Book - Text-Book of Embryology 8
| Embryology - 26 Feb 2026 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Bailey FR. and Miller AM. Text-Book of Embryology (1921) New York: William Wood and Co.
- Contents: Germ cells | Maturation | Fertilization | Amphioxus | Frog | Chick | Mammalian | External body form | Connective tissues and skeletal | Vascular | Muscular | Alimentary tube and organs | Respiratory | Coelom, Diaphragm and Mesenteries | Urogenital | Integumentary | Nervous System | Special Sense | Foetal Membranes | Teratogenesis | Figures
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
Development of the External Form of the Body
General Form
The vertebrate body is fundamentally cylindrical. The trunk is continued forward into the neck which in turn supports the head. The extremities are appendages of the trunk. This form arises during the development of the organism as a whole from the spherical egg cell. In Amphioxus the spherical form is retained until the gastrula begins to elongate; in the frog the same is true. In both these animals the simple elongation of the gastrula is the first step in the change to the cylindrical shape. In the bird the egg is spherical, but the cytoplasmic portion of the egg is a disk and out of this disk the early cylindrical body is established by a process of folding. The mammalian ovum also is spherical, but the part of the structure resulting from the early processes of development which gives rise to the body is a disk; and out of this disk the cylindrical body arises by folding in much the same manner as in the bird.
Since cleavage and the formation of the blastodermic vesicle in man has not been observed, it is necessary to take some other mammalian form for the early stages. In most mammals cleavage results in a solid mass of cells called the morula (Fig. 55, a). In certain forms, like the bat, the superficial cells of the mass become differentiated from those in the interior, the result being an enveloping layer and a central mass (Fig. 55, b). In the opossum during cleavage the blastomeres arrange themselves around a central cavity so that no definite morula is formed (Fig. 57) . In the case of the solid sphere, vacuoles appear within the central cells and then coalesce to establish a large cavity which occupies the greater part of the interior of the sphere. There remain then the enveloping layer and a few of the central cells which are attached to the enveloping layer over a small area and which comprise the inner cell mass (Fig. 55, c, d) . The cavity of the sphere in the mammal is probably not homologous with the blastocoel in the lower forms. The vacuolization of the central cells has been interpreted as an attempt at yolk formation. Whether the interpretation is correct or not, the cells surrounding the cavity behave in many respects as if yolk were present; and the cavity subsequently becomes the cavity of the yolk sac of the embryo.
Following the formation of the yolk cavity, the contiguous cells of the inner cell mass proliferate and migrate to form a complete lining for the cavity. These cells comprise the primitive entoderm. Meanwhile the central cells of the inner cell mass undergo vacuolization, leaving now only the enveloping layer and a single layer of cells applied to the entoderm. This single layer is the embryonic ectoderm and the newly formed space the amniotic cavity (Fig. 59, c). The entire structure is known as the blastodermic vesicle or blastocyst; the interior contains two cavities separated from each other by a plate or disk composed of ectoderm and entoderm and called the embryonic disk. At this point it must suffice to say, without entering into details, that the mesoderm appears as a third layer between ectoderm and entoderm in the embryonic disk and between entoderm and enveloping layer. The mesoderm increases rapidly and soon forms an extensive but loosely arranged tissue between the entoderm and the enveloping layer in the wall of the vesicle. The enveloping layer becomes known as the trophoderm because it comes in direct contact in the mammal with the uterine mucosa and through it must pass all the nutritive materials from the uterus to the interior of the vesicle.
Up to the time and stage when the mesoderm becomes a loosely arranged tissue filling much of the interior of the blastodermic vesicle, nothing is known of the development of the human ovum. What is probably the youngest human embryo, described by Bryce and Teacher, is shown in section in Fig. 73. The trophoderm is. the outer layer of the vesicle and has sent out numerous irregular projections into the uterine mucosa in which the vesicle is already embedded. The interior of the vesicle is occupied for the most part by the loose mesoderm. Embedded in the mesoderm are two cavities, the smaller being the yolk cavity lined by entoderm and the larger the amniotic cavity lined by ectoderm ; the cavities are separated from each other by the embryonic disk. This embryo was reckoned to be 13 or 14 days old.
A slightly older human embryo has been described by Peters (Fig. 74). It is now reckoned to be about 15 days old, although Peters regarded it at the time as being much younger. The trophoderm exhibits about the same characters as in the Bryce-Teacher embryo. The mesoderm shows a great cleft or space within it; a rather thin layer is applied to the trophoderm and also surrounds the yolk and amniotic cavities and forms the middle layer of the disk between the two cavities. The space within the mesoderm is the exoccelom or extraembryonic body cavity. The layer applied to the trophoderm is the somatic or parietal mesoderm which with the trophoderm itself comprises the chorion. The wall of the yolk sac is composed of entoderm and visceral or splanchnic mesoderm. The amniotic cavity is surrounded by ectoderm and parietal mesoderm. The embryonic disk is attached to the chorion at one side by a strand of mesoderm known as the belly stalk. The chief difference between this and the Bryce-Teacher embryo is the great cleft in the mesoderm.
Disregarding now the chorion and exoccelom, which are no longer involved in the form of the embryonic body, certain advances in development are seen in an embryo described by von Spee. In Fig. 77 a sagittal section shows the large yolk sac separated from the amniotic cavity by the embryonic disk. The anterior margin of the disk is bent ventrally by a fold of the germ layers. Figure 76 shows a dorsal surface view of the embryo; the amnion has been cut away. The embryonic disk is considerably elongated cephalocaudally. A gutter or groove surrounds the disk, and if compared with the sagittal section which has the fold at the cephalic end it can readily be seen that this groove is an early step in the constriction or pinching off of the disk from the yolk sac. The margins of the disc are being bent ventrally and tucked beneath the central portion; and since the disk is elongated the folding process will result in a cylindrical body form. Even now the impression is obtained that the yolk sac is suspended from the ventral side of the embryo by a narrower structure, the early yolk stalk. The dorsal surface of the disk is indented by the neural groove which extends nearly the whole length of the developing body.
