Book - Text-Book of Embryology 19
| Embryology - 28 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Bailey FR. and Miller AM. Text-Book of Embryology (1921) New York: William Wood and Co.
- Contents: Germ cells | Maturation | Fertilization | Amphioxus | Frog | Chick | Mammalian | External body form | Connective tissues and skeletal | Vascular | Muscular | Alimentary tube and organs | Respiratory | Coelom, Diaphragm and Mesenteries | Urogenital | Integumentary | Nervous System | Special Sense | Foetal Membranes | Teratogenesis | Figures
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
Foetal membranes
In all Vertebrates, with the exception of Fishes and Amphibians which lay their eggs in water, there begin to develop gr a very early stage certain accessory or extraembryonic structures which may be conveniently called jostal membranes. The development of these structures is very closely i elated to the development of the embryo itself, and their presence is apparently largely dependent upon the very considerable length of embryonic life in these forms, during which it is necessary for the embryo to maintain a definite relation to its food supply and to possess means of discharging waste products. The foetal membranes, therefore, have to do with the protection and nutrition of the growing embryo and also are connected with the care of the waste products of foetal metabolism.
Under the head of foetal membranes are to be considered (i) the amnion, (2) the allantois, (3) the chorion; also in connection with these, the yolk sac and the umbilical cord.
The development of these structures in Mammals and especially in man is extremely complex and can be best understood by comparison with their simpler development in Reptiles and Birds.
Foetal Membranes in Birds and Reptiles
Throughout these two classes there is such uniformity in the formation of the foetal membranes that the chick may be taken as typical. The chief characteristic of these classes, as influencing the form and structure of the foetal membranes, is the very large amount of yolk stored up within the egg for the nutrition of the embryo. This is made necessary by the early separation of the egg from the mother, in contrast to the close nutritional relationship between mother and foetus which obtains in Mammals (excepting Monotremes), where the young are retained within the body of the mother up to a comparatively late developmental stage.
The Amnion. Returning to that point in the development of the blastoderm of the chick where no trace of amnion has as yet appeared, we recall that the blastoderm at this stage consists of three layers, ectoderm, mesoderm and entoderm; that the medial line of the embryo is marked by the neural groove, flanked by the neural folds which are continuous with each other anteriorly; that on each side of the neural groove between ectoderm and entoderm the mesoderm is a solid mass of cells, while more laterally the mesoderm is split, its peripheral layer with the adjacent ectoderm forming the somatopleure, its central layer with the adjacent entoderm forming the splanchnopleure; that between somatopleure and splanchnopleure is the body cavity. Ventral to the neural groove is the notochord, while ventral to the latter is the primitive gut, the roof of which is formed of entoderm (Fig. 52),
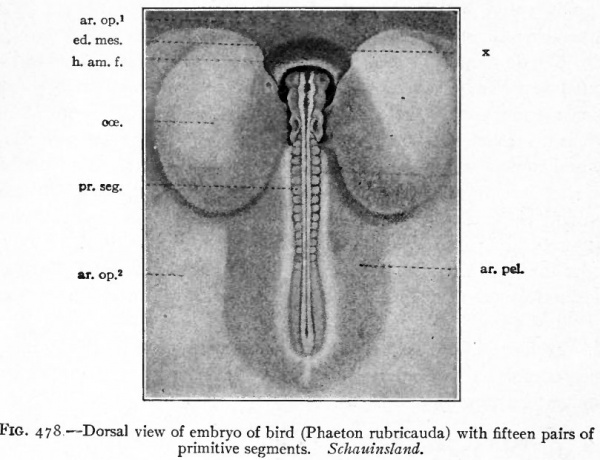
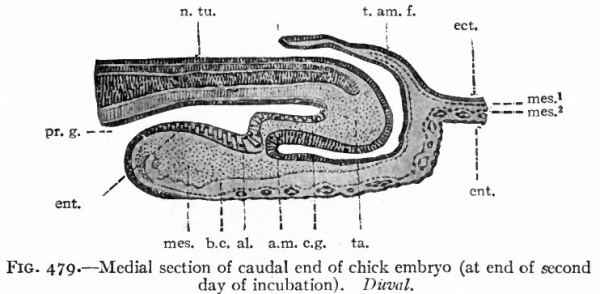
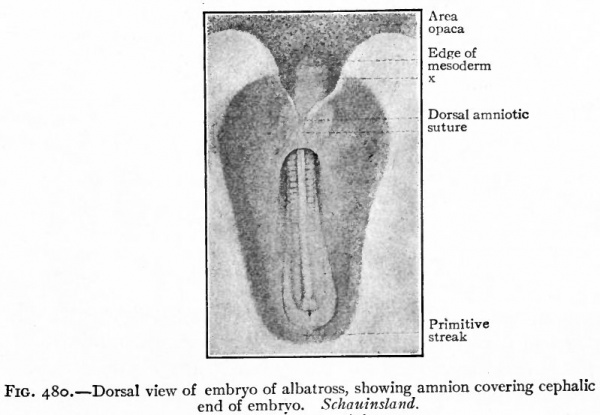
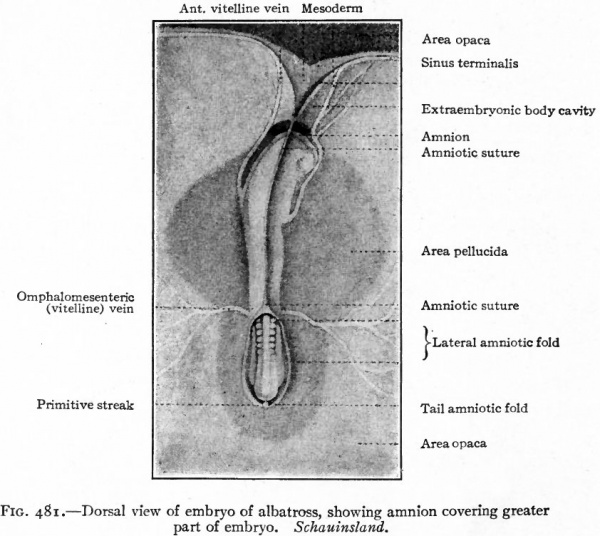
The first indication of amnion formation is the appearance of a fold the head amniotic fold just in front of the anterior union of the neural folds (Figs. 478 and 484, b) . This occurs during the second day of incubation. After the head fold has become well developed and extends back over the embryo like a hood (Fig. 480), similar lateral and tail folds make their appearance (Figs. 479 and 484, a and b). The folds continue to grow over the dorsum of the embryo and finally meet and fuse in the mid-dorsal line, forming the amniotic suture (Fig. 481).
Fig. 478. Dorsal view of embryo of bird (Phaeton rubricauda) with fifteen pairs of primitive segments. Schauinsland.
- ar. op* y Area opaca, portion in which mesoderm is not yet present; ar. op*, area opaca; ar. pel.-, area pellucida; cce., bladder-like dilatation of ccelom; ed. mes., edge of mesoderm; h. am. /., head amniotic fold; pr. seg., primitive segments; x, portion of amniotic fold containing no mesoderm.
The amniotic folds from the beginning involve the somatopleure, that is, the ectoderm and parietal mesoderm. But since they arise some distance from the developing embryonic body, the extraembryonic portions only are involved. At the same time a portion of the extraembryonic body cavity is also carried dorsally within the folds (Figs. 479 and 482) . When the folds unite over the embryo they break through at the line of contact, thus leaving the outer layers of the folds continuous and the inner layers continuous, with the extraembryonic body cavity continuous between the outer and inner layers.
Fig. 479. Medial section of caudal end of chick embryo (at end of second day of incubation). Duval.
- al^ Beginning of allantoic evagination; a.m., anal membrane; b.c., extraembryonic body cavity; e.g., caudal gut; ect., ectoderm; ent., entoderm; mes., mesoderm; mes. 1 , parietal mesoderm; mes. 2 , visceral mesoderm; n. tu., neural tube; pr. g., primitive gut; /. am. /., tail amniotic fold; to., tail.
Fig. 480. Dorsal view of embryo of albatross, showing amnion covering cephalic end of embryo. Schauinsland. x, Portion of blastoderm containing no mesoderm.
The result of the development of the amniotic folds is:
- That the embryo is completely enclosed dorsally and laterally by a cavity, the amniotic cavity, which is lined by ectoderm continuous with the ectoderm later epidermis of the embryo, the ectoderm lining the cavity and the overlying parietal mesoderm together constituting the amnion (Fig. 483).
- That the outer parts of the amniotic folds become completely separated from the inner the amnion to form a second membrane consisting externally of ectoderm, internally of mesoderm and called at first the serosa or jalse amnion, later the primitive chorion (Fig. 483).
- That the extraembryonic body cavity unites across the medial line dorsally, thus separating the amnion from the primitive chorion (Fig. 484, a, b and c).
During the formation of the amnion the chick embryo is becoming more and more definitely constricted off from the underlying large yolk mass which is liquefying and into which the embryo sinks somewhat. At the same time the amniotic cavity continues to increase in size and extends also ventrally beneath the embryo so that the embryo is everywhere enclosed within the amnion except at its narrow connection with the yolk (Fig. 484, c\ d).
Fig. 481. Dorsal view of embryo of albatross, showing amnion covering greater part of embryo. Schauinsland.
The amniotic cavity is filled with fluid, the liquor amnii, the origin of which is uncertain. In it the embryo floats freely, attached only by its ventral connection with the yolk. At about the fifth day of incubation rhythmical contractions of *he amnion begin. These are apparently due to the development of contractile fibers in its mesodermic tissue and give to the embryo a regular oscillating motion.
The Yolk Sac
The simplest type of yolk sac is found in Amphibians and Fishes. In Amphibians the yolk is enclosed within the embryo, the cells forming a part of the intestinal wall. The superficial cells are split off to form the yolk entoderm. Investing the yolk entoderm is the visceral mesoderm which is separated from the parietal mesoderm by the body cavity. Outside of the parietal mesoderm is the ectoderm (Fig. 33). In many of the Fishes the germ disk, as in Reptiles and Birds, is confined to one pole of the egg. Thus in these forms the embryonic body develops on the surface of the large yolk mass. As the embryo develops the germ layers simply grow around the yolk and suspend it from the ventral side of the embryo. At the same time a constriction appears between the embryo and the yolk mass, thus forming the yolk stalk. In this case the yolk is surrounded from within outward, by entoderm, visceral and parietal mesoderm, and ectoderm (Fig. 485) . The yolk furnishes nutriment for the embryo. This is conveyed to the tissues by means of blood vessels. Branches of the vitelline artery ramify in the wall of the yolk sac (in the mesodermal.tissue) ; the branches converge to form the vitelline veins which carry the blood back to the embryo.
