes its appearance at the level of the second pair of pharyngeal pouches. Toward the end of the fourth day the thyroid evagination has become saccular and retains its connection with the pharynx only by a narrow opening at the root of the tongue known as the thyro-glossal duct (Fig. 43). In mammaha the thyroid is contributed to by primordia which arise laterally from the fourth pharyngeal pouches as well as by a median evagination from the floor of the pharynx. It is possible that evaginations which in the chick arise from the fourth pharyngeal pouches are homologous with the lateral thyroid primordia of mammals. In the chick, however, these evaginations do not form typical thyroid tissue.
The thymus of the chick does not appear until after the fourth day of incubation. It takes its origin primarily from diverticula arising from the posterior faces of the third and fourth pharyngeal pouches. The original epithelial character of the thymus is soon largely lost in an extensive ingrowth of mesenchyme and the organ becomes chiefly lymphoid in its histological characteristics.
The Trachea
The first indication of the formation of the respiratory system is an outgrowth from the pharynx. In chicks of 3 days a mid- ventral groove is formed in the pharynx, beginning just posterior to the level of the fourth pharyngeal pouches and extending caudad. This groove deepens rapidly and by closure of its dorsal margins becomes separated from the pharynx except at its cephalic end. The tube thus formed is the trachea, and the opening which persists between the cephalic end of the trachea and the pharynx is the glottis (Fig. 43). The original entodermal evagination gives rise only to the epithelial lining of the trachea, the supporting structures of the tracheal walls being derived from the surrounding mesenchyme.
The Lung-buds
The tracheal evagination grows caudad and bifurcates to form a pair of lung-buds. As the lung-buds develop they grow into the loose mesenchyme on either side of the mid-line. The adjacent splanchnic mesoderm is pushed ahead of them in their caudo-lateral growth and comes to constitute the outer investment of the lung-buds. The entodermal buds give rise only to the epithehal Uning of the bronchi, and the air passages and air chambers of the lungs. The connective tissue stroma of the lungs is derived from mesenchyme surrounding the lung-buds, and their pleural covering from the investment of splanchnic mesoderm.
The Oesophagus and Stomach
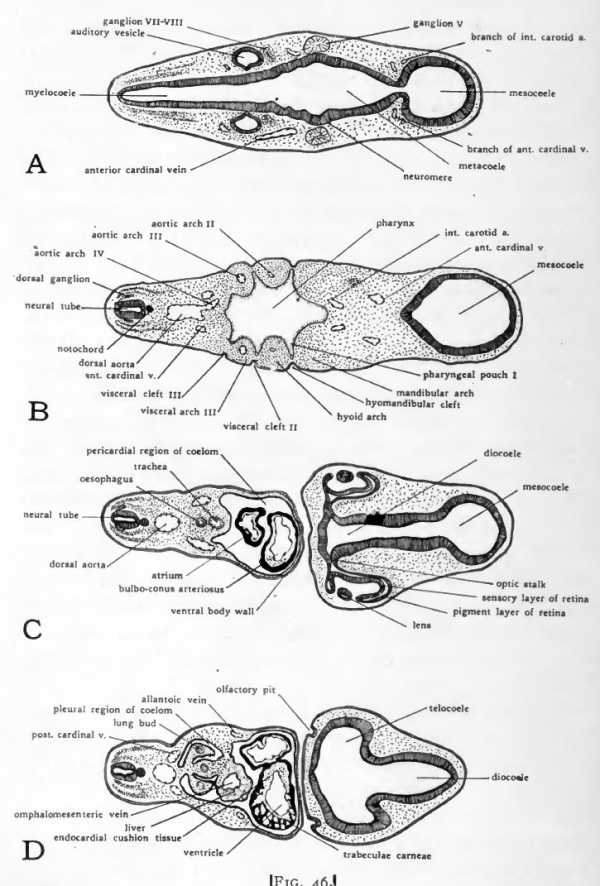
Immediately caudal- to the glottis is a narrowed region of the fore-gut which becomes the oesophagus, and farther caudally a slightly dilated region which becomes the stomach (Fig. 43). The concentration of mesenchyme cells about the entoderm of the oesophageal and stomach regions foreshadows the formation of their muscular and connective tissue coats (Fig, 46, C).
The Liver
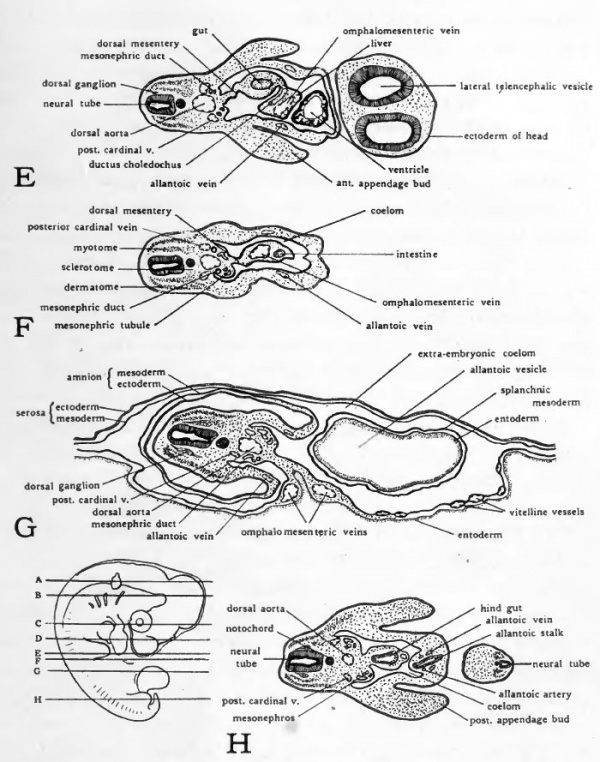
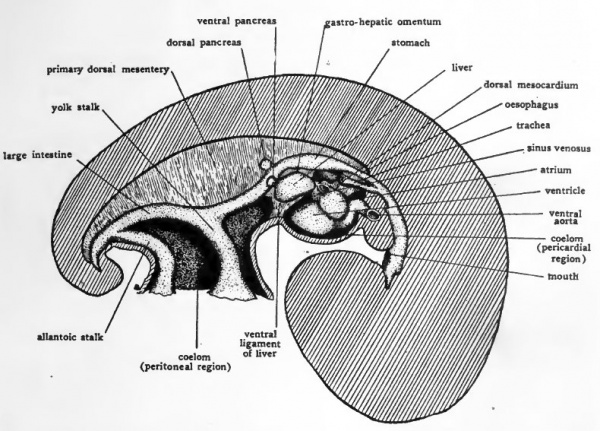
In all vertebrates the liver arises as a diverticulum from the ventral wall of the gut immediately caudal to the stomach region. In chick embryos the Uver diverticulum appears just as the part of the gut from which it arises is acquiring a floor by the concrescence of the margins of the anterior intestinal portal. As a result the liver evagination appears for a short time on the lip of the intestinal portal, and grows cephalad toward the fork where the omphalomesenteric veins enter the sinus venosus. As closure of the gut floor is completed, the liver diverticulum comes to lie in its characteristic position in the ventral wall of the gut. In embryos of four days the original evagination has grown out in the form of branching cords of cells and become quite extensive in mass (Fig. 43). In its growth the liver pushes ahead of it the splanchnic mesoderm which surrounds the gut, with the result that the liver from its first appearance is invested by mesoderm. (Fig. 46, E).
