2010 Group Project 3
Amniocentesis
- Links: Amniocentesis
Introduction
Amniocentesis is the process by which a thin needle is inserted through a womens abdomen into her womb, a sample of the amniotic fluid surrounding her growing fetus is taken out and analysed to acquire information about the baby's health.
This procedure is an important development in the technologies of prenatal diagnostic techniques as it allows parents an insight into possible diseases and complications that their baby might develop before birth at an early stage of its development. Since chromosomal abnormalities occur in 0.1%-0.2%[1] of live births, and the most common abnormality being Down syndrome, amniocentesis provides an opportunity for parents to make informed decisions about their pregnancy and gives them time to consider a number of options.
Although it is a very useful technique in diagnosing up to several hundred fetal complications there are also many risks associated with this invasive method of extraction of fetal cells as well as ethical issues concerning positive results of abnormalities and the possible use of stem cells.
It is shown to be a highly accurate technique but some women may be uncertain about the procedure due to the number of risks and may choose other diagnostic techniques such as chorionic villus sampling.
Advantages and disadvantages of Amniocentesis
| Advantages | Disadvantages |
|---|---|
| Gives the baby's sex.
Lower risk of miscarriage than CVS. Highly accurate, >99.4%.[2] Can detect several hundred disorders. Able to detect chromosomal as well as neural tube defects. Able to detect if the babies lungs will be mature enough for birth, by measuring the amount of surfactant and phospholipids in the amniotic fluid.[3] |
Risk of miscarriage.
Numerous other risks including infection, harm to the baby during procedure, and anxiety. Not 100% accurate, there is still a small chance of a false result, false positive or negative for a disorder can lead to undesirable outcomes. May be seen as an invasive procedure, whereas ultrasound is not. |
Historic Background
The removal of amniotic fluid using a needle has been recorded as early as 1930 by Menees, however it was not until 20 years later that the process was actually used in a diagnostic sense. The development of safe and effective techniques of the procedure took many years to evolve, including the necessary technologies to come to par such as ultrasound and chromosome mapping that are key features in ensuring safety and effectiveness in diagnosing the fetus.
This progress could not have been achieved without the work of several key scientists:
Douglas Bevis
Bevis's study of amniocentesis and hemolytic disease was considered a landmark event making the procedure wide spread and launching more interest and research into its possibilities. In February 1952 he published his study in the Lancet, The Antenatal Prediction of Hemolytic Disease of the Newborn[4] which was a revolutionary article at the time. Bevis experimented with the mixing of Rh+ and Rh- blood and what occurs when there is a clash between the mothers Rh factor and her fetus's Rh factor. This results in hemolytic disease, a disease in newborns where the mothers antibodies attack the fetal red blood cells when the antibodies cross the placenta where they are supposed to strengthen the babies immune system.[5][6] The rhesus factor is present and able to be identified in the fetus from the sixth week of development onwards.[7] The possibility of diagnosing this disorder prenatally was a critical discovery and paved the way for the discovery of hundreds of other disorders which are able to be prenatally diagnosed.
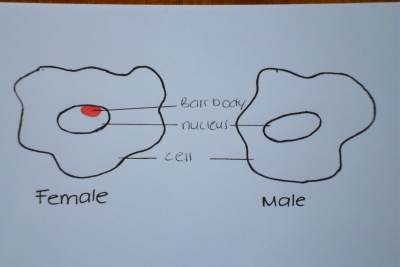
Murray Barr and Ewart Bartram
In the late 1940 's these two scientists began working together studying human sex cells. They discovered in 1949 that the sex of a fetus could be found by identifying an extra chromatin body which lies at the periphery of only female cells, named the Barr body, which is visible under the microscope. The significance of this in relation to amniocentesis as a diagnostic technique is that the possibility of sex linked diseases such as hemophilia, a blood clotting disorder, could be found out prenatally. Murray Barr explains the presence of the chromatin body in his paper Prenatal Sex Determination written in 1956[8], however he comments that puncturing the abdomen and uterus of the mother is not worth the associated risks just to determine the sex for the parents curiosity. The associated sex linked diseases which could be more accurately diagnosed prenatally from amniocentesis gave the procedure a truly valuable purpose in giving parents more insight into their babies health and more options in terms of treatment and termination. Until their discovery parents were given statistics based on their family histories of sex linked diseases which were nothing more than loose probability. With the sex of the baby able to be determined more reliable statistics were provided and doctors were able to lay out more options for parents in difficult situations.
- Case Study: A case study illustrating the use of sex chromatin analysis in prenatal diagnosis was carried out in 1971 by Ferguson-Smith, Nevin and Stone[9]. In a study of 30 pregnant women, one mother was a carrier of granulomatous disease, a sex linked disease carried on the X chromosome, giving a male fetus a 50% chance of being affected. Sex chromatin analysis showed no presence of a Barr body on the fetal cells, the fetus was thus concluded to be male and the decision was made to terminate the pregnancy 2 days after the amniocentesis procedure. Further chromosome analysis of the amniotic fluid showed an abnormal karyotype resulting in the baby actually having Down syndrome, therefore prenatal sex determination was proved successful, all due to the work of Barr and Bartram.
Steel and Breg
In 1966 Steel and Breg worked together studying the analysis of amniotic fluid. They were the first to successfully culture the cells from amniotic fluid and create a karyotype or chromosome mapping of the DNA of the fetus to study its genetic make up. Their study published in the Lancet in 1966, Chromosome analysis of human amniotic-fluid cells[10], was a major discovery in the progression of amniocentesis as they proved that chromosomal abnormalities are able to be prenatally diagnosed by creating a karyotype of fetus's DNA.