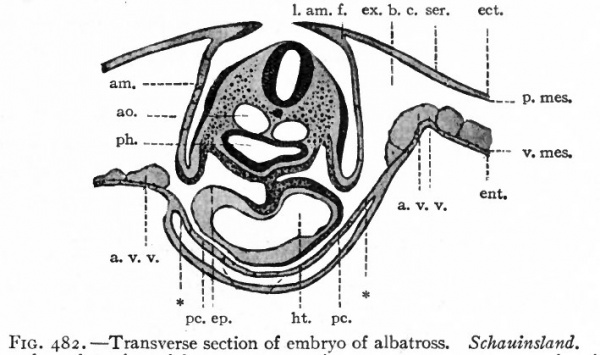
Fig. 482. Transverse section of embryo of albatross. Schauinsland.
- Section taken through region of heart, aw., amnion; ao., aorta; a. v.v., anterior vitelline veins; ect., ectoderm; ent., entoderm; ep., epicardium; ex. b. c., extraembryonic body cavity; ht., heart; /.aw./., lateral amniotic fold; pc., pericardium; ph., pharynx; p. mes., parietal mesoderm; ser.. serosa (chorion); v.mes., visceral mesoderm; * point at which extraembryonic body cavity passes over into the intraembryonic (or coelom proper).
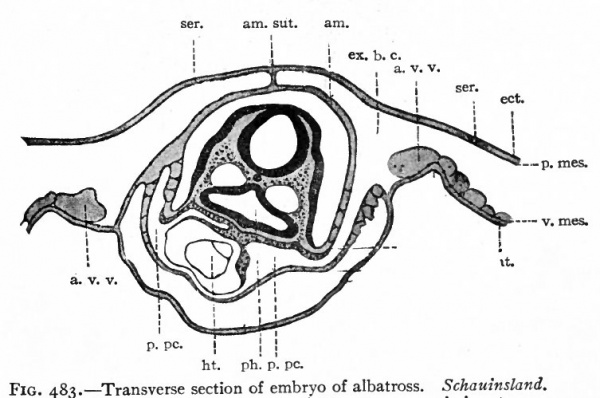
Fig. 483. Transverse section of embryo of albatross. Schauinsland.
- Section taken through region of heart, am., Amnion; am. sut., amniotic suture; a. v.v, anterior vitelline veins; ect., ectoderm; ent., entoderm; ex. b. c., extraembryonic body cavity; ht., heart; i> pc., primitive pericardial cavity; ph., pharynx; p. mes., parietal mesoderm; ser., serosa (chorion); v.mes, visceral mesoderm; * point at which extraembryonic body cavity passes over into intraembryonic (or coelom).
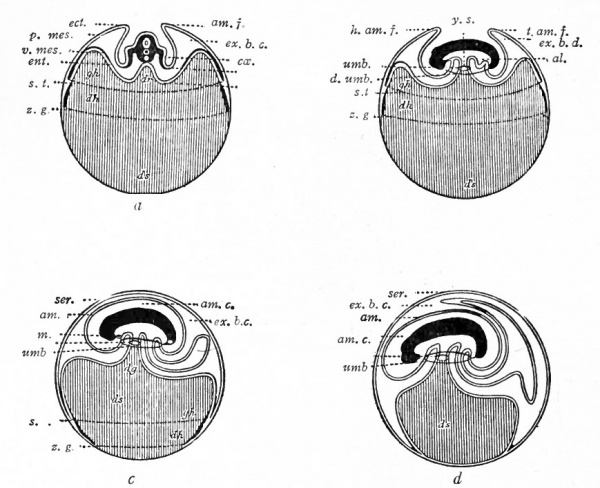
Fig. 484. Diagrams representing stages in the development of the foetal membranes in the chick. Hertwig.
- a, Transverse section; b, c, d, longitudinal sections; yolk represented by vertical lines, al., Allantois; am., amnion; am. c., amniotic cavity; cce., ccelom; dh., vitel line area between two dotted lines which represent the edge of the mesoderm (at s. /.) and entoderm (at z. g.}\ dg., yolk stalk; ds., yolk sac; d.umb., dermal umbilicus; ect., ectoderm; ent.> entoderm; ex. b. c., extraembryonic body cavity; gh., area vasculosa; h.am.f., head amniotic fold; m., mouth; p,mes., parietal mesoderm; s. t., sinus terminalis; ser., serosa (chorion); t.am./,, tail amniotic fold; umb., umbilicus; v mes., visceral mesoderm; z. g., dotted line represents edge of entoderm.
In the chick, while the amnion is forming, the inner germ layer gradually extends farther and farther around the yolk (Fig. 484, a, b, c and d). At the same time, as already noted (p. 566), the growth of the amnion ventrally results in a sharp constriction which separates the embryo from the underlying yolk. This constriction is emphasized by constant lengthwise growth of the embryo. Following the gradual growth of the entoderm around the yolk, the mesoderm also gradually extends around, at the same time splitting into visceral and parietal layers, so that the entoderm is closely invested by visceral mesoderm (Fig. 484, a, &, c and d). Finally, both entoderm and mesoderm enclose completely the mass of yolk. The yolk thus becomes enclosed in the yolk sac * which consists of two layers, entoderm and visceral mesoderm.^ The constricted v . connection between the yolk sac and the embryo is the yolk stalk. It is seen by reference to the diagrams (Fig. 484) that the entoderm lining the yolk sac is directly continuous through the yolk stalk with the entoderm lining theprimi-tive gut> The transition line between extra- and intraembryonic entoderm is sometimes referred to as the intestinal umbilicus, in contradistinction to the line of union, on the outside of the yolk stalk, of amniotic and embryonic ectoderm (the latter becoming later the epidermis) which is known as the dermal umbilicus.
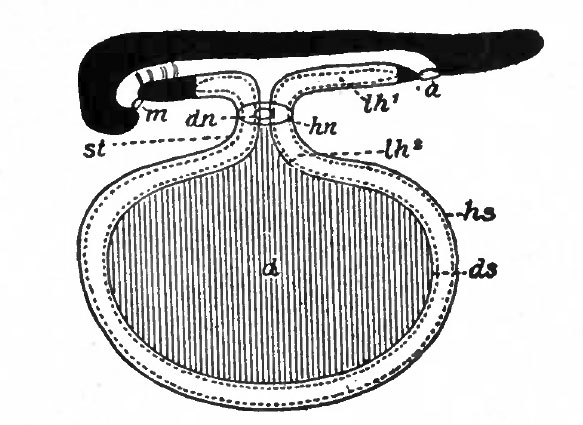
Fig. 485. Diagrammatic longitudinal section of selachian embryo. Hertwig. a., Anus; d., yolk sac; dn., intestinal umbilicus; ds., visceral layer of yolk sac; hs., parietal layer of yolk sac; hn., dermal umbilicus; lh l , coelom; lh 2 , exoccelom; m., mouth; st., yolk stalk.
As in Fishes and Amphibians, so also in Reptiles and Birds, the yolk furnishes nourishment for the growing embryo, and is conveyed to the embryo by the blood. At a very early stage the mesoderm layer of the yolk sac (visceral mesoderm) becomes extremely vascular. This vascular area is indicated by an irregularly reticulated appearance in the periphery of the blastoderm and is known as the area vasculosa (Fig. 51). The area vasculosa increases in size as the mesoderm grows around the yolk and its vessels become continuous with those in the embryo (Fig. 159). Some of these vessels enlarge as branches of two large vessels which are given off from the primitive aortae, the mtelline or omphalomesenteric arteries. (When the two aortae fuse to form a single vessel, the proximal ends of the vitelline arteries fuse likewise.) The branches of the arteries ramify in the mesoderm over the surface of the yolk and then converge, to form other vessels which enter the embryo as thevilellmeoromphalomesenteric veins (Fig. 160). As the mesoderm extends farther and farther around the yolk, the vessels extend likewise until the entire yolk is surrounded by a dense plexus of blood vessels in the wall of the yolk sac.
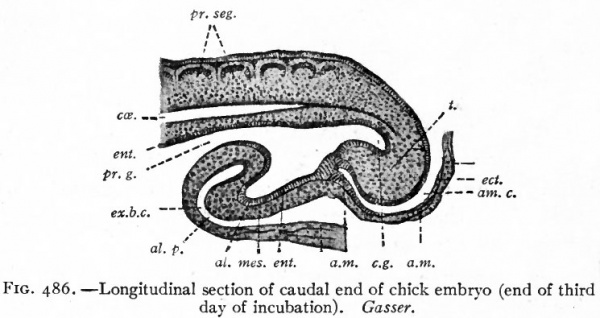
The Allantois. While the embryonic intestine is first assuming the form of a tube, there grows out ventrally from near its caudal end, during the third day of incubation, a diverticulum which is the beginning of the allantois (Fig. 486). This increases rapidly in size and pushes out into the extraembryonic body cavity behind the yolk stalk. As it is a diverticulum from the intestine, it consists primarily of entoderm. This pushes in front of it, however, the splanchnic (visceral) mesoderm which becomes the outer layer of the membrane. The connection between the intestine and the allantois is known as the urachus. In the chick the allantois attains a comparatively large size, pushing out dorsally between the amnion and the primitive chorion and ventrally between the latter and the yolk sac (Fig. 484, b, c and d). The inner wall of the allantoic sac blends with the amnion about the seventh day of incubation and with the yolk sac considerably later, while the outer wall joins the primitive chorion to form the true chorion, or as it is sometimes designated, the allanto-chorion (see p. 575) . As the allantois reaches the limit of the yolk, it leaves the latter, and pushing the primitive chorion before it, continues around close under the shell (Fig. 484) until it completely encloses the albumen at the small end of the egg.
Fig. 486. Longitudinal section of caudal end of chick embryo (end of third day of incubation). Gasser.
- al., Allantois; al. p., allantois prominence; a.m., anal membrane; am., amnion; am. c., amniotic cavity; e.g., caudal gut; cce., ccelom; ect., ectoderm; ent., entoderm; ex. b. c., extraembryonic body cavity; mes., mesoderm; pr. g., primitive gut; t., tail.
The allantois of the chick performs three important functions :
- It serves as a receptacle for the excretions of the primitive kidneys.