Somewhat more advanced than the von Spee embryo is one described by Eternod (Fig. 83). Eternod's embryo is 2.11 mm. in length and possesess eight primitive segments. The figure shows the amnion cut away on the dorsal side and the yolk sac on the ventral side. The body is more markedly cylindrical than the preceding stage, and more elongated. The constriction between the embryo and the yolk sac is well marked, and the narrower yolk stalk can be better appreciated. At the caudal end the belly stalk forms the attachment to the chorion. The neural folds are partly fused to form the neural tube. The cephalic end of the neural plate is notably larger, a character which already indicates the beginning of the head. One might say that the yolk stalk is becoming smaller; but as a matter of fact the diminution is more apparent than real. The apparent diminution is caused by the relatively more rapid increase in size of the embryonic body and yolk sac. At this point it should be mentioned that the bending and tucking under the body of the lateral body walls naturally results in the contact and eventual fusion of the two sides in the mid- ventral line. In this manner the ventral body wall is formed. The line of fusion is significant in its relation to certain malformation : For instance, the fusion is sometimes defective or incomplete, allowing some of the viscera to protrude. (See Chap. XX on "Teratogenesis") If the fusion is normal the ventral body wall is complete and closed except at the attachment of the umbilical cord through which pass the blood vessels that carry nutriment to the embryo and waste products away from it.
The changes that occur in the simple cylindrical body after the ventral body wall is closed comprise the differentiation of the head, neck and trunk regions and the development of the extremities as appendages of the trunk. Even in Eternod's embryo (Fig. 83) the region where the brain is developing is greater in diameter than the other part of the embryo. Thus the beginning of the head is indicated by an increase in size due primarily to the growth of the brain. The end of the head region is bent ventrally almost at a right angle to the long axis of the embryo, the bend occurring in the mid-brain and being known as the cephalic flexure. This is the first of the flexures that appear as development proceeds. On the cephalic side of the yolk sac attachment is a protrusion which indicates the position of the heart in what now may be called the cervical region or neck. Between the protrusion caused by the heart and the fore-brain there is~a depression which foreshadows the oral and nasal cavities and is now called the oral fossa.
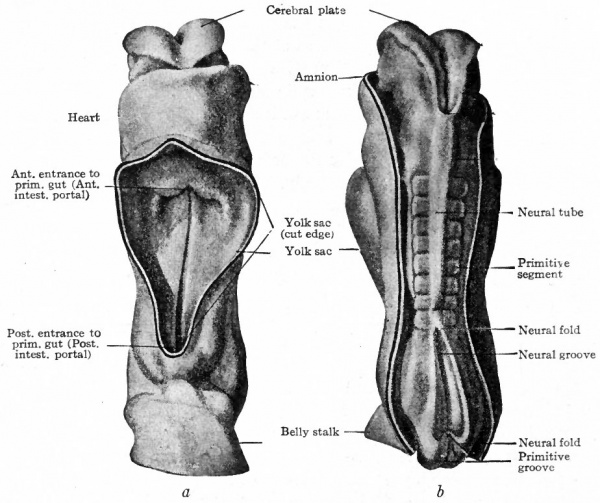
Fig. 83. (a) Ventral view; (b) dorsal view of human embryo with 8 pairs of mesodermal somites (2.11 mm). Eternod. From models by Ziegler.
- In b the amnion has been removed, merely the cut edge showing; in a the yolk sac has been removed.
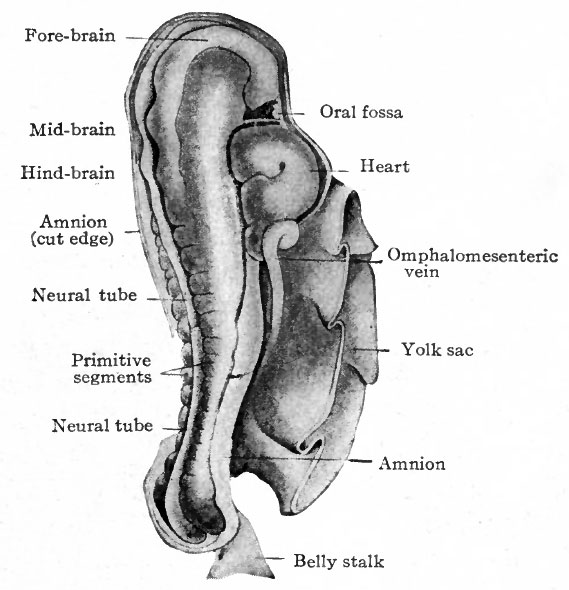
In Fig. 84, showing the dorso-lateral aspect of an embryo 2.5 mm. long and possessing 14 primitive segments, the beginning of the head, the cephalic flexure, the oral fossa, the protrusion in the cervical region caused by the heart, the belly stalk, and the constriction between body and yolk sac are all clearly indicated. It is worthy of note that the heart appears in the cervical region; during later development it recedes into thorax.
Fig. 84. Dorso-lateral view of human embryo with fourteen pairs of mesodermal somites (2.5 mm). Kollmann.