The proximal portion of the original evagination remains open to the intestine, and serves as the duct of the liver. This primitive duct later undergoes regional differentiation and gives rise in the adult to the common bile duct, to the hepatic and cystic ducts, and to the gall bladder. The cellular cords which bud off from the diverticulum become the secretory units of the liver (hepatic tubules).
The same process of concrescence which closes the floor of the fore-gut involves the proximal portion of the omphalomesenteric veins which, when they first appear, lie in the latei al folds of the anterior intestinal portal (Fig. 35). As the intestinal portal moves caudad in the lengthening of the fore-gut, the proximal portions of the omphalomesenteric veins are brought together in the mid-line and become fused. The fusion extends caudad nearly to the level of the yolk stalk (Fig. 47). Beyond this point they retain their original paired condition. In its growth the liver surrounds the fused portion of the omphalomesenteric veins (Figs. 43 and 46, D, and E). This early association of the omphalomesenteric veins with the liver fore-shadows the way in which the proximal part of the afferent vitelline circulation is to be involved in the establishment of the hepatic portal" circulation of the adult.
The Pancreas
The pancreas is derived from evaginations appearing in the walls of the intestine at the same level as the liver diverticulum. There are three pancreatic buds, a median dorsal, and a pair of ventro-lateral buds. The dorsal evagination appears at about 72 hours, the ventro-lateral evaginations toward the end of the fourth day. The dorsal pancreatic bud arises directly opposite the liver diverticulum and grows into the dorsal mesentery (Fig. 43). The ventro-lateral buds arise where the duct of the liver connects with the intestine so that the ducts of the liver and the ventral pancreatic ducts open into the intestine by a common duct (ductus choledochus) . Later in development the masses of cellular cords derived from the three pancreatic primorida grow together and become fused into a single glandular mass, but usually two and in rare cases all three of the original ducts persist in the adult.
The Mid-gut Region
In chicks of four days the enteric tract shows no local differentiation from the level of the liver to the cloaca except where the yolk-sac is attached. All of the gut tract between the stomach and the yolk-stalk, and the anterior third of the gut lying caudal to the yolk-stalk is destined to become the small intestine. The posterior two-thirds of the hind-gut becomes large intestine and cloaca.
The Cloaca
The beginning of the formation of the cloaca is indicated in chicks of four days incubation, by a dilation of the posterior portion of the hind-gut (Fig. 43). Although extensive differentiations in the cloacal region do not appear until later in development, certain of its fundamental relationships are established at this stage.
The cloaca of an adult bird is the common chamber into which the intestinal contents, the urine, and the products of the reproductive organs are received for discharge. The first appearance of the cloaca in the embryo as a dilated terminal portion of the gut establishes at the outset the relations of cloaca and intestine familiar in the adult.
Fig. 46. Diagrams of transverse sections-Dlarfour-day chick. The location of the sections is indicated on a small outline sketch of the entire embryo.
Although the urinary system is not at this stage developed to conditions which resemble those in the adult the parts of it which have been established are already definitely associated with the cloaca. The proximal portion of the allantoic stalk which is the homologue of the urinary bladder of mammals opens directly into the cloaca (Fig. 43). When the urinary system of the embryo is considered, we shall see that the ducts which drain the developing excretory organs also open into the cloacal region on either side of the allantoic stalk.
There is at this stage but little indication of the formation of the gonads. The relation of the sexual ducts to the cloaca can be made out only by the study of older embryos.
The Proctodaeum and the Cloacal Membrane
Indications of the formation of the cloacal opening to the outside appear during the fourth day of incubation. Its establishment is accomplished in much the same manner as the establishment of the oral opening. A ventral out-pocketing of the hind-gut arises just caudal to the point at which the allantoic stalk opens into the cloaca (Fig. 43) . At the same time a depression appears in the overlying ectoderm. The external depression which grows in toward the gut pocket is known as the proctodaeum. The double epithelial layer formed by the meeting of gut entoderm with proctodeal ectoderm is the cloacal membrane. The formation of the proctodaeum and the cloacal membrane cleaily indicate the location of the future cloacal opening although an open communication is not established by the rupture of the cloacal membrane until considerably later. The cloacal opening does not form at the extreme posterior end of the hind-gut and there is therefore a post-anal pocket of the hind-gut suggestive of the pre-oral pocket of the fore-gut.
The Circulatory System
The Functional Significance of the Embryonic Circulation
The arrangement of the embryonic circulation is difficult to understand only when its functional significance is overlooked. In the embryo as in the adult the main circulatory channels lead to and from the centers of metabolic activity. The circulating blood carries material from the organs of digestion and absorption to remote parts of the body; oxygen to all parts of the body from the organs which are specially constructed to take up oxygen from the surrounding medium; and waste materials from the places of their liberation, to the organs through which they are eliminated. The differences between the course of the circulation in the embryo and in the adult are due to the fact that their centers of metabolic activity are differently located.
The organs which in the adult carry out such functions as digestion and absorption, respiration, and excretion are extremely complex and highly differentiated structures. They are for this reason slow to attain their definitive condition and do not become functional until toward the close of embryonic life. Moreover the conditions by which the developing adult organs are surrounded during embryonic life are in some instances an absolute bar to their becoming functional were they sufficiently developed so to do. Suppose the lungs, for example, were fully formed at an early stage of development. The fact that the chick embryo is living submerged in the amniotic fluid would render them as incapable of functioning as the lungs of a man under water. Were the embryo dependent on the establishment of the organs which carry on metabolism in the adult, development would be at an impasse. To develop, the embryo must have not only the raw food material supplied it by the mother in the form of yolk, it must have a means of digesting the yolk, absorbing it, and carrying it to the places where it can be utilized. The utilisation of food material to produce the energy expressed in growth processes depends on presence of oxygen. For growth there must be a means of securing oxygen and carrying it, as well as food, to all parts of the body. Nor can continued growth go on unless the waste products generated by the growing tissues are eliminated. At the outset of its development the embryo must, therefore, establish organs for the digestion and absorption of food, the securing of oxygen, and the ehmination of waste products. These organs serve the embryo but temporarily and are different in structure and in location from the organs which carry out the corresponding functions in the adult, their nature and location depending on the exigencies of the embryo's living conditions.