Timeline for key events in development of Amniocentesis
| Year | Key developments |
|---|---|
| 1877 | Transabdominal amniocentesis recorded in literature by Prochownick, Von Schatz and Lambl. |
| 1919 | Scientist named Hinkel reported the release of amniotic fluid from a pregnant patient suffering from polyhydramnios (The condition of having too much amniotic fluid in the womb). |
| 1930 | Reported removal of amniotic fluid using a needle by Menees. |
| 1941 | Levine, Katzin and Burham showed that the RhD antigen could cause hemolytic disease and anemia in the child of a pregnant woman.[11] |
| 1949 | Murray Barr and Ewart Bartram discovered the presence of a chromatin body on the periphery of female cells that could be used to identify the sex of a fetus. |
| 1952 | Bevis's study "The Antenatal Prediction of Hemolytic Disease of the Newborn" was published, landmark event in launching the procedure's credibility, interest and use. |
| 1956 | Fuchs and Riis also reported in an article in "Nature" that they could determine the sex of the fetus by the presence of the Barr or chromatin body from cells of the amniotic fluid. |
| 1966 | Steel and Breg were the first to create a fetal karyotype from cultured amniotic fetal cells. This work was published in the Lancet, "Chromosome analysis of human amniotic-fluid cells". |
| 1968 | Nadler reported the first diagnosis of Trisomy 21. |
| 1972 | Brock and Sutcliffe discovered neural tube defects could be diagnosed due to the presence of increased amounts of alpha-fetoprotein (AFP) in the amniotic fluid. |
| 1970 | Nadler and Gerbie published the article "Role of amniocentesis in the intra-uterine diagnosis of genetic defects". From this time onwards, laboratories specializing in the analysis of amniotic fluid, including culturing and karyotyping were widespread and the procedure had become very well known and used. |
| 1972 | Use of ultrasound to guide the needle was reported by Jens Bang and Allen Northeved. Prior to this amniocentesis was done blind with no visual guidance. |
| 1988 | The Benacerraf group reported early amniocentesis procedures being performed (early amniocentesis occurs during the first trimester, to be explained below) however the higher fetal loss rate made this procedure less common and second trimester amniocentesis is now widely used. |
Procedure
WHO IS ELIGIBLE FOR THE TEST?
Since the procedure is invasive and not without a number of risks, the mother is tested before to determine whether she is at a higher risk than average of having a child with a chromosomal or neural tube defect. These tests include:
- Checking for abnormal ultrasound features.
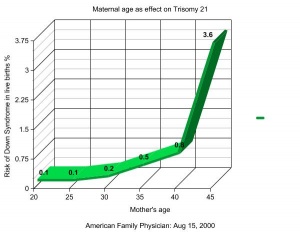
- Maternal age is considered, women older than 35 are at a higher risk of having children with chromosomal disorders.[13]
- History of chromosomal disorders in the family, testing if the parents are Rh positive or Rh negative.[14]
- History of previous children born with a genetic defect.
- Testing if the mother is a carrier of any X-linked diseases, if so the child may be at an increased risk of inheriting the disorder.
- If the mother is taking certain anti-seizure medications e.g. valproic acid or carbamazipine, this may put the fetus at a higher risk for spina bifida.[15]
WHEN CAN THE TEST BE TAKEN?
An amniocentesis test is most commonly performed between weeks 15 to 16 of gestation which is during the second trimester of pregnancy, however it is able to be performed anytime between weeks 14 to 20. Amniocentesis during the third trimester is strongly avoided as there is a high risk of inducing an early labor from premature rupture of membranes within the womb.[16]. Second trimester procedures are preferred over an early amniocentesis which is performed in the first trimester during weeks 11 to 14 as the earlier the test is performed the higher the risk of miscarriage, as much as 3 times greater. The reason an early amniocentesis option is offered to women is since the delay in tissue analysis of about 2 weeks means if an abortion is required it is physically and emotionally easier earlier on in the pregnancy. If an early diagnostic test is requested Chorionic Villus Sampling (CVS) is suggested as it carries less risk of miscarriage and complications than amniocentesis in the first trimester.[17]
STEPS OF THE PROCEDURE
Preparation
- Counseling for the mother is provided to investigate any family history of genetic disorders or any birth defects in previous children. The mother is given an assessment[18] on whether this procedure will benefit her based on her history and likelihood of baring a child with a fetal abnormality. She is also informed of the details and risks of the procedure and is able to make an informed decision to continue or not based on anxiety towards an invasive and risky procedure. [19][20]
- The next step is cleaning of the area before needle insertion with an iodine solution to ensure no contamination.
- Local anesthetic may be administered but since there is little pain it is usually omitted to avoid a second needle insertion.
Needle Insertion
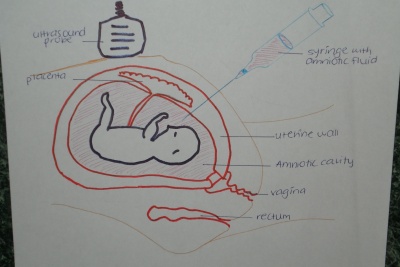
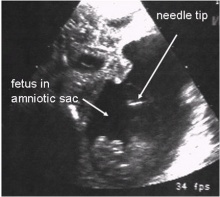
- The 20-22 gauge needle is inserted and 15 mL of amniotic fluid is sampled, it takes approximately 30 seconds to withdraw the fluid.[21]
- Before the needle is inserted and whilst the needle is inside the uterus ultrasound is used continuously to ensure no harm is caused to the baby or placenta.