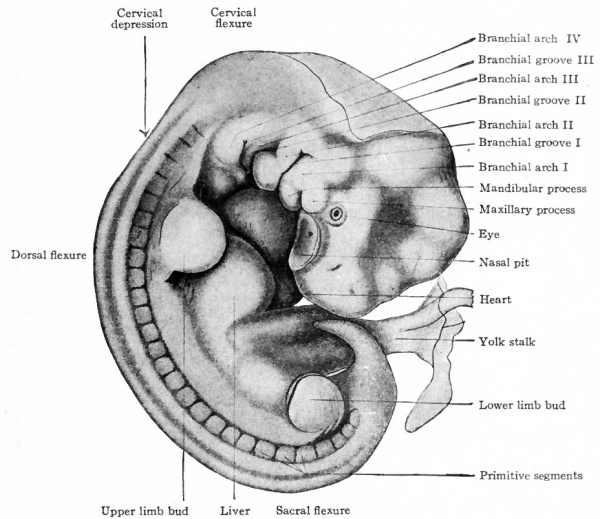
Fig. 85. Human embryo of 2.6 mm. His, from Keibel and Mall.
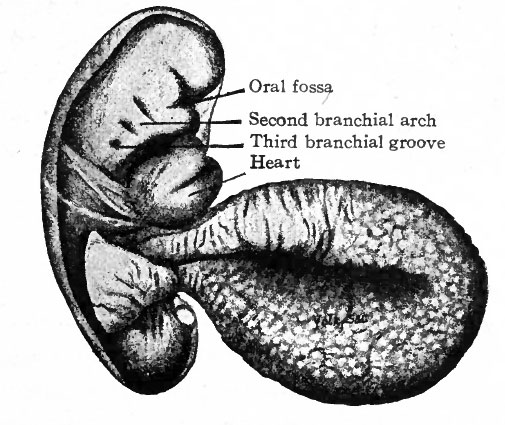
One of the early human embryos described by His is shown in Fig. 85. The veil-like structure around the embryo is the amnion. This embryo measures 2.6 mm. and was estimated to be 18-21 days old (the estimate in the light of more recent studies probably being too low). The body is more robust than in the preceding stage. In addition to the cephalic flexure the dorsum in profile is a curve, with three rather prominent regions of curvature; a cervical flexure, a dorsal flexure and a sacral flexure. The whole embryo is slightly twisted around its long axis, the head turned toward the left and the caudal end toward the right. In the cervical region are three vertical depressions which diminish in size from before backward. Alternating with these are prominences which also diminish from before backward. These alternating depressions and prominences are the branchial grooves and arches which are homologues of the gill slits and gill bars in fishes. The first arch lies in front of the first groove and bounds the oral fossa laterally; its two subdivisions, the mandibular process and maxillary process, with the notch between representing the future angle of the mouth, are already differentiated. Through the development of the first arch the depth of the oral fossa is considerably increased. The heart causes a conspicuous protrusion on the ventral side of the cervical region. The constriction between the body of the embryo and the yolk sac is marked, and this attenuated portion of the yolk sac is from now on spoken of as the yolk stalk. The structure attached caudal the yolk stalk and turned over the right side of the embryo is the belly stalk which later will be included in the umbilical cord.
Fig. 86. Human embryo of 4 mm. Rabl, from Kollmann's Atlas.
An embryo of 4 mm. is shown in Fig. 86. All the flexures are accentuated, so that the head and tail are close together. The fourth branchial arch has appeared behind the third groove and a fourth groove behind the fourth arch. The small structure behind the fourth groove may be the rudimentary fifth arch. The arches diminish rather uniformly from the first to the last. The rudiment of the eye is visible on the side of the forebrain region as a circular eminence surrounded by a slight groove. The heart protuberance is strikingly prominent. Certain new features have appeared at 'this stage, the limb buds. The fore-limb bud is a rounded eminence opposite the anterior part of the dorsal flexure; the hind-limb a similar structure opposite the sacral flexure. The limb buds, as tar as surface appearance goes, are Simply outgrowths from the body wall starting as small rounded eminences which, as development proceeds, become larger and finally differentiated into the various parts of the extremities.
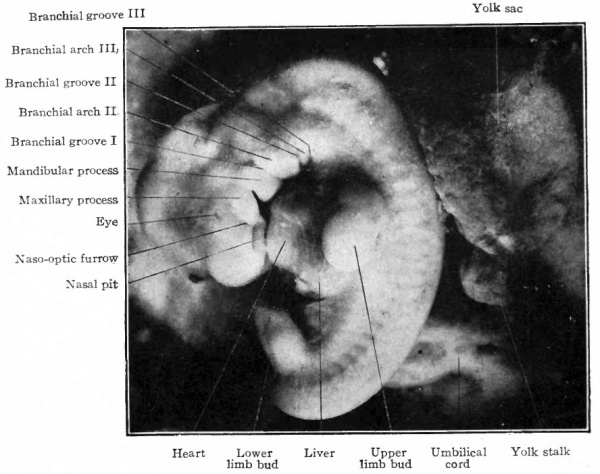
Fig. 87. Human embryo with twenty-seven primitive segments (7 mm., 26 days). Mall.
In a 7-mm. embryo described by Mall (Fig. 87), the flexures are slightly more accentuated than in the 4-mm. stage. The branchial arches and grooves are still prominent. The first groove, of which the dorsal part marks the site of the external auditory meatus, is at this time particularly well developed. The eye is a stronger feature than in the preceding stage. The distinct depression in front of the first arch is the nasal fossa. The limb buds are larger than in the 4 -mm. embryo. The general curvature of the embryo is so sharp at this stage that the rudimentary tail is almost in contact with the head.