- United with a part of the primitive chorion to form the albumen sac, its vessels take up the albumen as nourishment for the embryo. Because of this function and also because of the fact that little papillae sometimes appear on the inner surface of the albumen sac, evidently for the purpose of increasing its absorptive surface, this albumen sac has been compared by some to a placenta.
- It blends with the primitive chorion to form the true chorion and being extremely vascular and lying just beneath the porous shell, it serves as the most important organ of foetal respiration.
The allantois in the chick is an extremely vascular organ, the network of small vessels in the wall being composed of radicals of the allantoic or umbilical vessels of the embryo. Soon after the allantois begins to develop, two branches the umbilical arteries are given off from the aorta near its caudal end. These pass ventrally through the body wall of the embryo and thence out via the umbilicus to break up into extensive networks of capillaries in the mesodermal layer of the allantois. The capillaries converge to form the umbilical veins which pass into the embryo via the umbilicus and thence cephalad to the heart.
During the incubation period of the chick there are two extraembryonic sets of blood vessels. One set, the vitelline (omphalomesenteric) vessels (p. 187), is concerned with carrying the yolk materials to the growing embryo. The other set, the umbilical (allantoic) vessels, is chiefly concerned with respiration and carrying waste products to the allantois, but is' probably in part concerned with conveying the albumen to the embryo. When the chick is hatched, and the foetal membranes are of no further use and disappear, the extraembryonic portions of the blood vessels also disappear. The intraembryonic portions persist, in part, as certain vessels in the adult organism.
The Chorion or Serosa. This membrane is but little developed in the chick as compared with Mammals, especially the Placentalia. Its mode of origin as the outer leaves of the amniotic folds, cut off from the amnion by dorso-medial extension of the mesoderm and body cavity, has been described (P- S^S) It consists, as there shown, of extraembryonic ectoderm and parietal mesoderm (Fig. 483) . As first formed it is confined to the immediate region of the embryo and of the amnion to which it is later loosely attached. It soon extends ventrally around the yolk where it forms what is sometimes designated the skin layer of the yolk sac. The relation of the outer layers of the allantois to the chorion has been described on page 570, and is illustrated in Fig. 486.
Foetal Membranes in Mammals
The development of the foetal membranes in Mammals presents no such uniformity as is found in Birds and Reptiles where it was possible to describe their formation in the chick as typical for the two classes. In the different Mammals much variation occurs, not only in the first appearance of the membranes but also in their further development and ultimate structure.
In some forms (rabbit, for example) the amnion develops in a manner very similar to that in the chick; that is, by a dorsal folding of the somatopleure. There is, however, no head fold unless a temporary structure known as the proamnion be considered as such. The entire rabbit amnion is formed by an extension over the embryo of the tail amniotic fold. In other forms (bat and probably man) the amnion and amniotic cavity arise in situ over the embryonic disk, without any folding of the somatopleure.
Yolk is almost entirely lacking in most Mammals, but the yolk sac is always present although it soon becomes a rudimentary structure. The fact that the yolk sac is always present points toward the conclusion that Mammals are descended from animals which possessed large ova with abundant yolk. As a matter of fact the lowest Mammals, the Monotremes, possess large ova with large quantities of yolk. These are deposited by the female, are developed in a parchment-like shell, and are carried about in the brood-pouch.
The allantoic sac in many Mammals is a very rudimentary structure which, as in the chick, always arises as an evagination from the caudal end of the gut. The allantoic blood vessels, however, become vastly important since they here not only carry off waste products from the embryo, as in Reptiles and in Birds, but also assume the function of conveying nutriment from the mother to the embryo. In assuming this new function they are no longer concerned with the allantoic sac proper but enter into a new relation with the chorion.
The chorion is the most highly modified and specialized of all the mammalian foetal membranes. In some cases (the rabbit, for example) it arises in connection with the amnion, as in the chick, by a dorsal folding of the somatopleure. In other cases (bat and probably man) it arises at a very early stage, partly as a differentiation of the superficial layer of the .morula, partly as extraembryonic parietal mesoderm which develops later. In all cases where the embryo is retained in the uterus (except Marsupials) it forms a most highly specialized and complex structure which, in connection with the allantoic vessels, establishes the communication between the mother and the embryo.
For the sake of clearness it seems best to describe first the earlier stages of the foetal membranes in some case where the development resembles that of the chick; then later to consider the more specialized types of development, the ultimate structure of the membranes, especially the chorion, and their relation to the embryo and the mother.
Amnion, Chorion, Yolk Sac, Allantois, Umbilical Cord. Referring back to the mammalian blastoderm when it consists of the three germ layers, it will be remembered that the embryonic disk forms the roof, so to speak, of a large cavity the yolk cavity or cavity of the blastodermic vesicle (Fig. 75); that the ectoderm of the disk is continuous with a layer of cells which extends around the vesicle the extraembryonic ectoderm; that the entoderm of the disk is continuous with the entoderm lining the cavity of the vesicle; that the mesoderm extends peripherally beyond the disk between the ectoderm and entoderm (Fig. 81). It will be remembered also that the mesoderm later splits into two layers the parietal and visceral, of which the parietal plus the ectoderm forms the somatopleure and the visceral plus the entoderm forms-file splanchnopleure; and that the cleft between the two layers is the body cavity or ccelom.
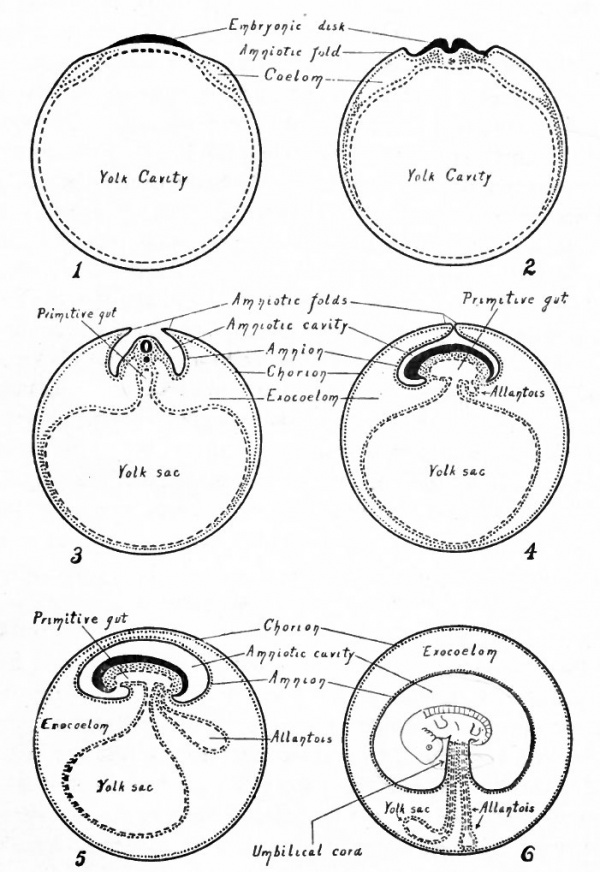
Fig. 487. Diagrams representing six stages in the development of the foetal membranes in a mammal. Modified from Kulliker.
- The ectoderm is indicated by solid black lines; the entoderm by broken lines; the mesoderm by dotted lines and areas.
In further development, along with the differentiation of the embryonic body, the somatopleure begins to fold dorsally at a short distance from the body (Fig. 487, 2). The folds amniotic folds appear cranially, laterally and caudally. These folds continue to grow dorsally (Fig. 487, 3) and finally meet and fuse above the embryo (Fig. 487, 4). They then break through along the line of fusion so that the extraembryonic body cavity which has been carried up dorsally over the embryo in the amniotic folds becomes continuous across the mid-dorsal line. A double membrane or rather two membranes are thus formed which extend over the embryo. The outer membrane is the- cjiojjon and is composed from without inward of ectoderm and parietal mesoderm. The inner membrane is the amnion and is composed from without inward of parietal mesoderm and ectoderm (Fig. 487, 5). Between the amnion and the chorion is a portion of the extraembryonic body cavity, which, as already mentioned, was carried dorsally with the amniotic folds (Fig. 487, 2, 3, 4 and 5).
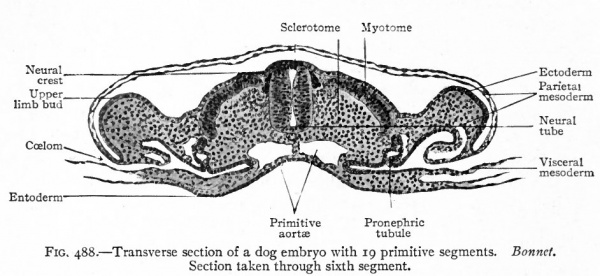
Fig. 488. Transverse section of a dog embryo with 19 primitive segments. Section taken through sixth segment. Bonnet.
In the manner just described the amnion becomes a sac which at first encloses the embryo laterally, and then laterally and dorsally (Efg- 488) . ^ Later as the embryo becomes constricted off from the underlying^ 1:avity, the amnion encloses it entirely except over a small area on the ventral side where the embryo is attached to the yolk sac (Fig. 487, 3, 4 and 5).
While the amnion is being formed, the mesoderm continues to extend around the vesicle between the ectoderm and the entoderm. At the same time it splits into parietal and visceral layers, of which the parietal is applied to the ectoderm, and the visceral to the entoderm. In this way the extraembryonic body cavity gradually extends farther and farther around the vesicle until finally the somatopleure is completely separated from the splanchnopleure (Fig. 487, 3, 4 and 5). The extraembryonic somatopleure now forms a complete wall for the vesicle and constitutes the chorion. The extraembryonic splanchnopleure forms a complete wall for the yolk cavity and constitutes the wall of the yolk sac. The proximal portion of the yolk sac becomes constricted to form the yolk stalk which connects the yolk sac with the ventral side of the embryonic body (Fig. 487, 5).
While the processes just described have been taking place, an evagination appears pushing out from the ventral side of the caudal end of the gut (Fig. 487,4). This evagination grows out into the extraembryonic body cavity (exocoelom), pushing before it the visceral layer of mesoderm, thus giving rise to a thin- walled sac which communicates with the gut the allantois (Fig. 487, 5). At this stage the embryonic body, with its surrounding amnion and appended yolk sac and allantois, lies within the large vesicle formed by the chorion. Up to this point the development resembles that in the chick.