- After the fluid is sampled the baby's heart beat is checked to ensure there was no harm.
The Amniotic Fluid
- The fluid removed contains fetal cells shed from the skin, and the lining of the gastrointestinal and respiratory tracts.
- These cells which will contain the DNA of the fetus is sent to a lab where it is cultured and the chromosomes are able to be mapped to investigate any chromosomal defects. Protein levels are also analyzed to investigate any neural tube defects.[22]
In the Lab
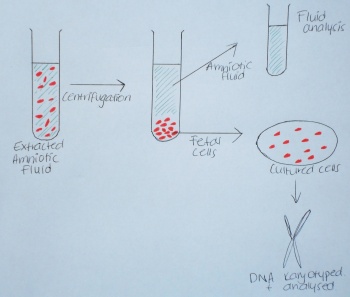
- In the lab the amniotic fluid sample is centrifuged to separate the fetal cells from the amniotic fluid containing lipids, proteins, glucose and electrolytes.
- After it is centrifuged the bottom half of the separated fluid containing the fetal cells is immersed in a culture medium designed to maximize colony growth of the embryonic cells. The cells are able to grow and then stored in an incubator until the time comes to harvest them.[23]
- The harvested cells are arrested in the metaphase stage of cell division which is the time of most condensation of the DNA. This means the chromosomes are able to be seen under a microscope after correctly dyed.
- The chromosomes are stained with certain chemicals that bind to the DNA to create a characteristic and recognizable banding pattern that will be different for the varying molecular structures of the chromosomes.
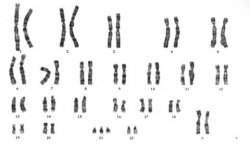
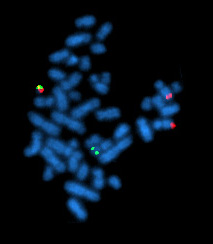
Creating a Karyotype
- The standard way of observing the stained chromosomes is by creating a karyotype.[23] A karyotype is a photograph taken of the chromosomes arranged from largest to smallest non-sex chromosomes (autosomal) numbered 1 to 22, with the 2 sex chromosomes placed at the end. This allows analysis to be done efficiently and any structural abnormalities to be clearly identified.
- Structural abnormalities that may be indicative of a genetic disorder include chromosome deletions, duplications, translocations and inversions.
Risks
There are risks involved in the procedure of amniocentesis. The steps that are attributed to the most risks include the insertion of the needle and withdrawal of amniotic fliud. However, there are other risks that must be considered, such as the mental health of the mother. The insertion of the needle and then withdrawing fluid is the most intrusive part of the procedure and problems can arise from this such as infection and hitting the feotus with the needle. Consequences include abnormal growth, injuring the mother or even miscarriage.
Miscarriage
There is a chance, however small, of a miscarriage. As stated before, only certain people should undergo the procedure because the procedure carries with it certain risks. Compared with Chronic Villus Sampling (CVS), the chance of a miscarriage is greater when amniocentesis is chosen for prenatal diagnosis. This is because CVS gives the option of needle insertion through the vaginal opening. [24]
A study undertaken dealt with specifically the rate of miscarriage due to amniocentesis being performed on the mother. It indicated that the number of pregnancies ending in miscarriage due to amniocentesis is as low as 1 in 1600.[25] Also, the number of stillbirths at 32 and 35 weeks is 3%.[26]
This sample space will differ slightly against the actual percentages of the population. What can be observed is that the leading reasons for amniocentesis are to find the chromosomal abnormality indicating Down Syndrome and at the request of the mother, possibly due to known family history.
The guidelines from the procedure section allow for properly measuring the risk of the procedure against knowledge that can be gained from it. A doctor or genetic counsellor should be consulted prior to the test.
Timing
Amniocentesis should be performed at a time when enough amount amniotic fluid can be taken for diagnosis without affecting the course of the pregnancy. Generally, this is around weeks 15 -19. As indicated under the procedure section. [15]
Sometimes there might not be enough amitotic fluid available to take for analysis. In this instance, more amniotic fluid may be taken at a later date.
Infection
There is a risk of infection with amniocentesis. It is intrusive as it requires contact to be made with the amniotic fluid by a needle through the skin, abdomen and uterine wall. The transfer of bacteria can occur if the needle and area of needle insertion (lower abdomen of the woman) are not properly sterile.
This risk is lowered by all persons involved in the procedure properly washing their hands and working in a sterile environment. Swabbing the area where the needle is inserted is the most common method of keeping these areas clean. [15]
This also leads to the possibility of transfer of blood plasma from the foetus to the mother. This can cause major issues in the instance where the rhesus (Rh) factor of the baby is positive and the mother is negative. The mother may develop antibodies to Rh factor due to blood transfusion or from a previous birth. The mother's antibodies can diffuse through the placenta and cause red blood cell agglutination. This has devastating effects on the development of the foetus. [27]
It is acceptable to use a needle for pain management of the mother during the procedure. The use of two needles generally increases the risk of infection, so it is more commonly practiced that the mother endures with the discomfort.