The main channels of the circulation in young embryos lead to and from their temporary organs of digestion and absorption, respiration, and excretion. The arrangement of the main vessels characteristic of the adult appears only as the organs characteristic of the adult develop. The changes by which the circulatory system acquires its adult arrangement are of necessity gradual. Any changes which were sufficiently abrupt to interfere with the circulation would result in disaster for the embryo. Even slight curtailment of the normal blood supply to any region would cause its growth to cease any marked local decrease in the circulation would result in local atrophy or malformation complete interruption of any important circulatory channel, even for a short time, would inevitably mean the death of the embryo. Consequently the arrangement of vessels characteristic of the embryo persists during the formation of the adult organs, and becomes altered only gradually as the adult organs and the vessels associated with them become ready to function.
If the various circulatory channels of young chick embryos are considered in the light of their functions, the differences between the embryonic and the adult circulations should not be troublesome. The circulation of young chick embryos involves three main arcs of which the heart is the common center and pumping station. One of these circulatory arcs, the vitelhne, carries blood to the yolk-sac where food materials are absorbed and then returns the food-laden blood to the heart for distribution within the embryo. Another arc carries blood to and from the allantois. The distal portion of the allantois lies close beneath the egg shell and the blood circulating in the allantoic vessels is thereby brought into a location where interchange of gases can be carried on with the air which penetrates the shell (Fig. 30, C and D). It is in the allantoic circulation that the blood gives off its carbon dioxide and acquires a fresh supply of oxygen. The allantoic circulation is also the embryo's means of eliminating nitrogenous waste material from the blood. The remaining circulatory arc is confined to the body of the embryo. The intra-embryonic circulation has many distributing and collecting vessels but all of them are alike in function in that they bring food material to, and carry waste material from, the various parts of the developing body. Nowhere in their course are the vessels of the intraembryonic circulation involved in adding food material or oxygen to that already contained in the blood they convey, and nowhere do they free the blood from waste materials until well along in development, when the nephroi become functional.
In the heart the blood from the three circulatory arcs is mingled. As it leaves the heart the mixed blood is not as rich in food material as the blood coming in through the omphalomesenteric veins, nor as free from waste materials and as rich in oxygen as the blood returned over the allantoic veins. Its condition of serviceability to the embryo is, however, constantly maintained at a good average by the incoming vitelline and allantoic blood.
There is a tendency among students who have done but little work on the circulation to regard any vessel which carries oxygenated blood as an artery, and any vessel which carries blood poor in oxygen and high in carbon dioxide content as a vein. This is not entirely correct even for the circulation of adult mammals on which the conception is based. In comparative anatomy and especially in embryology it is far from being the case. It is necessary, therefore, in dealing with the circulation of the embryo to eradicate this not uncommon misconception.
The differentiation between arteries and veins which holds good for all forms, both embryonic and adult, is based on the structure of their walls, and on the direction of their blood flow with reference to the heart. An artery is a vessel carrying blood away from the heart under a relatively high fluctuating pressure due to the pumping of the heart. Correlated with the pressure conditions in it, its walls are heavily reinforced by elastic and muscle tissue. A vein is a vessel carrying blood toward the heart under relatively low and constant pressure from the blood welling into it from capillaries. Correlated with the pressure conditions characteristic for it, the walls of a vein have much less elastic and muscle tissue than artery walls, and more non-elastic fibers reinforcing them.
The Vitelline Circulation
The earliest indication of blood and blood vessel formation is at the chick's source of food supply. Blood islands appear in the extra-embryonic splanchnopleure of the yolk-sac toward the end of the first day of incubation, and rapidly become differentiated to form vascular endothelium enclosing central clusters of primitive blood corpuscles (Fig. 25). By extension and anastomosing of neighboring islands a plexus of blood channels is formed in the yolk-sac. Further extension of the vitelhne plexus brings it into communication with the omphalomesenteric veins which have been developed in the embryo as caudal extensions of the heart (Fig. 21).
Toward the end of the second day of development the omphalomesenteric arteries establish communication between the dorsal aortae and the vitelline plexus. (See Chap. X and Figs. 29 and 35.) There is now a system of open channels leading from the embryo to the yolk-sac, and back again to the embryo. With the completion of these channels the heart begins to pulsate, circulation of the blood is thereby established, and the blood cells formed in the yolk-sac are for the first time carried into the body of the embryo.
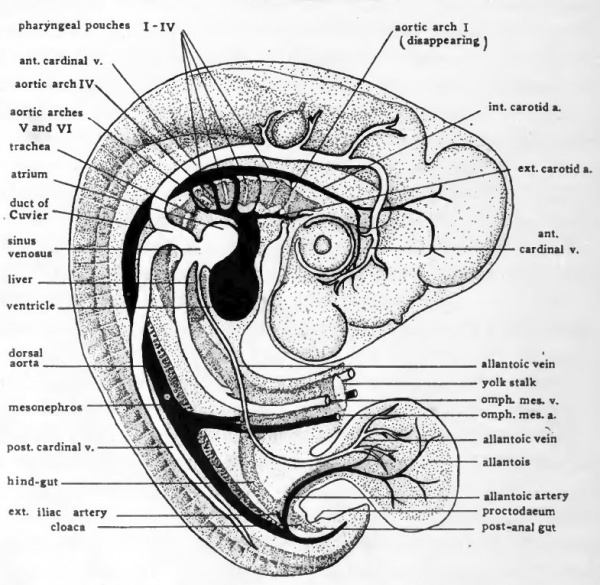
Fig. 47. Schematic diagram to show the location of the more prominent internal organs of the four-day chick. Except for the omphalomesenteric arteries and veins paired structures are represented only on the side toward the observer.
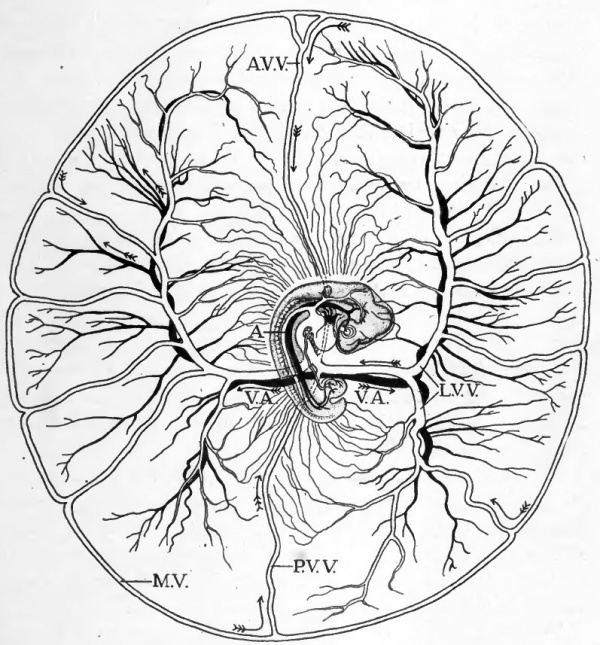
Fig. 48. Diagram to show course of vitelline circulation in chick of about four days. (After Lillie.)