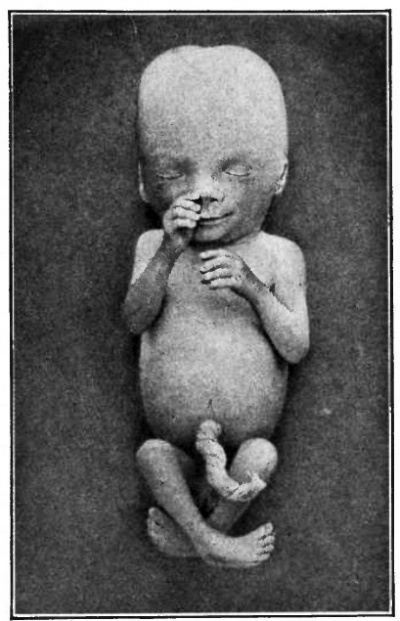
In Fig. 88, showing an embryo of 7.5 mm. with 27 primitive segments, the head is somewhat larger in proportion to the body. This character becomes accentuated as development proceeds and is especially noticeable up to the time of birth. The cervical and sacral flexures are still sharp, but the dorsal flexure is not quite so prominent. From now on, the body becomes more nearly straight. The rotundity of the ventral side of body is due to the heart and liver, the two organs now lying close together. The branchial arches are not actually smaller but appear less prominent. The second arch has enlarged and grown back over the third and fourth, partially hiding them. The limb buds' are larger; and the fore-limb bud now shows a transverse constriction dividing it into a proximal and a distal portion, the latter being the rudiment of the hand.
Fig. 88. Human embryo with 28 primitive segments (7.5 mm). Photograph.
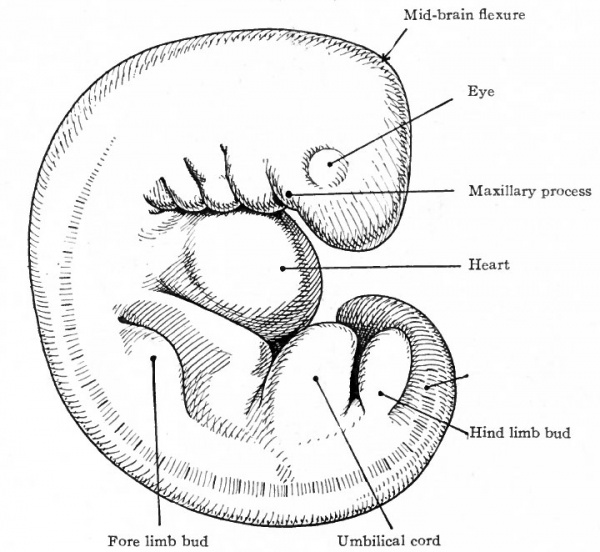
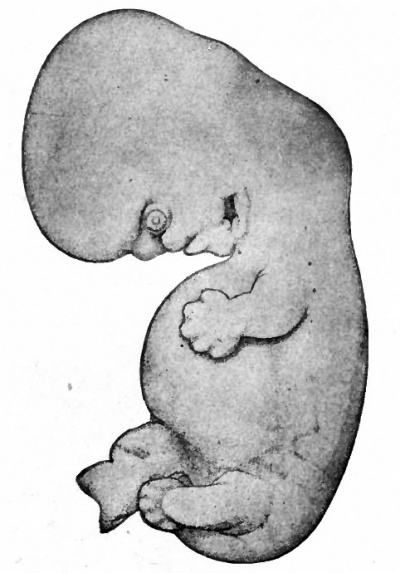
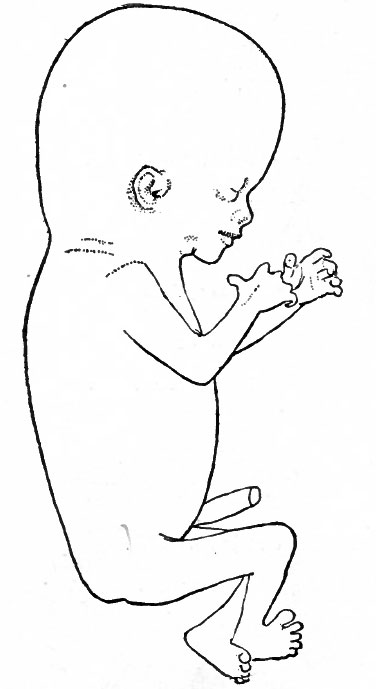
Figure 89 shows an embryo of 11 mm. All the flexures are slightly reduced except the cephalic. The cephalic flexure, which primarily affects the embryonic brain, persists as the mid-brain flexure of the adult. Two slight concavities have appeared in the dorsal profile, the occipital depression and the cervical depression. The latter becomes more conspicuous as development proceeds and persists as the depression at the back of the neck in the adult. The first branchial arch is a strong feature of the head, the maxillary process being especially prominent. This process has grown forward to form intimate contact with the nasal region. The second arch now hides the third and fourth arches, and the depression behind the second is known as the precermcal sinus. The first groove can be more readily appreciated as the site of the external auditory meatus, as can also the surrounding parts of the first and second arches be better appreciated as rudiments of the concha. The distal part of the fore-limb bud is flattened like a paddle, and the radial depressions in it mark the boundaries between the digits. In the proximal portion the fore-arm and arm are faintly indicated. The hind-limb bud is divided by a constriction into a proximal and distal portion; the latter is the beginning of the foot. During development the fore-limb is always at a slightly more advanced stage than the hind-limb. The ventral rotundity of the body is pronounced.