Use of Ultrasound
Ultrasound is used to show the foetus growing inside the uterus of the mother. The chance of the needle hitting the developing foetus is a risk factor. This can be avoided by constantly using ultrasound to see the position of the developing foetus. The constant vision allows for withdrawing fluid without physically hitting the foetus. It also gives the doctor confidence and reassurance that the needle is in the right place and not causing risk problems.
It also allows the doctor to see that the needle has entered the right area. This reduces the chance of the needle hitting the foetus. Futhermore, it reduces the occurrence of piercing other organs such as the bowel, which could lead to infection of the uterus, internal bleeding and other complications which could lead to miscarriage.
Psychological Risks
The emotional state of the mother can have affects on the outcome of the pregnancy. During pregnancy, hormone levels in the mother change to maintain the pregnancy, suppressing the degeneration of the endometrial lining which leads to menstruation.
The invasive nature of amniocentesis can invoke conditions such as anxiety in the mother. This can also be brought on by the results of amniocentesis coming back with undesirable results, leading to anxiety and depression.
Cortisol is a hormone produced in the adrenal gland that is triggered by emotional stressors. If it is produced excessively in the mother, some of the hormones can reach the foetus in the uterus. If this occurs there might be developmental effects on the foetus. [28] These include:
• Asymmetry of coordination of fingers, elbows and others
• Mental development
• Imperfections in nervous system
• Decreased perception, thinking and memory abilities
The presence of a partner during the pregnancy has been found to reduce the stress levels of the mother. However, the elevated anxiety levels brought on by amniocentesis were found not to have the same effect. [29] Therefore health care professionals must monitor the mother closely for signs of anxiety and help them better cope with the situation to reduce this.
Disorders Detected
There are many disorders that can be found from amniocentesis. These can be either chromosomal abnormalities or neural tube defects. The amniotic fluid sample taken during amniocentesis is centrifuged so the cells are separated from the plasma. From these cells, the chromosomes are able to be observed and from this, chromosomal abnormalities can be seen.
Chromosomal Abnormalities
The genetic disorders are diagnosed using karyotyping. Karyotyping from amniocentesis is taking the cells present in the amniotic fluid sample and mapping out the chromosomes in them. In a human, the normal number of chromosomes is 22 pairs of autosomal chromosomes and 1 pair of sex chromosomes. Doctors looking at these can compare the karyotype of a foetus against the normal karyotype for humans, and diagnose disorders based on these.
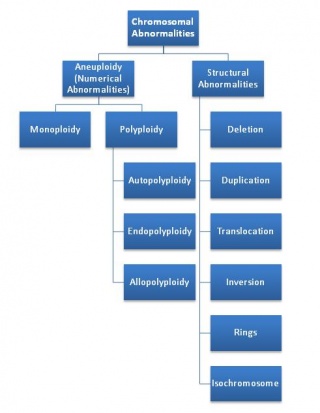
These abnormalities arise from errors during either mitosis or meiosis during reproduction. They can be broken down into sub categories based on how the chromosomes are different to a usual set.
Aneuploidy
These are the abnormalities where there are an atypical number of chromosomes. There may be the addition or subtraction of a chromosome from any of the chromosome pairs, resulting in an overall net difference between this set and the normal number of chromosomes. They can be divided into 3 categories based on these differences. Relatively common are disorders monoploidy and polyploidy abnormalities. The 3rd instance is the normal case, diploidy.
The instance where a chromosome is missing from a pair is called monoploidy. This can occur on any of the chromosomal pairs and the effects on development depend on which set it is present. A common example is Turner’s syndrome, where a sex chromosome is completely missing. [30]
Amniocentesis aims to find out whether disorders such as this are present in a foetus, so decisions can be made about whether to go ahead with the pregnancy. Polyploidy occurs when there is an increase in the number of chromosomes in a pair. This is the case in Down Syndrome, where there is an extra chromosome on the 21st pair.
Aside from these, the diploidy case is not abnormal, as this occurs when a pair contains two complete sets of chromosomes. As this leads to normal development, these foetuses do not have a numerical abnormality.
Structural abnormalities
Chromosomes are made up of genetic material. Structural defects occur when the size and shape of the chromosome itself is altered. This takes place when changes occur in the sequencing on the genetic material that makes up the chromosomes. The physical placement of genetic material can be altered through such things as deletions, duplications and translocations of base pairs within the sequences of DNA. Changing in the sequence of the DNA within these chromosomes leads to fundamental changes in the growth of the foetus.
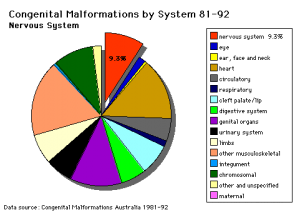
Neural Tube Defects
Neural tube defects arise from abnormal closure of the neural tube during foetal development. They are the most common, occurring in about 1 in every 2000 births. [31] They account for several birth disorders that can be pre-diagnosed using the amniotic fluid. In normal foetal development, the neural tube closes at the cranial and cordial neuropores at either end. The tube should be completely closed at stage 13 (week 4) of foetal development. If this doesn’t happen, any number of problems can arise depending on the type of non closure that occurred. Taking folic acid before conception decreases the reccurance and occurance of NTDs. [32]
Prenatal testing for this involves screening for levels of alpha fetoprotein. This indicates whether complications will rise due to the incomplete closure of the neural tube. Amniocentesis for these types of disorders can be carried out if there are found to be suspect ultrasound readings. [33] AFP levels are generally increased when the neural tube is open but lower than normal with Down Syndrome.