- For the intra-embryonic vessels see Fig. 47. Abbreviations; A, dorsal aorta; A.V.V., anterior vitelline vein; L.V.V., lateral vitelline vein; M.V., marginal vein (sinus terminalis); P.V.V., posterior vitelline vein; V.A., vitelline artery. The direction of blood flow is indicated by arrows.
The course of the vitelline circulation in chicks of four days is shown diagrammatically in Figures 47 and 48. Circulating in the rich plexus of small vessels on the yolk, the blood finally makes its way either directly into one or another of the larger vitelline veins, or to the sinus terminalis which acts as a collecting channel, and then over the sinus terminalis to one of the vitelline veins. The vitelline veins conVerge toward the yolk-stalk where they empty into the omphalomesenteric veins. The omphalomesenteric veins at first paired throughout their entire length have been brought together proximally by the closure of the ventral body wall and become fused to form a median vessel within the body of the embryo. It is through this vessel that the vitelline blood eventually reaches the heart. In the heart the blood of the vitelline, intra-embryonic, and allantoic circulations is mingled. The mixed blood passes out by the ventral aorta and the aortic arches into the dorsal aorta. Leaving the dorsal aorta through the vitelline arteries the blood is returned to the yolk-sac.
It should not be inferred that the blood stream "picks up" deutoplasmic granules and carries them to the embryo. The acquisition of food material by the blood depends on the activities of the entodermal cells lining the yolk-sac. These cells secrete digestive enzymes which break down the deutoplasmic granules. The liquified material is then absorbed by the yolksac cells and transferred to the blood. The blood carries the food material in soluble form to the embryo where it is finally assimilated.
The Allantoic Circulation
The allantoic arteries arise by the prolongation and enlargement of the segmental vessels arising from the aorta at the level of the allantoic stalk. Their size increases rapidly as the allantois increases in extent. From them the blood is distributed in a rich plexus of vessels which ramify in the mesoderm of the allantois (Fig. 47).
The situation of the allantois directly beneath the porous shell is such that the blood can carry on interchange of gases with the outside air (Fig. 30, D). It is in the rich plexus of small allantoic vessels where the surface exposure is very great that the blood gives off its carbon dioxide and takes up oxygen.
At a later stage of development the ducts of the embryonic excretory organs open into the allantoic stalk near its cloacal end. As the excretory organs become functional the allantoic vesicle becomes the repository for the nitrogenous waste materials eliminated through them. The watery portion qi the waste materials is passed off by evaporation. The remaining solids are deposited in the allantoic vesicle. They accumulate in the extra-embryonic portion of the allantois and there remain until that portion of the allantois is discarded at the close of embryonic life.
The blood from the allantois is collected and returned to the heart over the allantoic veins. From the distal portion of the allantois the smaller veins converge and unite into two main vessels, right and left, which enter the body of the embryo with the allantoic stalk (Fig. 46, H). After their entrance into the body the allantoic veins extend cephalad in the lateral body walls (Figs. 47 and 46, H to D). They enter the sinus venosus on either side of the entrance of the omphalomesenteric vein.
The Intra-embryonic Circulation
The earliest vessels of the intra-embryonic circulation to appear are the large vessels communicating with the heart. In chicks of 33 hours the ventral aorta leads off from the heart cephalically and bifurcates ventral to the pharynx giving rise to a single pair of aortic arches. The aortic arches pass dorsad around the anterolateral walls of the pharynx and are continued caudally along the dorsal wall of the gut as the paired dorsal aortae (Fig. 23).
When, toward the end of the second day of incubation, visceral clefts and visceral arches appear, the original pair of aortic arches comes to lie in the mandibular arch. In each of the visceral arches posterior to the mandibular, new aortic arches are formed connecting the ventral aortae with the dorsal aortae. By 55 hours three pairs of aortic arches are present and a fourth is beginning to form (Fig. 35).
At about this stage extensions of the dorsal aortic roots grow out anteriorly. The vessels thus derived extend cephalad in close association with the brain as the internal carotid arteries. In a later stage vessels arise from the ventral aortic roots and grow cephalad as the external carotid arteries (Fig. 47).
By the end of the fourth day of incubation two more pairs of aortic arches have appeared posterior to the four formed in 55 to 60-hour chicks. From their fia-st appearance the fifth aortic arches are very small and they soon disappear altogether. The first and second pairs of aortic arches have by this time suffered a great diminution in size which is indicative of their final disappearance. In many embryos of this age the first arches, and in a few the second also, have disappeared altogether. This leaves only the third, fourth, and sixth pairs of aortic arches. These arches persist intact for some time, and parts of them remain permanently, being incorporated in the formation of the aortic arch and the main vessels arising from it, and in the roots of the pulmonary arteries.
In reptiles, birds, and mammals the main adult vessels which connect the heart with the dorsal aorta are derived from the fourth pair of aortic arches of the embryo. The paired condition of these arches persists as the adult condition in reptiles, but in birds and mammals one of the arches degenerates before the end of embryonic life. In birds the left arch degenerates leaving the right one as the adult aortic arch; in mammals the right arch degenerates leaving the left as the aortic arch of the adult.
The dorsal aortae, at first paired, later become fused to form a median vessel. The fusion begins at about the level of the sinus venosus and progresses cephalad and caudad (Fig. 35). Fusion extends cephalad but a short distance, never involving the region of the aortic arches. Caudally the aortae eventually become fused throughout their entire length.
Early in development the aorta gives rise to a segmentally arranged series of small vessels which extend into the dorsal body wall. At the level of the anterior appendage buds a pair of the segmental arteries become enlarged and extend into the wing buds as the sub-clavian arteries. Coincident with the development of the allantois, segmental vessels opposite the allantoic stalk become enlarged and extend'into it as the allantoic arteries. The external iliac arteries to the posterior appendage buds arise as branches of the allantoic arteries close to their origin from the aorta (Fig. 47) .
The three main arteries which in the adult supply the abdominal viscera are represented in four-day chicks only by the omphalomesenteric arteries. The omphalomesenteric arteries arise as paired vessels (Fig. 35), but in the closure of the ventral body wall of the embryo they are brought together and fused to form a single vessel which runs in the mesentery from the aorta to the yolk-stalk (Fig. 47). With the atrophy of the yolk-sac the proximal part of the omphalo-mesenteric artery persists as the superior mesenteric of the adult. The coeliac and the inferior mesenteric arteries arise from the aorta independently at a later stage.
The cardinal veins are the principal afferent systemic vessels of the early embryo. They appear toward the end of the second day as paired vessels extending anteriorly and posteriorly on either side of the mid-line. At the level of the heart the anterior and posterior cardinal veins of the same side of the body become confluent in the ducts of Cuvier and turn ventrad to enter the sinus venosus (Figs. 24 and 35) . Chicks of four days show little change in the relationships of the cardinal veins (Fig. 47). Later in development the proximal ends of the anterior cardinals become connected by the formation of a new transverse vessel and empty together into the venous atrium of the heart. Their distal portions remain in the adult as the principal afferent vessels (jugular veins) of the cephalic region.