Fig. 89. Human embryo 11 mm. long (31-34 days). His.
In an embryo measuring 15.5 mm. (Fig. 90) the dorsal flexure is much reduced and the axis of the body is approaching the definitive line. The cervical flexure is still prominent, as is also the ventral rotundity of the body. The neck is now clearly differentiated. The external auditory meatus and the surrounding rudiments of the concha are plainly indicated. The limb buds are turned more nearly at right angles to the long axis of the body. The leg and thigh show early differentiation; the fingers are beginning to elongate and radial grooves on the foot indicate the boundaries between the toes. The tail, which was a prominent feature in the earlier stages, is proportionately small; in the human it is at most a rudimentary structure represented by the coccyx, and while in the early embryo it is fairly large it does not keep pace with the body during development.
Fig. 90. Human embryo of 15.5 mm (39-40 days). His.
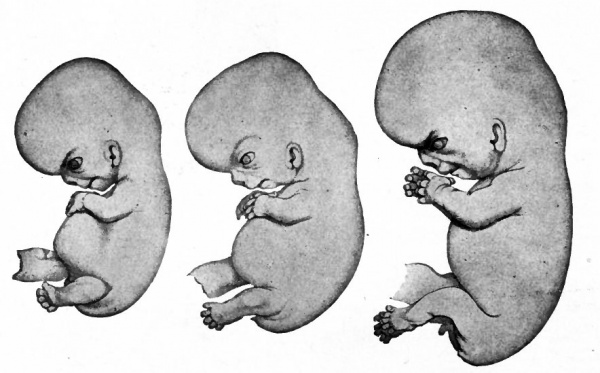
Fig. 91. Human embryo of 17.5 mm (47-51 days). Fig. 92. Human embryo of 18.5 mm (52-54 days). Fig. 93. Human embryo of 23 mm (2 months). His.
| Fig. 94. Human embryo of 78 mm (3 months). Minot. | Fig. 95. Human embryo of 4 months Kollmann. (original figure was printed at Natural size) |
After the stage shown in Fig. 90 the cervical flexure continues to diminish and the head comes to lie more nearly in line with the long axis of the body. The rotundity of the abdomen gradually becomes less as the heart and liver approach the proportions of the adult. The tail as an external structure disappears altogether, the buttocks increasing markedly. During the second month the external genitalia develop and the sex of the embryo can be distinguished. The general changes in form can be followed by comparing Figs. 91, 92, 93, 94 and 95.
In the early stages of human development, say during the first month, it is not uncommon to speak of all the membranes with the enclosed embryo as the ovum. During the first two months the developing organism itself is usually called an embryo. By the end of the second month when the embryo has reached the length of about an inch (25 mm.) it has acquired a form (Fig. 93) which in general resembles that of the adult and is henceforth referred to as a foetus.
The Face
When the fore-brain bends ventrally and the heart appears on the ventral side of the embryo in what will be the cervical region, there is thus produced between the two structures a depression or pit called the oral fossa (Fig. 84). This fossa is the rudiment of the oral and nasal cavities and around it the structures develop which- give rise to the face. Behind the fore-brain and dorsal to the heart, as the embryo develops, a series of slit-like depressions appear at right angles to the long axis of the body. Between the depressions are elevations. These structures are in the lateral wall of the embryonic pharynx, and are known as branchial grooves and arches (Figs. 85 and 86). It has been previously stated that they are homologous with the gill slits and gill bars of fishes. The first two arches and the first groove are involved in the formation of the face.
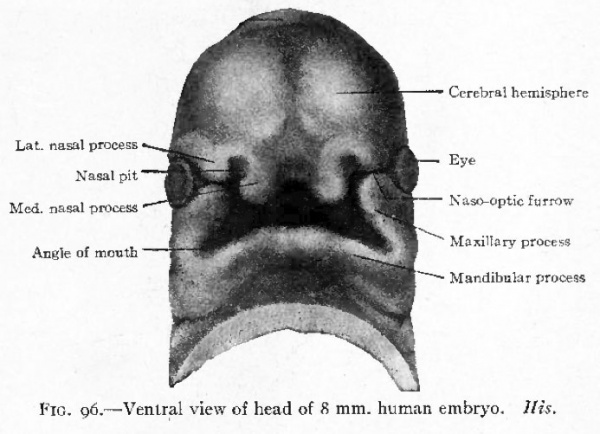
Fig. 96. Ventral view of head of 8 mm human embryo. His.
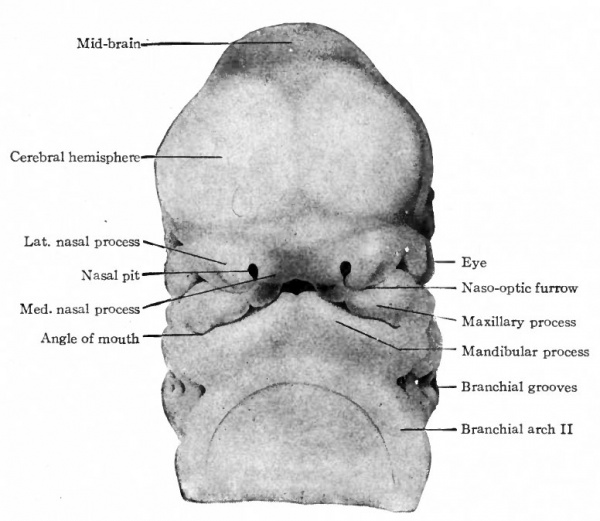
The first branchial arch becomes the largest of the series and, on account of its position, bounds the oral fossa laterally (Fig, 85). Its presence serves to deepen the fossa. Growing from the cephalic side of the arch, a strong process insinuates itself between the arch and the fore-brain region. This is called the maxillary process, while the original part of the arch is the mandibular process. The latter grows rapidly, extends ventrally and finally meets and fuses with its fellow of the opposite side in the midventral . line caudal to the oral fossa (Fig. 96). The maxillary process still bounds the oral fossa laterally. Meanwhile a broad downward projection from the front of the fore-brain region the naso-frontal process comes in contact laterally with the maxillary process (Fig. 96). Along the line of contact a furrow is left, which extends obliquely upward to the eye rudiment and is known as the naso-optic furrow.