The followling table shows some of the most commonly occuring chromosomal abnormalities and NTDs.
Disorders Detected using Amniocentesis
| Disorder | Cause | Main Caracteristics | Occurence per Birth |
| Down Syndrome (trisomy 21) | The presence of all or a part an extra 21st chromosome | Mental retardation
Abnormal musculoskeletal development Particular set of facial characteristics. |
1 per 1000 |
| Edwards Syndrome (trisomy 18) | The presence of all or a part an extra 18st chromosome | Kidney malformation
Structural heart defects Intestines outside body Mental retardation |
1 per 3000[34] |
| Turner syndrome (monosomy X) | This is several different conditions, the most common is that an entire sex chromosome is missing | Short stature
Swelling in limbs Webbed necks Low set ears |
1 per 2000 females[35] |
| Spina Bifida | Incomplete closure of the embryonic neural tube at the chorionic end | Baby born with protruding spinal chord
Different types, occulta is the mildest |
1-2 per 1000 |
| Anencephaly | Failure of neural tube closure at the cranial end | No forebrain
Baby born blind, deaf and unconscious |
1-5 per 1000[35] |
| Fragile X | Faulty X chromosome resulting in failure to express an essential protein for normal neural development from the FMR1 gen | Prominent ears, long face, low muscle tone, atypical social behaviour | males: 1 per 3600
females: 1 per 5000[36] |
Diagnostic Accuracy
A correctly gathered amniotic fluid sample of sufficient quantity generally contains shed skin, lung and bladder cells from only the foetus, and therefore should give genetic information pertinant to that foetus alone. Photographic evidence of the chromosomal arrangement allows future double checking of interpretation. Accuracy of traditional karyoptyping has been placed at above 99.4% [37]. Some studies place this as high as 99.8% accurate[38].
Molecular technologies such as fluorescence in situ hybridisation (FISH) and quantitative fluorescence polymerisation chain reaction (QF-PCR) have now become a commonly offered preamble or supportive test to traditional karyotype test (TKT). Due the molecular testing of uncultured amniocytes and the fast turn around time, the results can be available much sooner. These rapid genetic testing kits are used in association with pregnancies that have been assessed as having a low risk of genetic abnormalities, but ones that however warrant further investigation. A 1993 study of 4550 patients by using FISH analyisis testing for the most common autosomal abnormalities , trisomy 13,18 and 21 (Patau, Edward and Down syndromes respectively) and sex chromosome aberrations,found that the results were 99.8% accurate when checked against cytogenetic results [39]. These methods do not however give a full karyotype picture, and are unlikey to replace the comprehensive TKT testing in the near future.
Increasing risk of abnormalities with maternal age (35> yrs), carrier status of gene rearrangement in either parent and visible abnormal structural ultrasound diagnosis may make these molecular methods inappropriate to be offered as they currently cannot detect 20-30% of possible abnormalities [40]. There is debate as to the use of these methods as 'stand alone' tests, but are seen as rapid turn around diagnostic tools that can supplement TDK with a high degree of accuracy.
Due to the highly technical nature of the chromosome banding process and the physician's subsequent interpretation a small percentage of false negative and false positive results may occur. There is the potential for false results due to maternal blood contamination of the sample, however detection of maternal cell contamination (MCC) in amniotic fluid samples via PCR based assay may be helpful to screen against this[41].
A 1979 study of Canadian genetic centres placed alpha-1-fetoprotein (AFP) testing on amniotic fluid for diagnosis of neural tube defects 99.2% interpreted accurately for the condition[42].
Ethical Issues
The current Medico-legal stance on prenatal genetic testing sees amniocentesis as permissible according to law.
The risk of induced miscarriage or physical trauma to the foetus has been assessed as existing but minimal, however, such an invasive technique is generally today only offered to women after screening blood tests have made amniocentesis worth the risk to the mother and foetus.
Studies have shown that amniocentesis should only be performed after the start of the 15th week as that performing before this time has significantly higher miscarriage rates than the accepted proceedure related rate of 0.5-1.0%[43].
Amniocentesis does occur in the first to early trimester in some cases[44]. Issues of what constitutes an endangerment to the life of the foetus become evident.
Issues of informed consent to the procedure surround any invasive medical procedure.
Towards setting high ethical standards of practice pre and post genetic counselling by qualified staff is made available to anyone undertaking amniocentesis in Australia, monitored by the relevant state health services.
Privacy and freedom of information concerns may raise undue psychological hardships for the mother and therefore raises questions of ethics. The familial nature of genetic information may then be shared by those involved amongst a family group against an individuals wish.
The existence of proven false positive results in laboratory assays raises the ethical question of the validity of electing to voluntarily abort the pregnancy. Ethics of being certain of the results arise, for instance if it later becomes known that the results were incorrect, in terms of how will this impact on those concerned.
In Australia in 1969, abortion was not deemed unlawful by the supreme court if it could be proven that the pregnancy was endangering the mother's life or physical or mental health to a serious degree[45].
This raises questions as to the application of the criteria in favour of termination of pregnancy if Down syndrome is detected for instance, as compared to a neural tube defect present in the foetus[46].
Issues exist as to how comprehensive is the definition of what constitutes an acceptable threat to the mother from a legal and genetic point of view.