In succeeding stages a new connection is established between the embryo and the chorion in the following manner : The amnion enlarges and fills relatively more of the cavity within the chorion, while the yolk sac becomes smaller and the yolk stalk much attenuated (Fig. 487, 6). At the same time the allantois also becomes attenuated and its distal end comes in contact with the chorion (Fig. 487, 6). The growth of the amnion results in the pushing together of the attenuated yolk stalk and allantofs so that they lie parallel to each other (Fig. 487, 6), and are together invested by a portion of the amnion. As already described, both yolk stalk and allantois are composed of entoderm and mesoderm while the amnion is composed of mesoderm and ectoderm. Consequently when the three structures come together and fuse, there is formed a mass of mesoderm which contains the entoderm of the yolk stalk or vitelline duct and trie entoderm of the allantois or allantoic duct, and which is surrounded by the ectoderm of the amnion. The fusion of these three structures in this region thus produces a slender cord of tissue which forms the union between the embryo and the chorion and which is known as the umbilical cord (Fig. 487, 6).
In Mammals the yolk sac contains little or no yolk and consequently can furnish but little nutriment for the embryo; but the union of the allantois with the chorion, mentioned in the preceding paragraph, allows the allantoic blood vessels to come into connection with the chorion. And since in Mammals the chorion is the means of establishing the communication between the embryo and the mother, the allantoic (umbilical) vessels assume the function of carrying nutrient materials to the embryo and also of carrying away from the embryo its waste products. (See p. 192.)
Further Development of the Chorion
Up through the stages which have been described the correspondence in the development of the foetal membranes in Reptiles, Birds and Mammals is clear. From now on, the course of development in Mammals becomes more and more divergent. The extensive development of the yolk and yolk sac with its vascular system in the egg-laying Amniotes has been noted. This is dependent upon the fact that the embryo very early in its existence loses its nutritional connection with its mother and is therefore dependent for its food upon the yolk stored up within the egg. This condition obtains up through the lowest order of Mammals, the Monotremes, which are egg-laying animals. The Marsupials give birth to young of very immature development. In these two orders of Mammals the foetal membranes present essentially the same condition as in Birds and Reptiles. The chorion in Marsupials, however, lies in close apv position to the vascular uterine mucosa and perhaps provides for the passage of nutrition from the mother to the embryo. In all higher Mammals, however, no eggs are laid and the embryo early acquires an intimate nutritional relation to its mother. This relation is maintained until the embryo has reached a comparatively advanced stage of development. As would be expected therefore, there take place, coincidently with the change in nutritional relation between mother and embryo, and dependent upon this changed relation, the already noted decrease in, or entire loss of, yolk and at the same time the development of a special organ of relation between embryo and uterus. This organ is developed mainly from the chorion which becomes highly specialized as compared with the very simple chorion described in the chick.
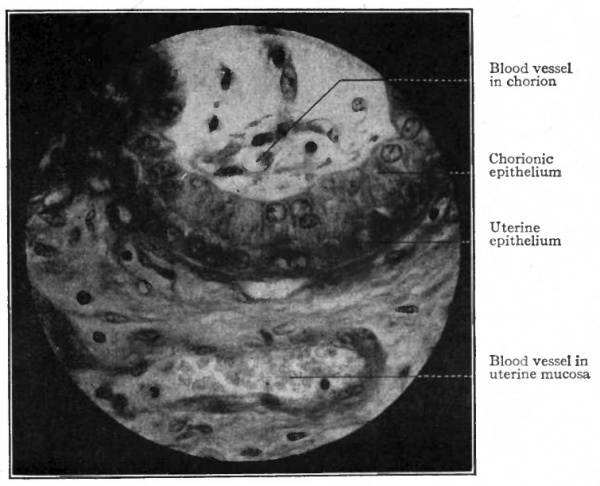
Fig. 489. Vertical section through wall of uterus and chorion of a pig. Photograph.
- Note especially the close apposition of the chorionic and uterine epithelium (and compare with Fig. 490); note also the enlarged blood vessels in the uterine mucosa.
In some Mammals (e.g., pig, horse, hippopotamus, camel) there develops a more intimate relation between the chorion and the uterine mucosa. In the pig, for example, the chorionic vesicle becomes somewhat spindle-shaped, and, except at its tapering ends, its surface is closely applied to the surface of the uterine mucosa. On that portion of the chorion which is in contact with the uterine mucosa small elevations or projections develop and fit into corresponding depressions in the mucosa. These projections involve the epithelial layer (ectoderm) of the chorion and the adjacent connective tissue (mesoderm) (Fig. 488) . Furthermore, the chorionic epithelial cells and the uterine epithelial cells acquire very intimate relations in that the ends of the former become rounded and fit into depressions in the ends of the latter (Fig. 490).
Fig. 490. From section through wall of uterus and chorion of a pig. Showing close relationship between the epithelium of the uterus and that of the chorion. Photograph.
The allantois and allantoic vessels in the pig afford a good example of the transition from the respiratory and excretory functions which they almost exclusively possess in Reptiles and Birds, to the additional nutritional function of these vessels in Mammals. The allantoic sac becomes large and applies itself to the inner surface of the chorion, so that the blood vessels of the allantois also grow into and ramify in the mesodermal layer of the chorion. This brings the allantoic (umbilical) blood vessels containing the foetal blood closer to the uterine vessels containing the maternal blood. The two sets of vessels never come in contact, however, being always separated by the chorionic an.d uterine epithelium and also by some connective tissue of the chorion and of the uterine mucosa (Fig. 490). Food materials for the embryo must, therefore, pass through the connective tissue and the two epithelial layers in order to get from the maternal to the foetal blood; and waste products from the embryo must also pass through the same tissues to get from the foetal to the maternal blood. When the foetal membranes of the pig are expelled at birth, the rudimentary chorionic villi simply withdraw from their sockets in the uterine mucosa and the chorion is cast off, leaving the uterine mucosa intact.
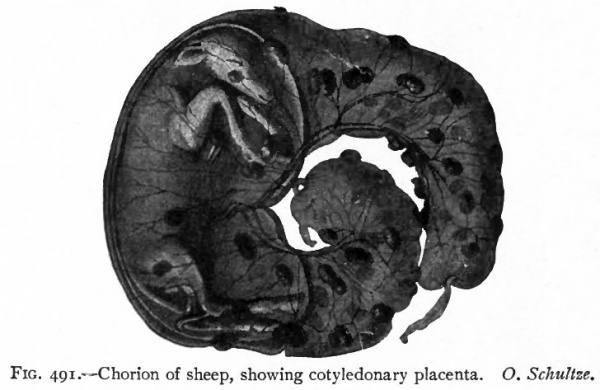
Fig. 491. Chorion of sheep, showing cotyledonary placenta. O. Schultze.
In other Mammals, the attachment of the chorion to the mucous membrane of the uterus is restricted to certain definite, highly specialized areas. This means that the villi which at first developed over the entire chorion, disappear from the greater part of it. Those villi which remain are limited to a definite area or areas and develop extensive arborizations. Moreover, they do not simply fit into depressions in the uterine mucosa, but become much more closely attached to it while the mucosa increases in thickness and in vascularity over the villous areas. There are thus formed two distinct though intimately associated parts of a structure which is known as the placenta the uterine part being designated the maternal placenta or placenta uterina, the foetal part the placenta foetalis. Such Mammals are grouped as Placentalia. In the sheep and cow a number of placentae multiple placenta are normally present (Fig. 104). In the dog and cat the placenta takes the shape of a band or a zone of specialized tissue encircling the germ vesicle. This is known as a zonular placenta. In man a single discoidal area develops discoidal placenta.
These different forms of placentae vary also in regard to the intimacy with which maternal and foetal parts are associated. Thus, for example, in the multiple placentae of the cow and sheep, the foetal placentae may be easily pulled away from the maternal placentae; while in the discoidal placenta of man, maternal and foetal parts are so closely related that both come away together as the after-birth or decidua.
The Foetal Membranes in Man
The foetal membranes in man are characterized by the early development of the amnion, the development of an extremely complicated discoidal placenta and the rudimentary condition of the yolk sac and allantois. The high development of the placenta the organ of interchange between foetal and maternal circulation is undoubtedly dependent upon the very long period of gestation during which the human foetus leads an entirely parasitic existence, being dependent wholly upon the mother for nutrition and respiration. The extensive development of the placenta in turn explains the rudimentary condition of the yolk sac and stalk and of the allantois, the nutritional and respiratory functions of these large and important organs in some of the lower animals, being in man taken up by the placenta.
The Amnion
In describing the development of the germ layers in the human embryo, comparisons were made between one of the youngest known human embryos that of Peters and the embryos of the bat and mole (p. 99). Reference to this description and to the figures shows that in the bat and mole the amnion is formed, not as in the chick and rabbit by dorsal foldings of the somatopleure and fusion of these folds, but in situ by a breaking down of some of the cells of the inner cell mass and consequent cavity formation. In Peters' embryo the amnion is already present as a closed cavity. The earlier stages in its formation are not known. As in the case of the germ layers, however, the appearances in sections are so closely similar as to suggest at least, that the human amnion is formed in the same manner as that of the bat and mole.
In Peters 7 ovum (Fig. 74), also in Bryce-Teacher's (Fig. 493), the amniotic cavity is seen already formed. It is roofed by a single layer of flat cells apparently analogous to the trophoderm of the bat (Fig. 59). As in the bat and chick this layer is continuous with the higher ectoderm of the embryo proper as represented here by the embryonic disk. The extraembryonic mesoderm is already present at this stage between the ectoderm of the amnion and the trophoderm, the epithelial cells of the latter being seen on the surface. Ventrally lies the yolk sac lined with entoderm, while laterally between the entoderm and ectoderm is seen the embryonic mesoderm. This formation of the amnion in situ considerably shortens the process of amnion formation as compared with that in most of the lower animals, where it is formed by dorsal foldings. This results in the very early formation of a complete amnion and amniotic cavity in such forms as the bat, mole and man.
The human amniotic cavity is at first small, the amnion covering only the dorsum of the embryo to which it is closely applied. The dorsal surface of the disk is at first concave, then flat, and later its margins curve ventrally as the flat disk becomes transformed into the definite shape of the embryonic body. As the margins of the disk bend ventrally they carry with them the attached amnion. As the embryo becomes constricted off from the yolk sac, the amnion is attached only ventrally in the region of the developing umbilical cord. With the exception of this attachment the embryo thus comes to lie free, floating in the amniotic fluid (Fig. 487, 6).