The posterior cardinals lie in the angle between the somites and the lateral mesoderm (Fig. 36, D, E). When the mesonephroi develop from the intermediate mesoderm, the cardinal veins lie just dorsal to them throughout their length (Figs. 52, C and 46, E to H). In young embryos the posterior cardinals are the main afferent vessels of the posterior part of the body. Later in development they are replaced by a new vesssel, the inferior vena cava. The changes by which posterior cardinals become reduced and broken up to form small vessels with new associations, belong to stages of development beyond the scope of this book.
The Heart
The heart in adult vertebrates is a ventral unpaired structure. Its origin in the chick from paired primordia is correlated with the way the young embryo lies spread out on the yolk surface. When the ventral body wall is completed by the folding together of layers which formerly extended to right and left over the yolk, the paired primordia of the heart are brought together in the mid-line. Their fusion estabUshes the heart as an unpaired structure lying in the characteristic ventral position (see Chap. IX and Figs. 26 and 27).
After the fusion of its paired primordia the heart is a nearly straight, double- walled tube (Figs. 49, A and 19). The primordial endocardium of the heart has the same structure and arises in the same manner as the endothehal walls of the primitive embryonic blood vessels with which it is directly continuous. The epi-myocardial layer of the heart is an outer investment which surrounds and reinforces the endocardial wall. As development progresses the epi-myocardium becomes greatly thickened and is finally differentiated into two layers, a heavy muscular layer, the myocardium, and a thin non-muscular covering layer, the epicardium.
In the apposition of the, paired primordia of the heart to each other the splanchnic mesoderm from either side of the body comes together dorsal and ventral to the heart. The doublelayered supporting membranes thus formed are known as the dorsal mesocardium and the ventral mesocardium, respectively (Fig. 26). The ventral mesocardium disappears shortly after its formation, leaving the heart suspended in the body cavity by the dorsal mesocardium (Fig. 26 E, D). Somewhat later the dorsal mesocardium also disappears except at the caudal end of the heart. Thus the heart comes to lie in the pericardial cavity unattached except at its two ends. The cephalic end of the heart remains fixed with reference to the body of the embryo where the ventral aorta lies embedded ventral to the floor of the pharynx, and the caudal end of the heart is fixed by the persistent portion of the dorsal mesocardium and the omphalomesenteric veins.
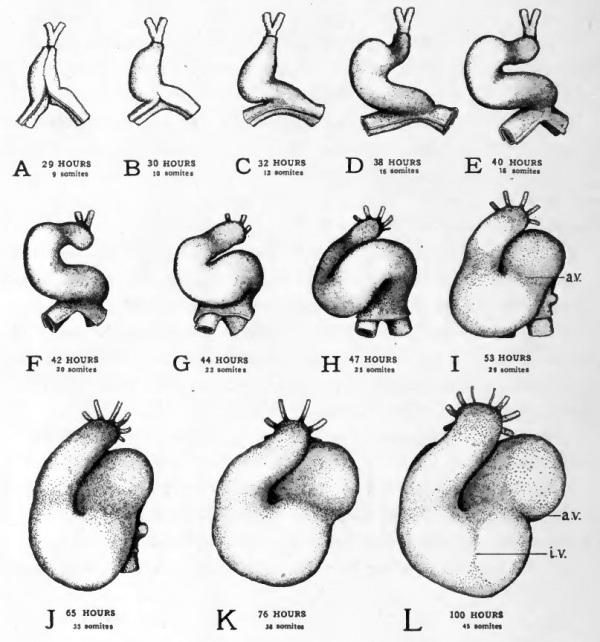
The straight tubular condition of the heart persists but a short time. The unattached ventricular region becomes dilated and is bent out of the mid-line toward the embryo's right while the fixed bulbo-conus arteriosus and the sinus venosus are held in their original median position (Fig. 49, A-E). This bending of the heart to form a U-shaped tube begins to be apparent in embryos of 30 hours and becomes rapidly more conspicuous, until by forty hours the ventricular region of the heart lies well to the right of the embryo's body (Cf. Figs. 21 and 22).
The bending of the heart to the side involves a considerable factor of "mechanical expediency". The initiation of the bending process depends on the fact that the heart is becoming elongated more rapidly than is the chamber in which it lies fixed by its two ends. The fact that the bending takes place to the side rather than dorsally or ventrally may be attributed to the impediment offered to its dorsal bending by the body of the embryo, and to its ventral bending by the yolk.
The lateral bending of the heart attains its greatest extent at about 40 hours of incubation. At this stage torsion of the body of the embryo changes the mechanical limitations in the heart region. As the embryo comes to lie on its left side the heart is no longer pressed against the yolk (Cf. Figs. 21 and 29). As a result the bend begins to swing somewhat ventrad and lies less closely against the body of the embryo (Figs. 49 and 50).
At about this stage of development a new factor affects the changes in the shape of the heart. The closed part of the U-shaped bend is forced caudad and at the same time becomes twisted on itself to form a loop (Figs. 49, F-I and 50, F-I). In the formation of the loop the atrial region is forced slightly to the left (i.e., toward the yolk) and the conus is thrown across the atrial region by being bent to the right {i.e., away from the yolk) and then caudad. The ventricular region constitutes the closed end of the loop. This twisting process reverses the original cephalo-caudal relations of the atrial and ventricular regions. The atrial region which was at first caudal to the ventricle now lies cephalic to it as in the adult heart.
The atrial region and the ventricular region which formerly were continuous without any line of demarcation, are by this time beginning to be marked off from each other by a constriction (Fig. 49, /, a.v.). As both the atrium, and the ventricle become enlarged, this constriction is accentuated (Fig. 49, L, a. v.). The constricted region is now . termed the atrio-ventricular canal.
During the fourth day the bulbo-conus arteriosus becomes closely applied to the ventral surface of the atrium. As the atrium gtows it tends to expand on either side of the depression made in it by the pressure of the bulbo-conus (Figs. 49, J-L and 50 J-L). These lateral expansions of the atrium are the first indication of the division of the atrium into right and left chambers which are later completely separated from each other. At the same time a slight longitudinal groove appears in the surface of the ventricle (Fig. 49, Z, i.v.) which indicates the beginning of the separation of the ventricle into right and left chambers. The division of the bulbo-conus to form the root of the aorta and the pulmonary artery does not appear until a later stage of development.
During the changes in the external shape of the heart which have been described, the whole heart has come to occupy a more caudal position with reference to other structures in the embryo. When the heart is first formed it lies at the level of the rhombencephalon. As development progresses it moves farther and farther caudad until at the end of the fourth day it lies at the level of the anterior appendage buds. Being unattached to the body, the ventricular region of the heart is carried farthest caudad (Cf. Figs. 19, 29, 34, and 40).