Abortion in itself is legal to some degree in many developed countries and the ethical issues involved are thus limited to personal choice.
Current Research
Amniocentesis remains the definitive way to obtain amniotic fluid for genetic testing. Whether amniocytes are to be cultured and examined using G-banding or other faster molecular diagnosis is carried out, the collection of prenatal amniotic fluid will continue to be relevant and necessary to diagnosing foetal genetic disorders.
Current research published in 2002 into non invasive isolation of foetal cells from the maternal blood stream via cell culturing techniques does aim to make the need for amniocentesis unnecessary for this purpose[47]. However it does not state that amniocentesis is likely to become obsolete in the near future.
A 2008 review of available literature outlines that further advancements into non invasive prenatal diagnosis (NIPD) have also looked at targeting and isolating cell free foetal nucleic acids that are present in the maternal circulation for a select few genetic conditions, that may form part of the screening process for Down's syndrome in particular[48].
Amniocentesis would then be requested by a practitioner to provide the conclusive evidence if the screening tests show the possibility of aneuploidy.
The refinement of current methods to detect chromosomal abnormalities and the desire for ever faster turn around times in diagnosis has seen the application of better and more accurate technology to the process of detection using amniocentisis. A 2007 study into the improvement of the analysis of FISH images via an automated dot counting algorithm via a camera and computer software has seen significant improvements in diagnostic accuracy if the original signal map as captured by the camera, is then further enhanced to give a greater spectrum contrast. This enhanced image is then reanalysed by the dot counting algorithm. An improvement of an average 9% across all colour channels was observed whilst reducing positive and negative dot counting errors [49].
Amniocentesis vs CVS
Amniocentesis and Chorionic Villus Sampling (CVS) are similar diagnostic techniques in many ways and may test for some similar defects. Many women weigh up the pros and cons to both these techniques to make a decision as to which one they are best suited to under their own circumstances.
CVS may be chosen over amniocentesis as the results can be obtained earlier, giving more time to undergo a safer and less painful abortion. Even though CVS carries a higher risk of miscarriage to a common second trimester amniocentesis, it is safer and carries a lower risk of miscarriage than a first trimester early amniocentesis. So for many women if an earlier test is required, CVS is usually the preferred choice.
However, amniocentesis has a number of advantages. It is able to detect neural tube defects as well as chromosomal, it is more commonly performed and more physicians are familiar with the procedure, it can detect the maturity of the babies lungs as well as the sex of the fetus.
Some similarities and differences are listed below:
| Similarities | Differences |
|---|---|
Test is taken for the same reasons:
Samples are taken using a needle. Ultrasound guidance is used to prevent harm to the baby, placenta or mother. There is a small chance that the results are difficult to analyze and obtain conclusive results and the test may need to be repeated. Risks are present and may include infection, miscarriage, pain and possible leakage of fluid. Are both invasive techniques. Counseling is provided prior to assess the need for the test, the risks and whether the mother is sure she wants to continue. |
Carry different risks of miscarriage:
CVS has a higher miscarriage risk since it is performed at an earlier stage of pregnancy. Are performed at different gestational weeks:
CVS is preferred by some women since the earlier procedure and results time will allow more time to make a decision about abortion Different fluids are sampled, one being amniotic fluid, the other being a sample from the chorionic villi of the placenta. Amniocentesis is only performed by a transabdominal procedure, CVS may be performed this way or through a transcervical technique where the needle is inserted through the vaginal opening and into the womb.[24] Accuracy:
Sample sizes:
|
Useful Links
More on Amniocentesis UNSW Embryology: Prenatal Diagnosis - Amniocentesis
Animated video of the procedure Video of Amniocentesis
Ultrasound video of procedure Ultrasound of procedure Note:You can see the needle insertion through the wall of the abdomen and uterus towards the top of the screen above the baby.
More images Google images of amniocentesis
Glossary
Agglutination - Term used to describe clumping together. Can be used for the changes that occur following sperm ejaculation or an immune response involving antibodies.
Allopolyploidy - A type of polyploid condition where the extra chromosome arises from a different species.
Amniocentesis - A prenatal diagnostic test involving sampling of amniotic fluid by needle aspiration for genetic analysis.
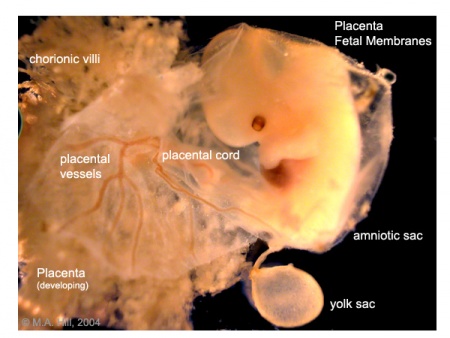
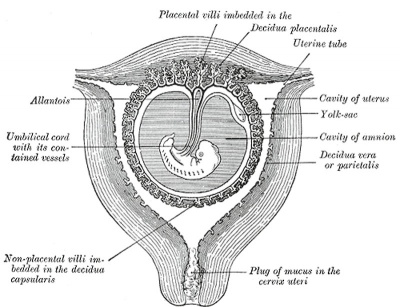
Amnion - An extraembryonic membrane ectoderm and extraembryonic mesoderm in origin and forms the innermost fetal membrane, produces amniotic fluid. This fluid-filled sac initially lies above the trilaminar embryo disc and with embryoic disc folding this sac is drawn ventrally to enclose (cover) the entire embryo, then fetus.