The amniotic cavity, at first small, increases rapidly in size and by the third month has reached the limits of the chorionic vesicle completely filling it. It then attaches itself loosely to the overlying chorion thus completely obliterating the extraembryonic body cavity. The amnion consists everywhere of two layers, an inner ectoderm, the cells of which are at first flat, later cuboidal or even columnar, and an outer layer of somatic mesoderm. At the dermal navel (p. 569) the amniotic ectoderm is continuous with the surface ectoderm (later epidermis) of the embryo. Some writers consider the fact that the epithelial covering of the umbilical cord is stratified as indicating that it is derived from embryonic ectoderm rather than from amniotic ectoderm, and describe the transition between the two as taking place not at the dermal umbilicus but at the attachment of the cord to the placenta. As in lower forms (p. 566) the walls of the amniotic cavity contain contractile elements which determine rhythmical contractions of the amnion.
The human amniotic fluid is a thin, watery fluid of slightly alkaline reaction containing about one per cent, of solids, chiefly urea, albumin and grapesugar. The origin of the fluid is not known. By some it is believed to be mainly a secretion of the maternal tissues, by others as largely of foetal origin. The urea it contains is probably excreted by the foetal kidneys.
When the amount of amniotic fluid is excessive the condition is known as hydramnios. If, as is sometimes the case, the amniotic fluid is present in very small amount, adhesions may form between the amnion and the embryo. These may result in malformations. With or without abnormality in the amount of amniotic fluid, bands of fibrous tissue may stretch across the cavity. If sufficiently strong these may produce such malformations as splitting of a lip or of the nose, or the partial or complete amputation of a limb.
In labor a portion of the amnion filled with fluid usually precedes the head through the cervical canal. It is rounded or conical, and becoming distended and tense with each uterine contraction or labor pain, serves as the natural and most efficient dilator of the cervix. When the cervix is partially or completely dilated, the amnion usually ruptures "rupture of the membranes" and all or a part of the amniotic fluid escapes as the "waters." Usually a varying amount of the fluid remains behind the embryo being kept there by the head completely corking the cervix. This escapes with the birth of the child. In some cases the amnion ruptures at the beginning of labor, before there has been any dilatation of the cervix. The dilating must then be done by the child's head or other presenting part. These are much less adapted to the purpose than the bag of membranes and the result is usually a difficult and protracted "dry" labor. Rarely the amnion fails to rupture during labor and the child is born within the intact bag of membranes. Such a child is said to be born with a "caul."
The Yolk Sac
In the human embryo the yolk sac is but a rudiment of the large and important organ found in some of the lower animals. It develops early and at the end of the second week is an almost spherical sac with a wide opening into the intestine (Fig. 85), there being but a slight constriction between the embryo and the yolk sac. During the third week the yolk sac becomes decidedly constricted off from the embryo, remaining connected, however, with the intestine by means of a long pedicle, the yolk stalk or vitelline duct (Fig. 87). As the placenta is formed, and at the same time the umbilical cord, the yolk sac becomes incorporated with the former, where it may sometimes be found by careful search after birth, while the yolk stalk becomes reduced to a strand of cells which traverses the entire length of the umbilical cord (p. 598).
Whatever function the rudimentary human yolk sac has, must be performed early, as both sac and stalk soon undergo regressive changes. Although no true yolk is present, the sac at first contains fluid and its thick outer mesodermal layer is the place of earliest blood and blood vessel formation. This would seem to indicate that like the larger yolk sac of lower animals, the human yolk sac serves temporarily as a blood-forming organ.
In about three per cent, of cases that portion of the yolk stalk which lies between the intestine and the umbilicus fails to degenerate, retaining its lumen and its connection with the intestine. It is then known as MeckeVs diverticulum and is of considerable surgical importance, as it may become invaginated into the small intestine and thus cause obstruction of the bowel. The blind end of the diverticulum may remain attached to the umbilicus, or it may become free, or in rare cases the stalk may retain a lumen from the intestine to the umbilicus, through which faeces may escape " faecal fistula." Occasionally a portion of the gut from which the yolk stalk is given off extends for a short distance into the cord. If, as is sometimes the case, this extension fails to retract before birth, a congenital umbilical hernia is the result (see Chap. XX).
The Allantois
The human allantois, while analogous to the allantois of Birds and Reptiles, shows certain marked peculiarities in its development, in its relation to surrounding structures and in its functions.
Its development is peculiar in that it does not push out, as, for example, in the chick, as an evagination from the primitive gut into the extraembryonic body cavity, for at the very early stage at which the human allantois first appears, the primitive gut is not as yet constricted off from the yolk sac and there is no extraembryonic body cavity into which the allantois can extend. It will be remembered that in the formation of the germ layers and in the development of the amnion the human embryo shows a marked tendency, as compared with lower forms, toward a shortening of the developmental process. This abbreviation and consequent very early formation applies also to the allantois. As the embryonic body assumes definite shape and the amnion is formed, -there is not the complete separation of amnion from the chorion seen, for example, in the chick, the embryo remaining connected posteriorly with the chorion by means of a short thick cord of mesodermic tissue. This is known as the belly stalk. Into this solid cord of mesodermic tissue which connects the embryo with the chorion, entodermic cells extend. These are derived from the embryonic entoderm before the constriction which differentiates the primitive gut from the yolk sac has made its appearance (Fig. 77). According to some there is a true evagination from the entodermic sac quite analogous to the evagination in the chick, resulting in a long slender tube lined by entoderm and extending from the embryo to the chorion. Others describe the entodermic outgrowth as a solid cord of cells. The mesodermic layer of the allantois is furnished by the mesoderm of the belly stalk. It is to be noted in this connection that the mesoderm of the belly stalk is embryonic mesoderm and that in Birds, for example, this portion of the mesoderm splits into two layers, somatic and splanchnic, with the extraembryonic body cavity between them. Into this extraembryonic body cavity the allantois extends. In man no such splitting occurs, so that there is no extraembryonic body cavity into which the allantois can extend. Instead, it grows out into the belly stalk.
The functions of the human allantois are somewhat different from those of the allantois of the chick. In the latter it is a direct respiratory organ in that it brings the embryo into relation with the outside air. In man the allantois, accompanied by the allantoic (umbilical) blood vessels, comes into relation with the placenta. As the placenta serves as the medium of exchange between foetal and maternal circulations, it acts as a modified organ of respiration. In the chick the allantoic cavity also serves for the reception of the excretions from the embryo, the allantoic fluid containing nitrogenous excretives. In man all such elimination is carried on through the placenta and there is consequently no need for the development of a large allantoic sac.
With development of the placenta, that part of the allantoic stalk which lies in the umbilical cord atrophies. Of the embryonic portion of the allantois, or the urachus, on the other hand, the proximal end communicates with the urinary bladder, while the remainder, which extends from the bladder to the umbilicus becomes transformed into a fibrous cord, the middle umbilical ligament (page 371). Rarely that portion of the allantoic stalk between the bladder and the umbilicus remains patent and opening upon the surface forms a "urinary fistula," allowing urine to escape.
In Reptiles and Birds the omphalomesenteric vessels, passing along the yolk stalk and ramifying in the mesodermal layer of the yolk sac, convey the nutrient materials of the yolk to the growing embryo. Since the allantois is an organ of respiration and excretion, the allantoic or umbilical vessels have nothing to do with the actual nourishment of the embryo (p. 191) . In Mammals the yolk sac is of less functional value. Consequently the vitelline vessels, although present (Fig. 162), play a less important role in conveying nutriment. The allantoic (umbilical) vessels, instead of ramifying in the wall of the allantois, as in the lower forms, come into connection with the chorion, passing primarily through the belly stalk. Since the chorion becomes the organ of interchange between the embryo and the mother, the allantoic vessels assume a new function, the allantoic (umbilical) vein carrying food material from the mother to the embryo, the arteries carrying waste products from the embryo to the mother. Thus in Mammals, as the yolk sac and vitelline vessels come to play a less important role in the nutrition of the embryo, the allantoic vessels, in connection with the chorion, become practically the only means by which the embryo receives its food-supply.
The Chorion and the Decidua
When the fertilized ovum reaches the uterus it becomes fixed or embedded In the uterine mucosa. Fixation usually occurs in the upper half of the uterus but may occur near the cervix. Rarely the ovum becomes fixed to the mucous membrane of the tube instead of to that of the uterus, and, developing there, gives rise to a "tubal" pregnancy one of the forms of extrauterine gestation.
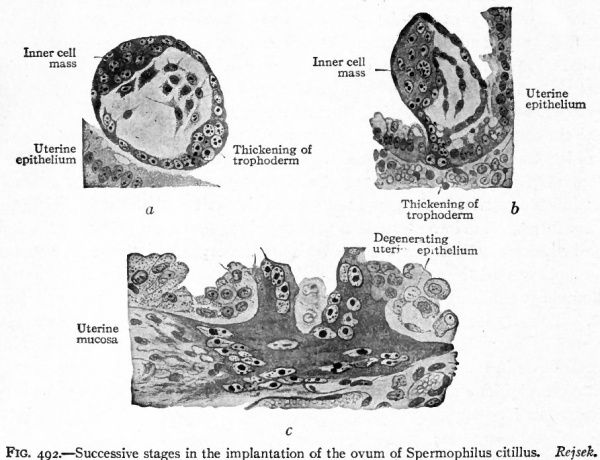
Until recently, it was believed that the ovum became attached to the surface of the mucous membrane. Recent studies upon some of the youngest human ova and upon those of some of the lower Mammals, however, seem to indicate that the ovum in some way pushes itself into buries itself in the uterine mucosa (Fig. 492). It is argued that if the ovum simply attaches itself to the surface of the mucosa, one would expect to find, for a time at least, epithelium between the attached surface and the stroma. In a very young human ovum no such epithelium was found and the ovum had the appearance of having penetrated the stroma by which it was surrounded (Fig. 493). Thus, for the first two weeks of gestation, the ovum lies embedded in the stroma of the uterine mucosa, giving so little surface indication of its presence that it is practically impossible to locate it except by serial sections of the entire mucosa. After two weeks the position of the ovum begins to be indicated by a slight prominence of the mucous membrane, the summit of the prominence being marked by an entrance plug consisting of coagulum, cast off cells and fibrin (Fig. 74). In the Bryce-Teacher ovum no such entrance plug was found (Fig. 493). At this stage the plug contains no glands or blood vessels. Later it becomes organized and replaced by connective tissue. Whatever the mode of fixation of the ovum to the uterus, there immediately result important changes in the uterine mucosa which lead to the formation of the decidua. These changes are both destructive and constructive. They are destructive in that the epithelial covering of the ovum, the trophoderm, has some solvent action on the uterine mucosa and breaks down the walls of the maternal blood vessels thus allowing the blood to flow around the ovum (Fig. 493). They are constructive in that they result in the formation of the decidua.