The various structures that have been mentioned bound the oral fossa which has now become a deep quadrilateral pit. Cranially (above) the fossa is bounded by the broad, rounded, unpaired naso-frontal process; caudally (below) it is bounded by the paired mandibular processes; laterally by the paired maxillary processes. Between the maxillary and mandibular processes on each side a notch marks the future angle of the mouth. In general it may be stated that the naso-frontal process gives rise to the nose and middle of the upper lip, the maxillary processes to the lateral parts of the upper lip and the cheeks, the mandibular processes to the lower jaws, chin and lower lip.
Fig. 97. Ventral view of head of 11.3 mm human embryo. Rabl.
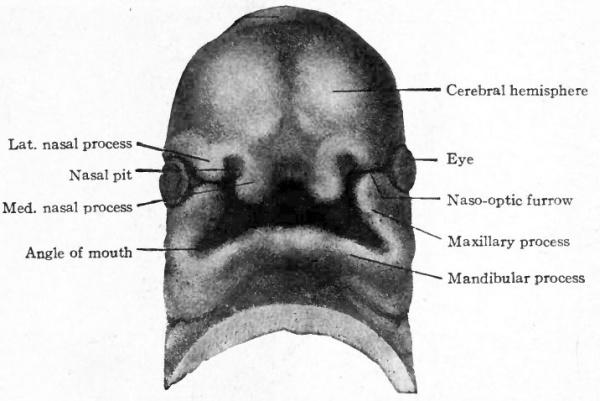
The naso-frontal process extends farther downward toward the mandibular processes, so that the oral fossa becomes more nearly enclosed and the entrance to it reduced to a slit. At the same time two secondary processes develop on each side from the naso-frontal, one, the medial nasal process, near the median line and the other, the lateral nasal process, more laterally. Between the two processes the nasal pit marks the entrance to the future nasal cavity (nostril). The maxillary process grows farther toward the medial line and forms contact with the lateral and medial nasal processes (Figs. 96 and 97).
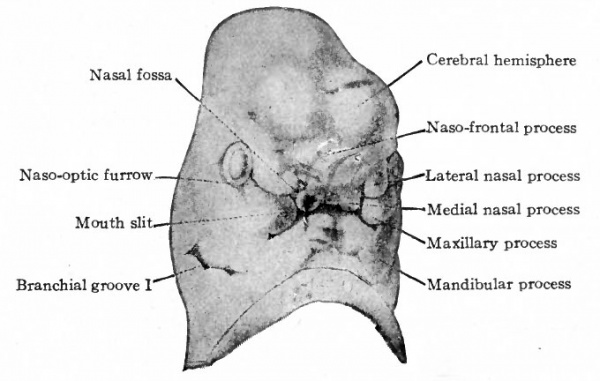
Fig. 98. Ventral view of head of 13.7 mm human embryo. His.
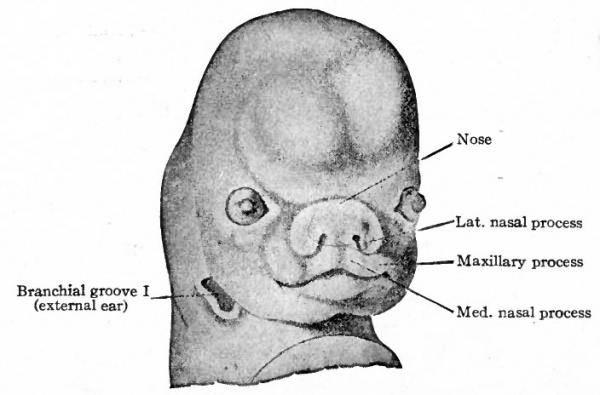
Further development consists essentially of fusions between the various elements already present and of changes in the relative proportions of these elements. The two medial nasal processes come close together to form a single medial process which gives rise to the middle portion of the upper lip and the adjacent portion of the nasal septum (Figs. 98 and 99). The maxillary process expands generally to form the cheek and lateral portion of the upper lip and at the same time fuses with the nasal processes. This fusion obliterates the naso-optic furrow and shuts off the connection between the nasal pit and mouth slit (Figs. 98 and 99). Internally the concomitant formation of the palate separates the oral and nasal cavities. The lateral nasal process gives rise to the wing of the nose. The nose at first is a broad, flat structure but later becomes elevated, elongated and narrower. The lower jaw, lower lip and chin are formed by the mandibular processes. At first the chin is short vertically but broad transversely. Later it becomes longer and a transverse furrow divides the middle portion into the lower lip and chin.
Fig. 99. Ventral view of head of human embryo of 8 weeks. His.
The Extremities
The rudiments of the extremities or limbs appear in human embryos about the end of the third week. They arise as small rounded protuberances on the lateral body wall, the upper limb buds just caudal to the level of the cervical flexure and the lower opposite the sacral flexure (Fig. 86) . The first to appear are the upper buds, the lower appearing a little later. The difference in time of appearance is reflected through embryonic and fcetal life in the slight advance in the degree of development of the upper over the lower.