Amniotic cavity - The fluid-filled (amniotic fluid) extraembryonic coelom (cavity) formed initially by epiblast and then ectoderm and surrounding extraembryonic mesoderm. In humans, it forms the innermost fetal membrane, produces amniotic fluid expanding to fuse with the chorionic membrane during week 8 of development. This fluid-filled sac initially lies above the trilaminar embryo disc and with embryoic disc folding this sac is drawn ventrally to enclose (cover) the entire embryo, then fetus.
Amniotic fluid - The fluid that fills amniotic cavity totally encloses and cushions the embryo. This fluid is sampled in the prenatal diagnostic test amniocentesis.
Aneuploidy - Atypical number of chromosomes.
Antibody - Produced by the body and circulated to protect against antigens. They form part of the immune response of the body.
Autopolyploid - Type of polyploid. Arises when a pair has more than two sets of chromosomes as a result of redoubling.
Cell culture - The maintenance or growth of dispersed cells in a medium after removal from the body. [52]
Centrifuge - Any of various rotating machines that separate liquids from solids or dispersions of one liquid in another, by the action of centrifugal force.[53]
Chorionic villus sampling - (CVS) The taking a biopsy of the placenta, usually at the end of the second month of pregnancy, to test the fetus for genetic abnormalities.
Chromatin - A diffuse material within the nucleus of a non-dividing eukaryotic cell; consists of DNA and proteins.
Chromosomes - Double stranded DNA coiled around histones. Condenses during mitosis and meiosis.
Deletion (chromosomal) - Part of the genetic material making up a chromosome is deleted. Results in frameshift errors and a decrease in genetic material.
Duplication (chromosomal) - Part of the genetic material is copied, increasing the amount of genetic material in a chromosome
Diploidy - Describes a diploid cell. This is a cell that has 2 complete chromosomes in a pair.
Endopolyploidy - A type of polyploidy which comes from the replication of chromosomes without division of the cell nucleus.
Fetus - In mammals, term describes the period of development following the embryonic period. In humans, the development week 9 to 36 is the fetal stage (second and third trimester). (see fetal period above). This term is also used non-scientifically to describe the human conceptus at both embryonic and fetal stages of development.
FISH - Abrev. Fluorescent In Situ Hybridisation. A Molecular genetic diagnostic test.
Gestation - The period of time from conception to birth. A pregnancy with multiple fetuses is referred to as a multiple gestation.
Inversion (chromosomal)- Genetic material within a chromosome breaks off and reattaches upside down relative to the DNA sequence. This is a chromosomal abnormality.
Isochromosome - A chromosome that has lost a complete arm and replaced it with an exact copy of its other arm. this forms a mirror image at the centromere.
Karyotype - Term used to describe the chromosomal (genetic) makeup (complement) of a cell by the number and appearance of chromosomes.
Metaphase - One of the 5 mitosis phases (prophase, prometaphase, metaphase, anaphase, and telophase) of nuclear cell division, generating two diploid daughter nuclei. Originally based on light microscopy of living cells and electron microscopy of fixed and stained cells. At metaphase kinetochore microtubules align chromosomes in one midpoint plane. Metaphase ends when sister kinetochores separate.
Mitosis - The normal division of all cells, except germ cells, where chromosome number is maintained (diploid). In germ cell division (oocyte, spermatozoa) meiosis is a modified form of this division resulting in reduction in genetic content (haploid). Mitosis, division of the nucleus, is followed by cytokinesis the division of the cell cytoplasm and the cytoplasmic contents. cytokinesis overlaps with telophase.
Monoploidy - The condition where a chromosome pair is missing one chromosome.
Polyhydramnios - The condition of having too much amniotic fluid in the womb, the peak should be 800-1000mL, which may increase to as much as 3 times the amount with this condition.[54]
Polyploidy - The condition where there is more than 2 chromosomes to a pair. This is a numerical abnormality.
Prenatal diagnosis - any of the diagnostic procedures used to determine whether a fetus has a genetic abnormality.
Rh factor - The protein on surface of red blood cells in some blood types (Rh+) and absent in others (Rh-). Can cause erythroblastosis fetalis in second pregnancy if fetal/maternal blood of opposite groups mix on first pregnancy.
Rings (chromosomal) - Occurs when genetic material which is part of a chromosome breaks off and fuses end-to-end with itself, forming a ring.
Second trimester - Clinical term used to describe and divide human pregnancy period (9 months) into three equal parts of approximately three calendar months. The first trimester corresponds approximately to embryonic development (week 1 to 8) of organogenesis and early fetal. The second and third trimester correspond to the fetal period of growth in size (second trimester) and weight (third trimester), as well as continued differentiation of existing organs and tissues.
Stillbirth - A fetus or infant delivered without signs of life after 20 weeks or more of gestation.
Transabdominal - Insertion of the needle across the abdominal wall or through the abdominal cavity.[55]
Translocation (chromosomal) - A genetic abnormality where genetic material is swapped between chromosomes.
TDK - Abrev. Traditional Karyotype. Cytological diagnostic testing of chromosome.
Ultrasound - A non-invasive technique for visualizing and prenatal diagnosis of several features of development including: follicles in the ovaries, the gestational sac, fetus in the uterus, fetal parameters, and the placenta. The technique uses high-frequency sound waves that are reflected off internal structures. These reflections can then be analysed and displayed by computer. Modern ultrasound machines can also carry out 3 dimensional reconstructions and measure "flow" (blood) using doppler measurements.