Fig. 49. Ventral views of the heart at various stages to show its changes of shape and its regional differentiation.
- All the drawings were made from dissections with the aid of camera lucida outlines. The outer of the two layers shown is the epi-myocardium ; the inner, the endocardium. In the stages represented in Figs. E-H torsion of the embryo's body is going on at the level of the heart. Since torsion involves the more cephalic regions first and progresses caudad the transverse axis of the body of the embryo is at different inclinations to the yolk at the cephalic end and at the caudal end of the heart. In drawing these figures their orientation was taken from the body at the level of the conus region of the heart, the sinus region therefore appears inclined.
- Abbreviations: a.v., constriction between atrium and ventricle; i.v., interventricular groove.
Fig. 50. Dextral views of the same series of hearts shown in ventral view in Fig. 49.
- The heart drawings in Figs. 49 and 50 should be compared with actual specimens or with drawings of entire embryos of corresponding age for the relation of the heart to the body of the embryo.
The changes which take place in the heart wall can be seen best in sections. The endocardium in the heart of a four-day chick is still a single cell layer hning the lumen. The original epi-myocardium at this stage can be differentiated into an inner myocardial portion and an outer epicardial portion. The myocardium has become greatly thickened and the cells in it are elongated and beginning to show the histological characteristics of developing muscle cells. Their arrangement in bundles which project toward the lumen fore-shadows the formation of the muscle bands (trabeculae carneae) which ridge the inner wall of the adult heart. The cells of the epicardial portion of the epi-myocardium are becoming flattened to form the epithelial and connective tissue covering of the heart (epicardium) . Lying between the endocardium and the myocardium in the region of the atrio-ventricular canal and of the opening of the ventricle into the bulbo-conus, there are loosely aggregated cells which are mesenchymal in characteristics. These cells constitute what is called endocardial cushion tissue. They later take part in the formation of the septa which divide the heart into chambers and in the formation of the connective tissue frame- work of the cardiac valves.
The Urinary System
The General Relationships of Pronephros, Mesonephros and Metanephros
In the development of the urinary system of birds and mammals there are formed in succession three distinct excretory organs, pronephros, mesonephros, and metanephros. The pronephros is the most anterior of the three, and the first to be formed. It is wholly vestigial, appearing only as a slurred-over recapitulation of structural conditions which exist in the adults of the most primitive of the vertebrate stock. The mesonephros is homologous with the adult excretory organs of fishes and amphibia. It makes its appearance in the embryo somewhat later than the pronephros, and is formed caudal to it. The mesonephros is the principal organ of excretion during early embryonic life, but it also disappears in the adult except for parts of its duct system which become associated with the reproductive organs. The metanephros is the most caudally located of the excretory organs, and the last to appear. It becomes functional toward the end of embryonic life when the mesonephros is disappearing, and persists permanently as the functional kidney of the adult.
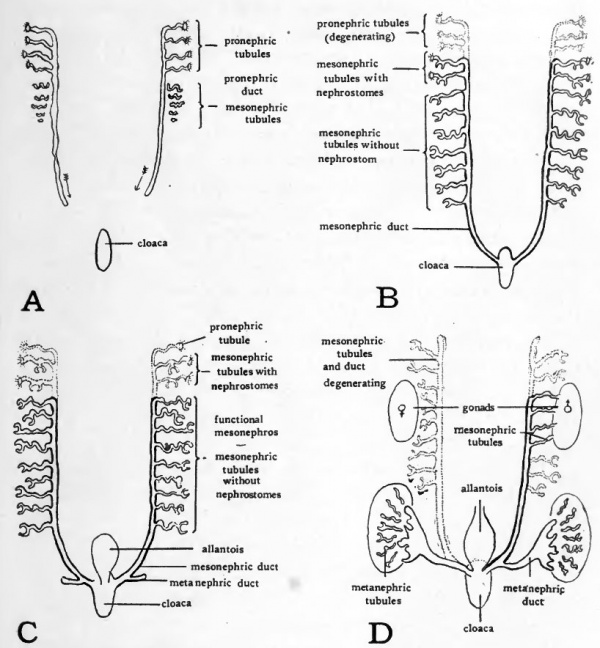
Figure 51 shows schematically some of the main steps in the embryological history of the nephric organs, which it will be helpful to have in mind before taking up in detail any of the phases of their formation in the chick. The pronephros, mesonephros and metanephros are all derived from the intermediate mesoderm, and are all composed of units which are tubular in nature. In the different nephroi these tubules vary in structural detail but their functional significance is in all cases much the same. They are concerned in collecting waste materials from the capillary plexuses which are developed in connection with them. In the accompanying diagrams conventionalized tubules have been drawn to represent the three nephric organs. No pretense is made of representing either the exact shape or the actual number of the tubules.
Fig. 51. Schematic diagrams to show the relations of pronephros, mesonephros, and metanephros at various stages of development. For explanation see text.
In the first stage represented (Fig. 51, A) only the pronephros has been established. It consists of a group of tubules emptying into a common duct, called the pronephric duct. The pronephric ducts of either side are formed first at the level of the pronephric tubules and then extend caudad, eventually reaching and opening into the cloaca (See arrows in Fig. 51, A).
As the pronephric ducts are extended caudal to the level at which pronephric tubules are formed they come in close proximity to the developing mesonephric tubules. In their growth the mesonephric tubules extend toward the pronephric ducts and soon open into them (Fig. 51, B). Meanwhile the pronephric tubules begin to degenerate. Thus the ducts which originally arose in connection with the pronephros are appropriated by the developing mesonephros. After the degeneration of the pronephric tubules these same ducts are called the mesonephric ducts because of their new associations (Fig. 51 , C).
At a considerably later stage outgrowths develop from the mesonephric ducts near their cloacal ends (Fig. 51, C). These outgrowths form the ducts of the metanephroi. They grow cephalo-laterad and eventually connect with the third group of tubules developed from the intermediate mesoderm, the metanephric tubules (Fig. 51, D). With the establishment of the metanephroi or permanent kidneys the mesonephroi begin to degenerate. The only parts of the mesonephric system to persist, except in vestigial form, are some of the ducts and tubules which in the male are appropriated by the testis as a duct system.