During the fourth week the limb buds become elongated, and a transverse constriction on each bud marks off a distal from a proximal portion. The proximal portion remains approximately cylindrical while the distal portion becomes flat like a paddle (Fig. 88) . The flattened end-piece is the beginning of the hand (or foot), which is thus the first part of the extremity to be differentiated. During the sixth week the proximal portion, which in the meantime has become still longer, is marked off by a bend at the elbow (or knee) into two segments, the fore-arm and arm (or leg and thigh).
During the fifth week the rudimentary digits (fingers and toes) appear on the flattened distal segments of the developing extremities. They are first indicated by radial depressions on the flat surface, the digits being the elevations between the depressions (Figs. 89 and 90). The digits grow rapidly in thickness and length thus producing an apparent deepening of the interdigital grooves and also forming protrusions around the distal free borders of the limb-buds (Fig. 90). The depressed areas comprise a series of membranes resembling the web in the feet of certain aquatic animals. The digits grow more rapidly than the web, the latter eventually being confined to the proximal part of the interdigital spaces. After the seventh week the angle between the thumb and second digit approaches a right angle; a lesser degree of approach to a right angle holds true in case of the great toe (Figs. 91, 92 and 93).
As the limbs first elongate their long axes lie nearly parallel with the long axis of the body (Fig. 89) . Later they are directed ventrally nearly at right angles to the body axis (Fig. 92). The radial margins of the upper extremities are turned toward the head (cephalad) , as are the tibial margins of the lower. The palmar surfaces of the hands and plantar surfaces of the feet are turned inward or toward the projected sagittal plane of the body. The elbow is turned slightly outward and toward the tail, the knee slightly outward and toward the head. From these conditions it is inferred that the radial side of the upper extremity is homologous with the tibial side of the lower extremity, and the palmar surface of the hand with the plantar surface of the foot.
In order to acquire positions relative to the body as found in post-natal life, the extremities must undergo further changes. These consist of torsion around their long axes and rotation through an angle of 90 degrees. The right upper extremity twists to the right, and the right lower to the left. The left upper twists to the left, and the left lower to the right. At the same time they swing backward through an angle of 90 degrees so that they come to lie parallel with the long axis of the body. The result is that the radial side of the upper extremity is turned outward or away from the sagittal plane of the body and the tibial side of the lower inward or toward the sagittal plane of the body. In the upper extremity this is, of course, the supine position of the fore-arm in which the radius and ulna are parallel.
Age, Length and Weight of the Body
The age of human embryos is a matter of importance to the embryologist who desires to trace development in general or that of any organ, and to the obstetrician who is interested in the scientific phase of his work. Development commences when fertilization occurs and the age of the embryo should be dated from that time. Unfortunately it is not possible to determine exactly when fertilization takes place. This is impossible for several reasons : first, because fertilization cannot be. directly observed; second, because even if the time of cohabitation is known the period required by the sperm to reach the ovum cannot be exactly determined; and third, because the time that the ovum escapes from the ovary and passes into the oviduct is not known.
In view of these uncertain factors which cannot be controlled, the age of a human embryo can be determined only within certain limits. In a few cases on record the time of coitus was known, and assuming that an ovum was in the tube ready for fertilization, the copulation age could be reckoned. This, however, does not give the exact fertilization age, which is the actual age, because it requires some time for the spermatozoa to pass through the uterus and oviducts. In the Bryce-Teacher embryo, for instance, coitus occurred 16 days prior to abortion. The fertilization age was computed to be about 14 days. An embryo described by Eternod was aborted 21 days after coitus, and the fertilization age was reckoned at about 19 days. Other cases of a similar nature have been studied and the actual age of the embryo in each case was regarded as two days less than the copulation age. Such reckoning could be done only in those cases where the time of coitus and of abortion are both known.
In the majority of cases the only datum available is the time that the last menstrual flow occurred. In these cases the age of the embryo is reckoned from the beginning of the last menstrual period, and is known as the menstrual age. This at best can be only an approximate age because even if the date of coitus is known the date of ovulation is undetermined and therefore the time that the sperm and ovum meet is unknown. It has been shown that in perhaps the majority of cases ovulation occurs in the intermenstrual period, on the average about the middle of its duration, and therefore fertilization would not bear a direct time relation to menstruation. The condition of the corpus luteum, if it could be determined, would be of great value in reckoning the age of the embryo resulting from the development of the ovum that escaped from that particular follicle. The method of reck : oning from the first day of the last menstruation i? used by obstetricians for determining the probable time of birth.
In statistics collected by Issmer the average duration of pregnancy in 1220 cases was found to be 280 days, when estimated from the first day of the last menstrual period; and in 628 cases it was found to be 269 days from the date of fruitful cohabitation. It would seem therefore, on the basis of these statistics, that deducting about 10 days from the menstrual age would give the approximate copulation age which is not far removed from the actual age.
The length of an embryo can be determined by direct measurement. It is the general practice to take the greatest length of the embryo in a straight line in its natural attitude, not including the extremities. In embryos between 4 and 14 mm., when the body is much curved, one point of measurement lies on the apex of the cervical flexure and the other on the apex of the sacral flexure. (See Figs. 87 and 88.) This is known as the neckrump length. In later stages where the curvature is reduced and the body is more nearly straight, the measurement falls between the apex of the cephalic flexure and the apex of the sacral flexure. (See Figs. 90 and 91.) This is known as the crown-rump length. The relation of length to age has been the subject of much study, but no formula for deducing the age from the length, which can be determined, has been wholly satisfactory. The formula given by Mall some years ago was later abandoned by him as unreliable. This formula (for embryos from i to 100 mm.) was that the age can be expressed by the square root of the length in millimeters multiplied by 100 (Vlength in millimeters X 100). At present it can be said in general that embryos of 2 to 3 mm. will fall within the fourth week of development, embryos of 5 to 6 mm. will come about the end of the fifth week, those of 10 mm. at the end of the sixth week, and those of about 25 mm. at the end of the eighth week.