X linked - Term used to refer to genes, and genetic diseases, located on the X chromosome. Therefore more likely to be expressed in males, where there is only a single maternal X chromosome.
References
- ↑ <pubmed>11209176</pubmed>
- ↑ <pubmed>989112</pubmed>
- ↑ <pubmed>15301283</pubmed>
- ↑ <pubmed>14898745</pubmed>
- ↑ <pubmed>18730250</pubmed>
- ↑ <pubmed>14869844</pubmed>
- ↑ <pubmed>4255486</pubmed>
- ↑ <pubmed>20325291</pubmed>
- ↑ <pubmed>4255487</pubmed>
- ↑ <pubmed>4159775</pubmed>
- ↑ <pubmed>16837685</pubmed>
- ↑ A short History of Amniocentesis, Fetoscopy and Chorionic Villus Sampling, Dr. Joseph Woo. http://www.ob-ultrasound.net/amniocentesis.html
- ↑ <pubmed>6220164</pubmed>
- ↑ <pubmed>15832534</pubmed>
- ↑ 15.0 15.1 15.2 Prenatal Testing: An Amniocentesis Primer. (2004). http://www.plus-size-pregnancy.org/Prenatal%20Testing/prenataltest-amnios.htm
- ↑ <pubmed>18191913</pubmed>
- ↑ Prenatal Testing: An Amniocentesis Primer. (2004). http://www.plus-size-pregnancy.org/Prenatal%20Testing/prenataltest-amnios.htm
- ↑ <pubmed>9084385</pubmed>
- ↑ Brajenović-Milić B, Babić I, Ristić S, Vraneković J, Brumini G, Kapović M. Womens Health Issues (2008) Pregnant women's attitudes toward amniocentesis before receiving Down syndrome screening results.PMID: 18180167
- ↑ <pubmed>15284930</pubmed>
- ↑ <pubmed>8236967</pubmed>
- ↑ North East Valley Division General Practice Victoria, Australia.http://www.nevdgp.org.au/info/melb_us/Amniocentesis_melb.htm
- ↑ 23.0 23.1 <pubmed>5273905</pubmed>
- ↑ 24.0 24.1 24.2 <pubmed>8236967</pubmed>
- ↑ http://www.ncbi.nlm.nih.gov/pubmed/17077226
- ↑ <pubmed>PMC2464303</pubmed>
- ↑ Principles of Human Physiology(2002) William J. Germann Cindy L. Stanfield
- ↑ <pubmed>1694152</pubmed>
- ↑ http://www.ncbi.nlm.nih.gov/pubmed/20401956
- ↑ http://www.nytimes.com/2009/09/15/science/15chrom.html?_r=2&pagewanted=1&hpw
- ↑ <pubmed>11147289</pubmed>
- ↑ <pubmed>11147289</pubmed>
- ↑ <pubmed>9852645</pubmed>
- ↑ 2007, July 07). MedlinePlus medical encyclopedia: Trisomy 18. Retrieved November 7, 2008, from MedlinePlus medical encyclopedia Web site: http://www.nlm.nih.gov/MEDLINEPLUS/ency/article/001661.htm#Causes,%20incidence,%20and%20risk%20factors
- ↑ 35.0 35.1 Robbins and Cotran Pathological Basis of Disease (7th edition); Vinay Kumar, Abul K. Abbas, Nelson Fausto, copyright Elsevier Inc(2005)
- ↑ http://www.who.int/genomics/public/geneticdiseases/en/index2.html
- ↑ <pubmed>12968936</pubmed>
- ↑ <pubmed>18492228</pubmed>
- ↑ <pubmed> 8488836</pubmed>
- ↑ <pubmed>17075785</pubmed>
- ↑ <pubmed>< 7897630</pubmed>
- ↑ <pubmed>86382</pubmed>
- ↑ <pubmed>20051662</pubmed>
- ↑ <pubmed>17106178</pubmed>
- ↑ <pubmed>16969440</pubmed>
- ↑ <pubmed>12663637</pubmed>
- ↑ <pubmed>12498422</pubmed>
- ↑ .<pubmed>18945714</pubmed>
- ↑ <pubmed>17970921</pubmed>
- ↑ Prof Kristine Barlow-Stewart, Mona Saleh. Prenatal Testing – CVS and Amniocentesis (2007). http://www.genetics.com.au/pdf/factsheets/fs17c.pdf
- ↑ <pubmed>8236967</pubmed>
- ↑ The Free Dictionary. (2007). http://medical-dictionary.thefreedictionary.com/cell+culture
- ↑ The Free Dictionary. (2007). http://www.thefreedictionary.com/centrifuge
- ↑ Baby Center (2008). http://www.babycenter.com.au/pregnancy/complications/polyhydramnios/
- ↑ The Free Dictionary (2007). http://medical-dictionary.thefreedictionary.com/transabdominal
2010 ANAT2341 Group Projects
Project 1 - Ultrasound | Project 2 - Chorionic villus sampling | Project 3 - Amniocentesis | Group Project 4 - Percutaneous Umbilical Cord Blood Sampling | Project 5 - Fetal Fibronectin | Project 6 - Maternal serum alpha-fetoprotein | Group Assessment Criteria
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2026, February 26) Embryology 2010 Group Project 3. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/2010_Group_Project_3
- © Dr Mark Hill 2026, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G