Fig. 492. Successive stages in the implantation of the ovum of Spermophilus citillus. Rejsek.
- a- Ovum (blastodermic vesicle) lying free in the uterine cavity, b, Later stage in which the syncytial knob (thickening of trophoderm) has penetrated the uterine epithelium as far as the basement membrane, c, Still later stage in which the trophoderm has penetrated the uterine stroma; the cells of the uterine epithelium at the point of entrance are degenerating.
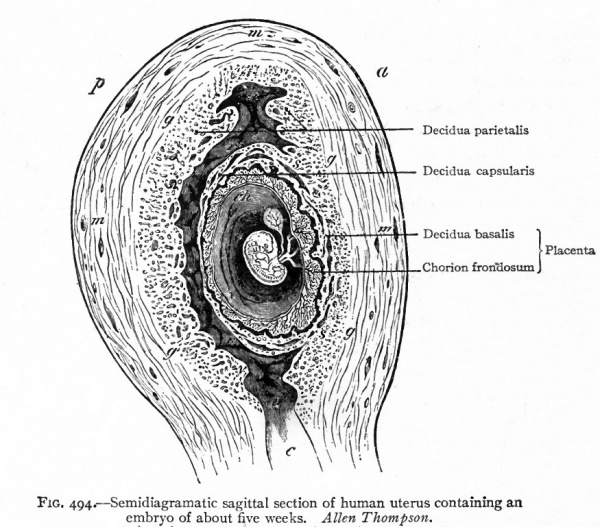
From their relation to the ovum and to the uterus, the deciduse (by which is meant the uterine mucosa of pregnancy) have been divided into the decidua parietalis or decidua vera t the decidua basalis or serotina, and the decidua cap sularis or reflexa.
cap., Capillary; cyt., cellular layer (cyto-trophoderm); ep., uterine epithelium; g/., uterine gland ; n. z., necrotic zone of decidua (uterine mucosa); P. e., point of entrance of the ovum; tro., syncytium (plasmodium, plasmodi-trophoderm); tro. 1 , masses of vacuolating syncytium invading capillaries. The cavity of the blastodermic vesicle is completely filled by mesoderm, and embedded therein are the amniotic. and entodermic (yolk) vesicles. The natural proportions of the several parts have been observed.
The decidua parietalis is the changed mucosa of the entire uterus with the exception of that portion to which the ovum is attached. The decidua basalis is that portion of the mucosa to which the ovum is attached and which later becomes the maternal part of the placenta. The decidua reflexa is either the extension of the mucosa over the ovum or that part of the mucosa under which the ovum buries itself (Fig. 494).
It will be remembered that surrounding the entire young ovum is the chorion and that this membrane consists of two layers, an outer ectoderm (trophoderm) and an inner mesoderm. In the youngest known human embryo the chorion is a shaggy membrane, its entire surface being covered with small projections or villi. Later these villi disappear from all of the chorion except that part of it which becomes attached to the uterine mucosa and forms the foetal part of the placenta. The latter is known as the chorion frondosum, while the smooth remainder of the chorion is known as the chorion laeve.
There are thus to be considered:
- The decidua parietalis.
- The decidua capsularis.
- The decidua basalis
- The chorion frondosum forming the placenta.
Fig. 494. Semidiagramatic sagittal section of human uterus containing an embryo of about five weeks. Allen Thompson.
- a, Ventral (anterior) surface; c, cervix uteri; ch, chorian; g, outer limit of decidua; m, muscularis; p, dorsal (posterior) surface.
The Decidua Parietalis
The changes in the uterine mucosa which result in the formation of the decidua parietalis are similar to, though more extensive than, the changes which take place during the earlier stages of menstruation. There is congestion of the stroma with proliferation of the connective tissue elements and increase in the length, breadth and tortuosity of the glands. These changes result as in menstruation in thickening of the mucosa so that at the height of its development the decidua parietalis has a thickness of . about i cm. It extends to the internal os where it ends abruptly, there being no decidua formed in the cervix.
In the superficial part of the mucosa the glands wholly or almost wholly disappear and their place is taken by the proliferating connective tissue of the stroma. The result is a layer of comparatively dense connective tissue the compact layer. Beneath this layer are found remains of the uterine glands in the shape of widely open, somewhat tortuous spaces which extend for the most part parallel to the muscularis. Some of these glandular remains retain part of their epithelium. Lying in the proliferating stroma, these spaces give to this layer the structure which has led to its being designated the spongy layer.
During the latter half of pregnancy the decidua parietalis becomes greatly thinned, due apparently to pressure from the growing embryo with its membranes. With this thinning, the few remaining glands of the compact layer disappear. The character of the spongy layer changes, the glands collapsing or being reduced to elongated, narrow spaces parallel to the muscularis. The entire tissue also becomes much less vascular than in early pregnancy.
If the foetal membranes are in situ the compact layer is in contact with the ectodermic (epithelial) layer of the chorion. Next to this lies the mesodermic (connective tissue) layer of the chorion. Delicate adhesions connect the mesodermic tissue of the chorion with the mesodermic layer of the amnion. Covering the latter is the amniotic ectoderm (epithelium).
The Decidua Capsularis
Early in its development this has essentially the same structure as the decidua parietalis. Its older or more common name, decidua reflexa, indicates the earlier idea that this portion of the decidua represents a growing around or reflection of the uterine mucosa upon the attached ovum. Peters, after examining the very early ovum which bears his name, came to the apparently warranted conclusion that instead of the uterine mucosa growing out around the ovum, the ovum buries itself in the mucosa, and that by the time the ovum had reached the size of the one he examined (i mm.), it was almost entirely covered over by the mucosa (Fig. 74). See also Fig. 493. In Peters' ovum a coagulum consisting of blood cells, other cast off cells and fibrin marked the point at which the ovum probably entered the stroma. Later this is replaced by connective tissue and for a considerable time the point is marked by an area of scar tissue.
By about the fifth month the rapidly growing embryo with its membranes has filled the uterine cavity, and the decidua capsularis, now a very thin transparent membrane, is everywhere pressed against the decidua parietalis. It ultimately either disappears (Minot) or blends with the decidua parietalis (Leopold, Bonnet).
The Decidua Basalis
As the decidua basalis is that part of the mucosa to which the chorion frondosum is attached, it is convenient to consider the two structures together.
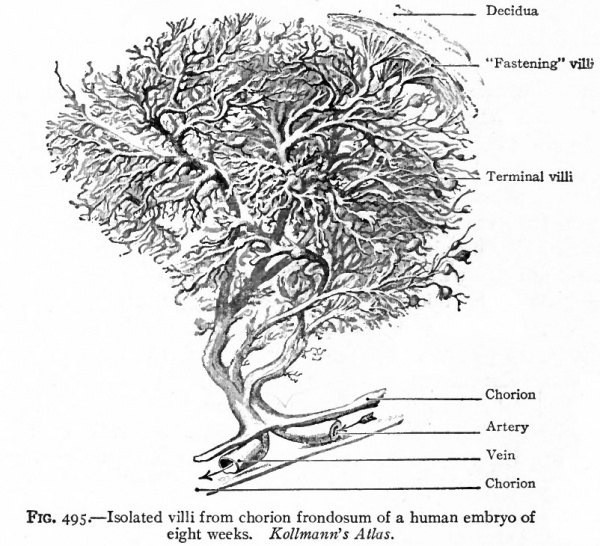
Fig. 495. Isolated villi from chorion frondosum of a human embryo of eight weeks. Kollmann's Atlas.
At a very early stage, villi develop over the entire surface of the chorion (Fig. 493). Very soon, however, the villi begin to increase in number and in size over the region of the attachment of the ovum and to disappear from the remainder of the chorion, thus leading to the already mentioned distinction between the chorion frondosum and the chorion laeve (p. 586) .
THE CHORION FRONDOSUM or foetal portion of the placenta consists of two layers which are not, however, sharply separated.
- The compact layer. This lies next to the amnion and consists of connective tissue. " At first the latter is of the more cellular embryonal type. Later it resembles adult fibrous tissue.
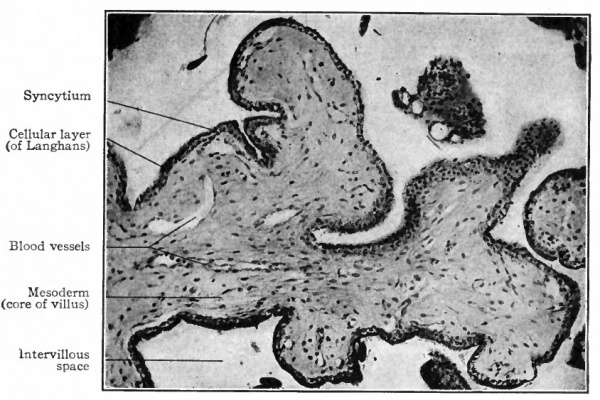
- The villous layer. The chorionic villi, when they first appear, are short simple projections from the epithelial layer of the chorion and consist wholly of epithelium. Very soon, however, two changes take place in these projections. They branch dichotomously giving rise to secondary and tertiary villi, forming tree-like structures (Fig. 495). At the same time mesoderm grows into each villus so that the central part of the originally solid epithelial villus is replaced by connective tissue, which thus forms a core or axis. This connective tissue core is at first free from blood vessels, but toward the end of the third week terminals of the umbilical (allantoic) vessels grow out into the connective tissue and the villus becomes vascular. Each villus now consists of a core of vascular mesodermic tissue (embryonal connective tissue) covered over by trophoderm (epithelium). At first the epithelium of the villus consists of distinctly outlined cells. Very soon, however, the epithelium shows a differentiation into two layers. The inner layer lying next to the mesoderm is called the layer of Langhans or cyto-trophoderm. Its cell boundaries are distinct and its nuclei frequently show mitosis. The outer covering layer consists of cells the bodies of which have fused to form a syncytium the syncytial layer or plasmoditrophoderm. This is a layer of densely stained protoplasm of uneven thickness (Figs. 496 and 497). It contains small nuclei which take a dark stain. As this layer is constantly growing, and as these nuclei do not show mitosis, it has been suggested that they probably multiply by direct division.