The following table gives the approximate greatest length at the ends of the lunar months following the second month:
| Lunar Month (4 weeks) | Crown Rump Length (mm, CRL) |
| 3d lunar month | 70- 90 mm |
| 4th lunar month | 100-170 mm |
| 5th lunar month | 180-270 mm |
| 6th lunar month | 280-340 mm |
| 7th lunar month | 350-380 mm |
| 8th lunar month | 425 mm |
| 9th lunar month | 467 mm |
| l0th lunar month | 490-500 mm |
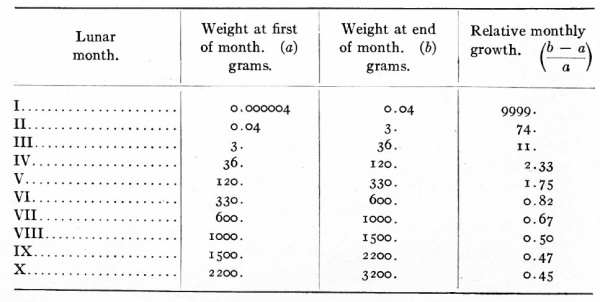
Some interesting observations have been made on the increase in volume of the embryo (and foetus) and of its parts during development. It has been estimated that the human ovum, which is about 0.2 mm. in diameter weighs about four millionths of a gram (0.000004 gm.) and that at the end of the first month the embryo weighs about four hundred ths of a gram (0.04 gm.). It thus appears that the human ovum increases approximately 10,000 times in weight during the first month of development. On the same basis the relative growth rate which is the ratio of the gain during a given period to the weight at the beginning of the period during the second calendar month is 74, during the third month n, during the fourth month 1.75, during the fifth month 0.82, during the sixth month 0.67, during the seventh month 0.50, during the eighth month 0.47 and during the ninth month 0.45 (Jackson). The accompanying table taken from Jackson's work gives the relative growth rate of the embryo in each of the ten lunar months of pregnancy.
The head attains its maximum relative size during the second month when its weight is about 45 per cent, of the total weight of the embryo. Thereafter it gradually diminishes in relative size until birth when it constitutes about 26 per cent, of the total weight. The trunk is relatively largest during the first month when its weight is about 65 per cent, of the total and then decreases to between 40 and 45 per cent, in the later fcetal months. The extremities gradually increase in relative size after their appearance; the upper extremities forming about 10 per cent, of the total weight at birth and the lower about 20 per cent.
Although the relative growth rates of the various organs have also been estimated it does not seem desirable to include them in the chapter which treats of the external form c Two organs might be mentioned, however, which have a marked influence on the contour of the body ; that is, the heart and liver. In embryos about 10 mm. long the heart constitutes nearly 4 per cent, of the total body volume. Thereafter it diminishes in relative size to less than i per cent, at birth. Figures 8 5 and 86 show the marked protrusion produced by this organ on the ventral side of the body. In embryos of around 30 mm. the liver forms more than 10 per cent, of the total volume of the body; in fact it is relatively large during both the second and third months. Figures 87 and 88 show the effect of the organ on the contour of the body, in conjunction with the heart.
References for Further Study
BRYCE, T. H. and TEACHER, J. H.: Early Development and Imbedding of the Human Ovum. Glasgow, 1908.
His, W.: Anatomic menschlicher Embryonen. With atlas, 1880-1885.
JACKSON, C. M.: On the Prenatal Growth of the Human Body and the Relative Growth of the various Organs and Parts. Am. Jour, of Anat., Vol. 9, 1909.
KEIBEL. F.: Die Entwickelung der ausseren Korperform der Wirbeltierembryonen, inbesondere der menschlichen Embryonen aus der ersten 2 Monaten. In Band I, Teil II of Hertwig's Handbuch der vergl. und experiment. Entwickelungslehre der Wirbeltiere, 1902.
KEIBEL, F. and ELZE, C.: Normentafel der Entwickelungsgeschichte des Menschen. Jena, 1908.
KEIBEL, F. and MALL, F. P.: Manual of Human Embryology. Chap. VIII, 1910.
KOLLMAN, J.: Handatlas der Entwickelungsgeschichte des Menschen. Jena, 1907.
MALL, F. P.: On the Age of Human Embryos. Am. Jour, of Anat., Vol. 23, 1918.
RABL, C.: Die Entstehung des Gesichtes. Leipzig, 1902.
TRIEPEL, H.: Alter menschlicher Embryonen und Ovulationstermin. Anat. Anzeiger, Bd. 48, 1915.
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
Text-Book of Embryology: Germ cells | Maturation | Fertilization | Amphioxus | Frog | Chick | Mammalian | External body form | Connective tissues and skeletal | Vascular | Muscular | Alimentary tube and organs | Respiratory | Coelom, Diaphragm and Mesenteries | Urogenital | Integumentary | Nervous System | Special Sense | Foetal Membranes | Teratogenesis | Figures
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2026, February 26) Embryology Book - Text-Book of Embryology 8. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Book_-_Text-Book_of_Embryology_8
- © Dr Mark Hill 2026, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G