The Pronephric Tubules of the Chick
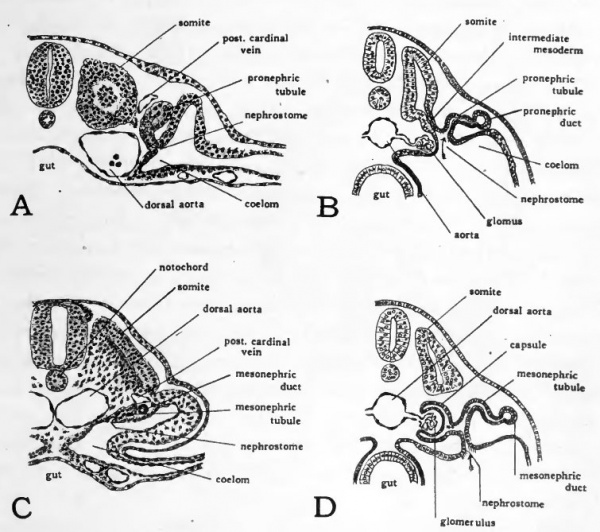
The pronephros in the chick is represented by tubules which first appear at about 36 hours of incubation. The pronephric tubules arise from the intermediate mesoderm, or nephrotome, lateral to the somites. They are paired, segmentally arranged structures, a tubule appearing on either side opposite each somite from the fifth to the sixteenth. Transverse sections passing through the loth to 14th somites of an embryo of about ^S hours show the pronephric tubules favorably. Each tubule arises as a solid bud of cells organized from the intermediate mesoderm near its junction with the lateral mesoderm (Fig. 52, vl). At first the free ends of the buds grow dorsad, passing close to the posterior cardinal veins. Later the end of each tubule is bent caudad coming in contact with the tubule lying posterior to it. In this manner the distal ends of the tubules give rise to a continuous cord of cells, the primordium of the pronephric duct. The pair of cell cords thus formed continue to extend caudad beyond the pronephric tubules and soon become hollowed out to form open ducts. When they eventually reach the level of the cloaca they turn ventrad and open into it.
The significance of the rudimentary structures in the chick which represent pronephric tubules, can be most readily understood by comparing them with fully developed and functional pronephric tubules. Figure 52, B, shows the scheme of organization of a functional pronephric tubule. The ciliated nephrostome draws in fluid from the coelom. As the fluid passes the capillaries of the glomus, waste materials from the blood are transferred to it. The nephric duct serves to collect and discharge the fluid passing through the tubules. Vestiges of a nephrostome opening into the coelom appear in the pronephric tubules of the chick (Fig. 52, A) but the tubules never become
Fig. 52. Drawings to show nephric tubules.
- A, drawing from transverse section through twelfth somite of 16 somite chick to show pronephric tubule. {After Lillie.) B, schematic diagram of functional pronephric tubule. (After Wiedersheim.) C, drawing from transverse section through seventeenth somite of 30-somite chick, to show primitive mesonephric tubule; D, schematic diagram of functional mesonephric tubule of primitive type. (After Wiedersheim.) For a later stage of the mesonephric tubules of the chick see Fig. 53.
The Mesonephric Tubules
The mesonephric tubules develop from the intermediate mesoderm caudal to the pronephros. The early steps in their formation are well shown in transverse sections of chicks of 29 to 30 somites (about 55 hours). In the posterior somites conditions are less advanced than they are more anteriorly. Consequently by studying the posterior sections of a transverse series first and then progressing cephalad a graded series of developmental stages may be obtained.
The mesonephric tubules appear first as cell clusters formed in the intermediate mesoderm. They he ventro-mesial to the cord of cells which is the primordium of the pronephric duct. The cells of the developing tubules acquire a more or less radial arrangement, and at the same time become more distinctly isolated from the surrounding mesoderm cells. By 55 hours of incubation the primordial cell cord representing the pronephric duct has become hollowed out to establish a definite lumen. The most anterior of the mesonephric tubules also have acquired a lumen. The growth of the^tubules brings them in close association with the duct. In some of the more differentiated tubules indications can be made out of their opening into the duct which is soon to be definitely established. The more posterior mesonephric tubules do not become associated with the duct until somewhat later, but remain as a series of isolated vesicles.
Figure 52, shows the scheme of organization of a functional mesonephric tubule of primitive type. As is the case with the pronephric tubule, its ciliated nephrostome draws in fluid from the coelom. The mesonephric tubule differs from the pronephric chiefly in its relation to the blood vessels associated with it. It develops a cup-like outgrowth into which a knot of capillaries is pushed. The cup-shaped outgrowth from the tubule is called the capsule (of Bowman) and the tuft of capillaries, a glomerulus. Waste-laden fluid is extracted from the capillaries of the glomerulus, mingles with the fluid coming in by way of the nephrostome, and is eventually discharged into the nephric duct. In mesonephric tubules of a more highly differentiated type the nephrostome becomes closed and all the fluid passing through the tubule is drawn from the glomerulus and other capillaries adjacent to the tubule.
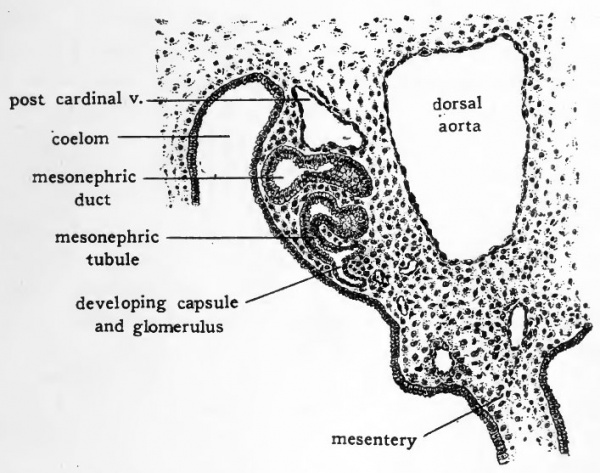
In the chick a few of the more anterior mesonephric tubules are of the primitive type and show vestiges of a nephrostome opening into the coelom (Fig. 52, C). These anterior mesonephric tubules, however, persist for but a short time, do not attain the characteristic relation to a glomerulus and never become functional. Even in chicks of four days' incubation the mesonephric tubules have not attained their full development. It is possible, however, to make out most of their fundamental parts (Fig. 53). The tubules lying in the ventro-lateral portion of the mesonephros have been longest established and are somewhat more advanced in development than those lying in the dorso-mesial portion. Nearly all of the tubules have become elongated and somewhat coiled. At one end they open into the mesonephric duct or a diverticulum of the duct which acts as a collecting tubule. At their other end a cluster of closely packed cells indicates the place at which the capsule and glomerulus will appear. The glomeruli develop very rapidly. Circulation is usually established in them by the fifth day. From this time until about the eleventh day of incubation the functional activity of the mesonephros is at its height. After the eleventh day the developing metanephros begins to become active and the mesonephros degenerates. The establishment of the metanephros and the development of the genital organs occur in stages which are too advanced to come within the scope of this book.
Fig. 53. Drawing from transverse section of four-day chick to show mesonephric tubule and duct. For the location of the area drawn consult Fig. 46, F.
The Coelom and Mesenteries
In adult birds and mammals the body cavity consists of three regions, pericardial, pleural and peritoneal. The pleural division is paired, each of the pleural chambers being a laterally situated, sac containing one of the lungs. The pericardial chamber containing the heart, and the peritoneal chamber containing the viscera, other than the lungs and heart, are unpaired. These regions of the adult body cavity are formed by the partitioning off of the primary body cavity or coelom of the embryo.