Fig. 496. Section of proximal end of villus from chorion frondosum of human embryo of two months. Photograph. In the space above the villus is a mass of cells such as are invariably found among or attached to the villi (see text, page 594).
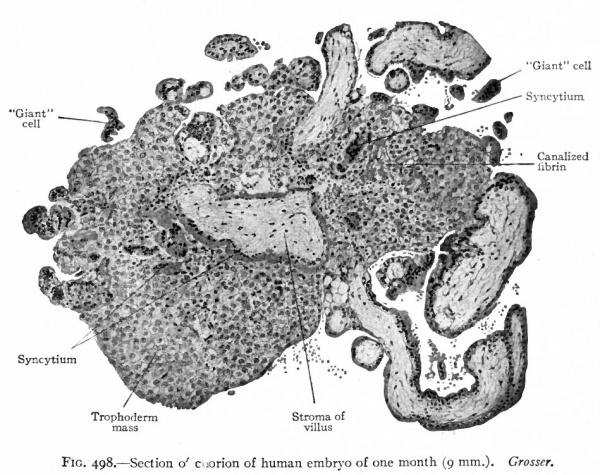
At an early stage large masses of cells appear among the villi, sometimes being attached to the villi (Figs. 496 and 498) . The origin of these masses is not known with certainty. They may represent thickenings of the syncytium in which the cell boundaries have reappeared, or they may represent outgrowths from Langhans' layer. In some cases the cells are small with darkly staining nuclei, in other cases large and homogeneous with large vesicular nuclei. Large multinuclear cells, or giant cells, with homogeneous cytoplasm, also appear. In some cases they apparently lie free in the intervillous spaces although it is claimed by some investigators that they merely represent sections of tips of the syncytial masses. A structure known as canalized fibrin (which takes a brilliant eosin stain) begins to develop in the earlier months of pregnancy and gradually increases in amount during the later stages. It is found in relation with the large cell masses among the villi and is probably a degeneration product of these masses.
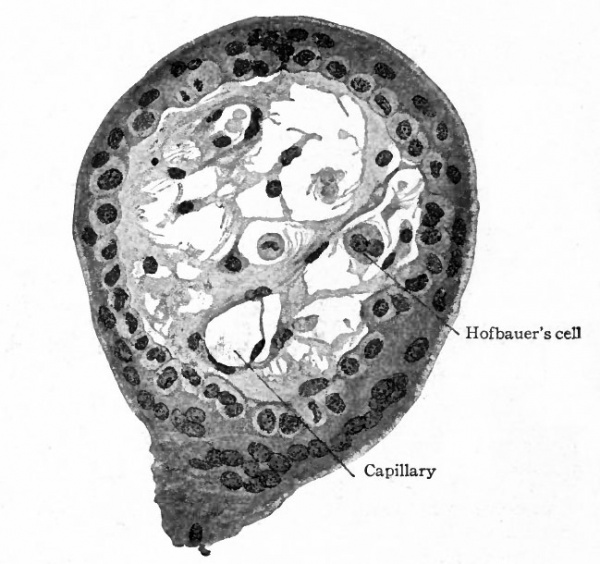
Fig. 497. Transverse section of chorion villus from human embryo of two months. Showing mesodermal core of villus and surrounding cellular layer (cyto-trophoderm) and syncytium (plasmodi-trophoderm). Hofbauer's cell is an example of large cells found in the villi, but the significance of which is not known. From retouched photograph. Grosser.
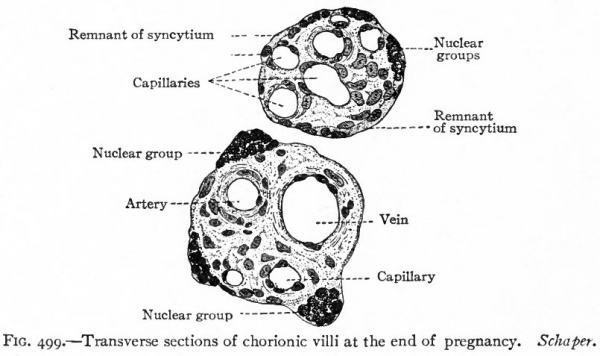
In the later months of pregnancy the covering layer of the villi loses its distinctly epithelial character, the cyto-trophoderm or cellular layer disappearing and the plasmodi-trophoderm or syncytial layer becoming reduced to a thin homogeneous membrane. At points in this membrane are knob-like projections composed of darkly staining nuclei. These are known as nuclear groups, or proliferation islands, and probably represent the proximal portions of the large cell masses already described (compare Figs. 497 and 499).
Certain of the uterine stroma cells increase greatly in size and become the decidual cells. These are large cells 30 to 100 microns and vary in shape. Late in pregnancy they acquire a brownish color and give this color to the superficial layer of the decidua parietalis. Each cell usually contains a single large nucleus. Some contain two or three nuclei. A few are frequently multinuclear.
Fig. 498. Section of chorion of human embryo of one month (9 mm). Grosser.
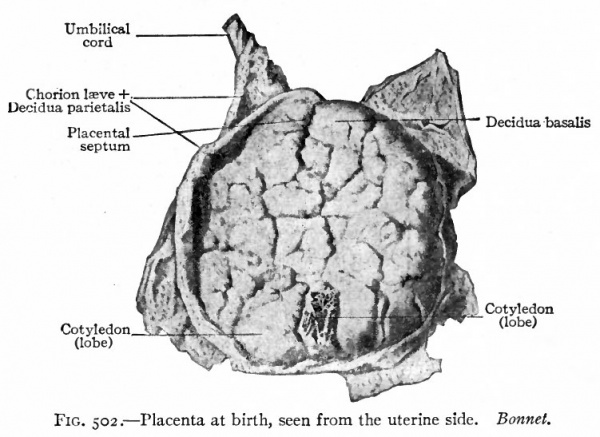
Some of the chorionic villi float freely in the blood spaces of the maternal placenta floating villi; others are attached to the maternal tissue fastening villi. The villi are separated into larger and smaller groups or lobules by the growth of connective tissue septa from the maternal placenta down into the decidua basalis. These are known as placental septa, while the groups of chorionic villi are known as cotyledons (Figs. 500 and 502).
Both decidual cells and chorionic villi are important from a diagnostic standpoint, as the finding of them in curettings or in a uterine discharge may be accepted as proof of pregnancy.
During the early months of pregnancy first four months the decidua basalis has essentially the same structure as the decidua parietalis. Its surface epithelium disappears very early, perhaps even before the attachment of the ovum. The glandular elements and the connective tissue undergo the same changes as in the decidua parietalis and here also result in the differentiation of a compact layer and a spongy layer. Both layers are much thinner than in the decidua parietalis.
As already noted, connective tissue septa pass from the superficial layer of the decidua basalis down into the foetal placenta subdividing the latter into cotyledons. At the margin of the placenta the decidua basalis passes over into the thicker decidua parietalis and here the chorion is firmly attached to the decidua basalis.
Fig. 499. Transverse sections of chorionic villi at the end of pregnancy. Schaper.
There still remains to be considered what may be called the border zone between the decidua basalis and the chorion frondosum. The whole purpose of the placenta is the interchange of materials between the maternal and foetal circulation. It is in the border zone that this interchange takes place. The entire structure of this zone is for this function, while all the rest of the placenta serves to transport the blood to and from this area. We have considered on the maternal side the structure of the superficial (compact) layer of the decidua basalis (p. 587) , on the foetal side the structures of the villous layer of the chorion frondosum (p. 588) . Unfortunately, this border zone has an extremely complicated structure which is difficult of interpretation in the usual microscopic section. This has led to much confusion in description and many differences of opinion as to actual structure. We can here consider only the more generally accepted facts, referring the student to special articles on the subject for further details.
In the fully developed placenta, the chorionic villi lie either free (floating villi) or attached to the decidua (fastening villi) in what are known as intervillous spaces (Fig. 500). In sections the villi are, on account of their structure, cut in all directions, many sections of villi being entirely free from their basal connections. The villi thus present the appearance of projections, peninsulas, or islands lying in spaces filled with blood (Fig. 501).
Fig. 501. Vertical section through wall of uterus and placenta in situ about seven months' development. Minot.
Branches from the arteries of the uterine muscularis enter the decidua basalis. They take very tortuous courses through the latter and in it lose their connective tissue and muscular coats, and, while of considerably larger diameter than most capillaries, become reduced to endothelial tubes. These follow the intervillous (placental) septa in which they branch and from which they finally open directly into the intervillous spaces along the edges of the cotyledons. The maternal blood is thus poured into the intervillous spaces at their periphery. After flowing through them it passes into veins which leave the intervillous spaces near the center of the cotyledons (Fig. 500).
Fig. 502. Placenta at birth, seen from the uterine side. Bonnet.
The relation of these spaces to the maternal blood vessels is not easy to make out in ordinary sections, but many observations have established the fact that both arteries and veins open directly into the spaces. The entire system of intervillous spaces may thus be considered as a part of, or an appendage to, the maternal vascular system, the maternal blood flowing from the arteries into these spaces and returning from these spaces to the mother through the veins. The foetal blood, on the other hand, circulates in the capillaries of the connective tissue of the villi separated from the maternal blood of the intervillous spaces by the epithelial villous covering already described (p. 589) . It is between the maternal blood of the intervillous spaces and the foetal blood in the villous capillaries that the interchange of material takes place. Both the maternal and foetal vascular systems are closed systems so that no blood can pass directly from mother to foetus or from foetus to mother. This can be absolutely proved in early pregnancy by the fact that nucleated red cells are at this stage constantly present in the blood of the foetiis but never normally present in the maternal circulation. The normal circulation of blood through spaces unlined by endothelium is such a remarkable exception in histology that repeated attempts have been made to demonstrate an endothelial lining to the intervillous spaces but, up to the present time, no such lining has been found.
The manner in which the intervillous spaces are formed still remains the subject of much controversy. The similarity of development in the human ovum and in the ovum of the bat has already been noted. In the bat the chorion when first formed consists of two thin layers, an inner mesodermal layer and an outer ectodermal layer (trophoderm). From analogy there is every reason to believe that the early human chorion has the same structure. Proof of this is, however, as yet wanting, as in the earliest human ova the trophoderm is already a thick layer. There are also present over the entire surface of the chorion and thus in contact not only with the future decidua basalis but also in contact with the entire future decidua capsularis, well developed villi, each consisting of a core of mesoderm and of a thick covering of trophoderm (Fig. 74). Between the villi, bounded by the villi and by the decidua, are ; pools of maternal blood. Peters suggested that rapid proliferation of the cells of the trophoderm might result in an opening up of the maternal vessels with which they came in contact and give rise to repeated effusions of maternal blood. This blood would be poured out mainly within the trophoderm but bounded externally by the decidua. The blood pools thus formed would represent the first stage in the formation of the intervillous spaces. According to Bonnet and others the chorionic villi of the developing placenta are constantly opening up new decidual vessels, the trophoderm eroding or dissolving more and more decidual tissue, so that the intervillous spaces are constantly increasing in size with growth of the placenta.
The placenta at birth is a discoid mass of tissue between 15 and 20 cm. in diameter, about 3 to 4 cm. thick and weighs from 500 to 1200 grms. As its area of attachment marks the point where the ovum becomes fixed to the uterine mucosa and as the point of fixation of the ovum varies, the placenta may be attached to any portion of the uterine wall. It is most frequently attached in the region of the fundus and more frequently to the posterior wall than to the anterior. If the fixation of the ovum is sufficiently low, the placenta may partly or completely close the internal os, thus giving rise to what is known as placenta praevia.
The Umbilical Cord. As the amnion grows and extends ventrally with the ventral bending of the embryonic disk, the yolk stalk and sac, now very much attenuated, become pressed against the cord of mesodermal tissue which connects the embryo with the chorion, and incorporated with it to form the umbilical cord (Figs. 81 and 82).
The umbilical cord thus consists of (Fig. 503) :
- Amnion. This is attached to the embryo at the navel. It is at first loosely connected with the underlying tissue of the cord so that it is easily peeled off; later it becomes firmly adherent. The epithelium of the amniotic covering of the cord is stratified and is described by some (Minot, McMurrich) as of embryonic ectodermic origin instead of as part of the amnion.
- What may be called the ground substance or substantia propria of the cord. This is an embryonic connective tissue often described as "mucous tissue." It consists of a soft gelatinous intercellular substance and irregular, branching stellate cells. On account of its consistency it has been called "Wharton's jelly."
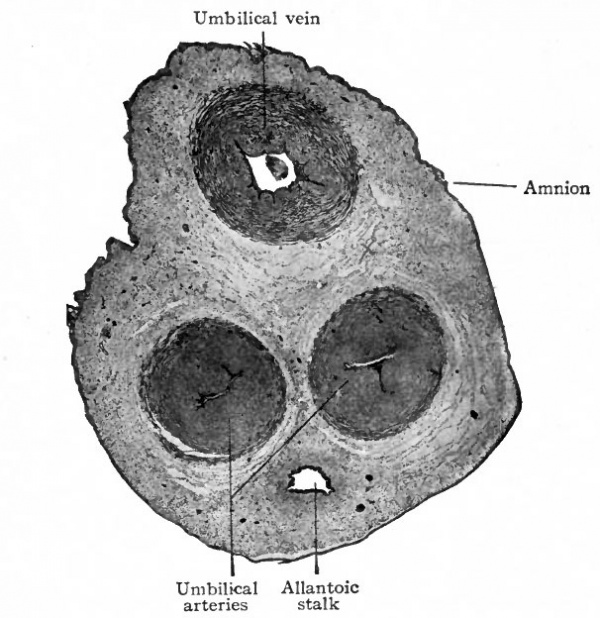
- Three umbilical vessels two arteries and one vein. All these vessels are thick walled and the developing smooth muscle is in bundles separated by considerable connective tissue. The two umbilical arteries carry venous blood from the foetus to the placenta where their branches ultimately give rise to the capillaries of the chorionic villi. From the villi the blood enters the terminals of the umbilical vein and returns as arterial blood to the foetus (Fig. 504). As they traverse the cord the arteries make a number of spiral turns around the vein and give to the cord the appearance of being spirally twisted. The cause of this twisting is not known. In places where the turns are quite abrupt and there are considerable accumulations of connective tissue, the cord has a knotted appearance. These points are known as false knots. Rarely the cord is actually tied into a more or less complex knot true knot probably due to movements of the foetus.
- Remnants of the allantoic stalk and of the yolk stalk. These, if present, are continuous or broken cords of epithelial cells. Rarely one or the other may retain its lumen or some of the yolk stalk vessels may remain.
As the yolk stalk is carried around to be incorporated as part of the umbilical cord there is enclosed with it a small part of the extraembryonic body cavity.
Fig. 503. Transverse section of umbilical cord of a pig embryo six inches in length. Photograph. The human umbilical cord averages 50 cm. in length and has a diameter of about 1.5 cm.
The Expulsion of the Placenta and Membranes
After the birth of the child, the uterine contractions usually cease temporarily and the uterine walls remain contracted around the placenta. In the course of a few moments the uterine contractions are resumed and the placenta and membranes are expelled as the after -birth.
The line of separation of the placenta and of the decidua parietalis from the uterine mucosa is through the deeper part of the spongy layer (Fig. 500). By this separation many blood vessels are opened, the hemorrhage being controlled by the firm contractions of the uterine muscle. The condition of the uterine mucosa, after child-birth, has been described as an exaggeration of its condition at the end of menstruation. Reconstruction of the mucosa takes place by proliferation of the still remaining connective tissue and of the glandular elements,
Anomalies
The manner in which the placenta is formed by excessive development of the decidua and chorion over a limited area and atrophy of the chorion throughout the remainder of its extent suggests the most frequent variations from the normal.
The villi instead of developing over the usual discoid al area may develop along a band-like area which more or less completely encircles the chorion. This gives rise to an annular placenta similar to that seen in the Garnivora. Continued development of the villi over the entire chorion may occur. This results in a thin "placenta membranacea." Such a placenta is apt to be adherent and may thus cause a serioiu postpartum condition. Failure of the villi to atrophy and their continued development over more than a single area give rise to variations in form and number of placenta. When there are two not very distinctly separated areas the condition is known as placenta bipartita. Two completely separated placentae with distinct branchings of the umbilical vessels to supply them are known as placenta duplex. Placenta triplex and up to placenta septuplex have been described. When one or more placental lobules develop at a little distance from the main placental mass but connected with the latter by blood vessels, the result is the not uncommon placenta succenturiata. Placenta spuria is applied to such an accessory lobule when it has no vascular connection with the main placenta and consequently no function.
Anomalies of the placenta associated with multiple pregnancies and with anomalies of the foetus will be found under their respective heads.
Anomalies of the cord are for the most part dependent upon anomalies of the foetus and of the placenta.
- Next: Teratogenesis
References for Further Study
BENEKE: Sehr junges menschliches Ei. Monatsschr. f. Geburtshilfe u. Gyndkologie, Bd. XXII, 1904.
BONNET, R.t Lehrbuch der Entwickelungsgeschichte des Menschen. Berlin, 1907.
BRYCE, T. H.: Embryology. In Quain's Anatomy, nth ed., Vol. I, 1908.
BRYCE, T. H., and TEACHER, J. H.: An Early Ovum Imbedded in the Decidua. Glasgow, 1908.
CRAGIN, E. B.: Text-book of Obstetrics. 1915.
FRASSI, L.: Uber ein junges menschliches Ei in situ. Arch.f. mik. Anat., Bd. LXX, 1907.
GROSSER, O.: Die Eihaute und der Placenta. 1908.
HERTWIG, O.: Lehrbuch der Entwickelungsgeschichte des Menschen und der Wirbeltiere. Berlin, 1906.
HOFBAUER, J.: Biologic der menschlichen Placenta. Wien and Leipzig, 1905.
HUBRECHT, A. A. W.: Placentation of Erinaceus Europaeus. Quart, Jour, of Mic.Sci., Vol. XXX, 1889.
VON HUEKELOM, S. : Ueber die menschliche Placentation. Archiv. fur Anat. und Physiol., Anat, Abth., 1898.
KEIBEL, F., and MALL, F. P: Manual of Human Embryology. Vol. I, 1910.
KOLLMANN, J.: Lehrbuch der Entwickelungsgeschichte des Menschen. Jena, 1898.
KOLLMANN, J.: Handatlas der Entwickelungsgeschichte des Menschen. Bd. I, 1907.
LEOPOLD, G.: Ueber ein sehr junges menschliches Ei in situ. Leipzig, 1906.
MARCHAND, F.: Beobachtungen an jungen menschlichen Eiern. Anat. Hefte, Bd. XXL 1903.
McMuRRiCH, J. P.: The Development of the Human Body. Philadelphia, 1907.
MINOT, C. S.: Uterus and Embryo. Jour, of Morphol., Vol. II, 1889.
Minot CS. A Laboratory Text-Book Of Embryology. (1903) Philadelphia:P. Blakiston's Son & Co.
PETERS, H.: Ueber die Einbettung des menschlichen Eies und das frUheste bishei bekannte menschliche Placentationsstadium. Leipzig, 1899.
MERTTENS, J.: Beitrage zur normalen und pathologischen Anatomic der menschlichen Placenta. Zeitschr. f. Geburtshilfe u. Gynakologie, Bd. XXX, XXXI, 1894.
REJSEK, J.: Anheftung (Implantation) des Saugetiereies an die Uteruswand, insbesondere des Eies von Spermophilus citillus. Arch. f. mik. Anat., Bd. LXIII, 1904.
Rossi DORIA, T.: Ueber die Einbettung des menschlichen Eies, studirt an einem kleinen Eie der zweiten Woche. Arch. f. Gynak., Bd. LXXVI, 1905.
SELENKA, E.: Studien iiber die Entwickelungsgeschichte der Tiere; (Menschenaffen) . Wiesbaden, 1901-1906. Parts 8- 10.
STRAHL, H. : Die Embryonalhiillen der Sauger und die Placenta. In Hertwig's Handbuch der vergleich. u. experiment, Entwickelungslehre der Wirbeltiere. Bd. I, Tei) II, 1902.
WEBSTER, J. C.. Human Placentation. Chicago, 1901,
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
Text-Book of Embryology: Germ cells | Maturation | Fertilization | Amphioxus | Frog | Chick | Mammalian | External body form | Connective tissues and skeletal | Vascular | Muscular | Alimentary tube and organs | Respiratory | Coelom, Diaphragm and Mesenteries | Urogenital | Integumentary | Nervous System | Special Sense | Foetal Membranes | Teratogenesis | Figures
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2024, April 28) Embryology Book - Text-Book of Embryology 19. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Book_-_Text-Book_of_Embryology_19
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G