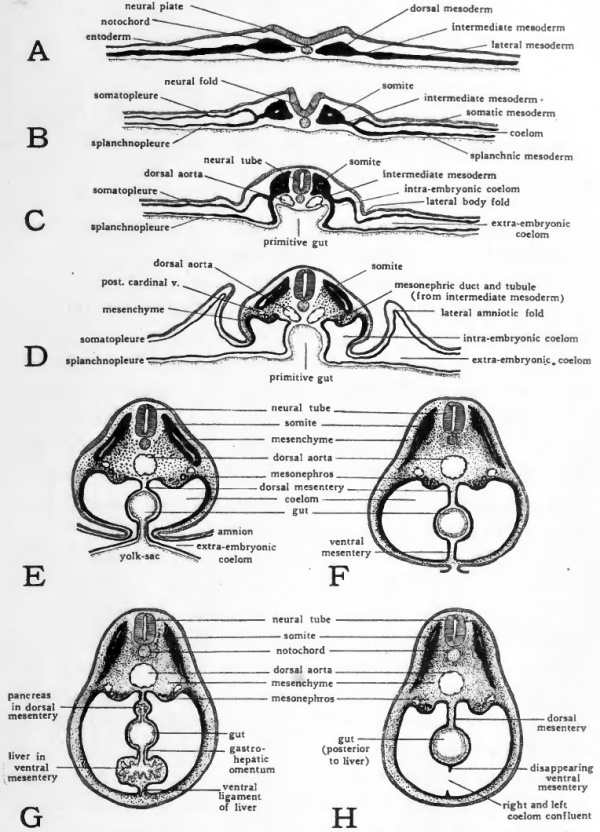
In the chick the coelom arises by a splitting of the lateral mesoderm of either side of the body (Fig. 54, A, B). It is therefore, primarily a paired cavity. Unlike the coelom of some of the more primitive vertebrates, the coelom of the chick never shows any indications of segmental pouches corresponding in arrangement with the somites. The right and left coelomic chambers extend antero-posteriorly without interruption through the entire lateral plates of mesoderm. This difference in the formation of the coelom does not imply any lack of homology between the coelom of the chick and that of more primitive forms. The process of coelom formation in the chick may be considered as being accelerated with a resultant slurring over of the early phases. The coelom first appears in a condition which is comparable with the coelom of more primitive forms at that period of differentiation when the segmentally arranged coelomic pouches have broken through into each other and their cavities have become confluent.
The coelomic chambers are not limited to the region in which the body of the embryo is developing. They extend on either side into the mesoderm, which in common with the other germ layers, spreads out over the yolk surface. A large part of the primitive coelomic chambers thus comes to be extra-embryonic in its associations. (See Chapter XI and Figures 30 and 32.) The portion of the coelom which gives rise to the embryonic body cavities is first marked off by the series of folds which separate the body of the embryo from the yolk (Fig. 54, C, D). As the closure of the ventral body wall progresses (Fig. 54, E, F) the embryonic coelom becomes completely separated from the extra-embryonic. The delayed closure of the ventral body wall in the yolk-stalk region, results in the embryonic and extra-embryonic coelom retaining their open communication at this point for a long time after they have been completely separated elsewhere.
Fig. 54. Schematic diagrams of cross sections at various stages to show the establishment of the coelom and mesenteries. For explanation see text.
The same folding process which estabhshes the vejitral body wall completes the gut ventrally (Fig. 54, C to F) . Meanwhile the right and left ccelomic chambers are expanded mesiad. As a result the newly closed gut comes to lie suspended between the two layers of splanchnic mesoderm which constitute the mesial walls of the right and left coelomic chambers, respectively. The double layers of splanchnic mesoderm which thus become apposed to the gut and support it in the body cavity are known as mesenteries. The mesentery dorsal to the gut, suspending it from the dorsal body wall is the primary dorsal mesentery, and that ventral to the gut, attaching it to the ventral body wall is the primary ventral mesentery.
When the dorsal and ventral mesenteries are first established they constitute a complete membranous partition dividing the body cavity into right and left halves. The primary dorsal mesentery persists in large part but the ventral mesentery early disappears bringing the right and left ccelomic chambers into confluence ventral to the gut and establishing the unpaired condition of the body cavity characteristic of the adult.
In considering the early development of the heart (Chapter IX) the formation of the dorsal and ventral mesocardia was taken up. In their relation to the other mesenteries of the body, the mesocardia are to be regarded as special regions of the primary ventral mesentery. In the most cephalic part of the body cavity, the gut lies embedded in the dorsal body wall instead of being suspended by the primary dorsal mesentery as it is farther caudally (Cf. Fig. 26, E and Fig. 54, F). The ventral mesentery is, however, developed in the same manner anteriorly as it is posteriorly and when the heart is formed it is suspended in the most anterior part of the primary ventral mesentery. The dorsal and ventral mesocardia are the parts of the primary ventral mesentery lying dorsal to the heart, and vontral to the heart, respectively (Fig. 26, D).
When the ventral mesocardium, and a little later the dorsal mesocardium, breaks through, the primary right and left coelomic chambers become confluent to form the pericardial region of the body cavity (Figs. 24 and 55). Later in development the ventral mesentery farther caudally disappears so that caudally as well as cephahcally an unpaired condition of the coelom is brought about (Fig. 54, H).
In the liver region the ventral mesentery does not disappear. The liver arises as an outgrowth from the gut and in its development extends into the ventral mesentery (Fig. 54, G). The portion of the ventral mesentery dorsal to the liver persists as the gastro-hepatic omentum, and the portion ventral to the liver persists as its ventral ligament (falciform ligament) (Fig- 55)
Fig. 55. Schematic lateral view of dissection of four-day chick to show the body cavity and the more important mesenteries.
The primary dorsal mesentery persists and forms the supporting membranes of the digestive tube. In the adult its different regions are named according to the parts of the digestive tube with which they are associated, as for example, mesogaster that part of the primary dorsal mesentery which suspends the stomach, mesocolon, that part of the primary dorsal mesentery supporting the colon, etc.
The separation of the body cavity into pericardial, pleural, and peritoneal chambers is accomplished by the formation of septa growing in from the body wall. Consideration of the details of their formation would lead us into stages of development beyond the scope of this book. Those interested in following the later embryology of the chick will find in the appendix references to more exhaustive books, and to a few of the more recent original papers on its development.
- Last Page: Return to Contents
The Early Embryology of the Chick: Introduction | Gametes and Fertilization | Segmentation | Entoderm | Primitive Streak and Mesoderm | Primitive Streak to Somites | 24 Hours | 24 to 33 Hours | 33 to 39 Hours | 40 to 50 Hours | Extra-embryonic Membranes | 50 to 55 Hours | Day 3 to 4 | References | Figures | Site links: Embryology History | Chicken Development
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2026, March 1) Embryology Book - The Early Embryology of the Chick 13. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Book_-_The_Early_Embryology_of_the_Chick_13
- © Dr Mark Hill 2026, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G