1987 Developmental Stages In Human Embryos - Stage 18
| Embryology - 26 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
O'Rahilly R. and Müller F. Developmental Stages in Human Embryos. Contrib. Embryol., Carnegie Inst. Wash. 637 (1987).
| Online Editor Note |
|---|
| O'Rahilly R. and Müller F. Developmental Stages in Human Embryos. Contrib. Embryol., Carnegie Inst. Wash. 637 (1987).
The original 1987 publication text, figures and tables have been altered in formatting, addition of internal online links, and links to PubMed. Original Document - Copyright © 1987 Carnegie Institution of Washington.
|
- 1987 Stages: Introduction | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | References | Appendix 1 | Appendix 2 | Historic Papers | Embryonic Development
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
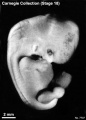
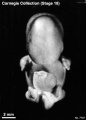
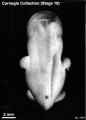
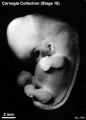
Stage 18
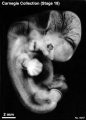
Stage 18 (8097)
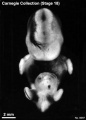
Stage 18 (8097)
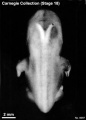
Stage 18 (8097)
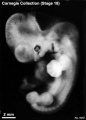
Stage 18 (8097)
Stage 18 (7707)
Stage 18 (7707)
Stage 18 (7707)
Stage 18 (7707)
Summary
External: the body is more of a unified cuboidal mass, and both cervical and lumbar flexures are indicated; the limbs are longer, the digital plate of the hand is definitely notched, the elbow region is usually discernible, and toe rays can be identified in some specimens; eyelid folds are present in the more advanced embryos; a distinct tip of the nose can be seen in profile; auricular hillocks are being transformed into specific parts of the external ear.
Internal: in the heart, septum secundum and the associated foramen ovale are appearing; the membranous part of the interventricular septum is beginning to take form; the vomeronasal organ is represented by a groove; choanae develop; some subsegmental buds develop in the bronchial tree; collecting tubules develop from the calices; testicular cords may begin to appear in the male gonad; the paramesonephric duct grows rapidly down through the mesonephros; 1–3 semicircular ducts are present in the internal ear.
Size and Age
Less-advanced embryos of stage 18 may be expected to have a greatest length of about 14.5 mm, whereas more-advanced examples would in most instances be about 16 mm. Nearly two-thirds of all embryos at this stage range from 14 to 16 mm.
Although the data are not extensive (only eight specimens), the greatest diameter of the chorion usually ranges from 40 to 51 mm, and all three principal diameters are approximately equal.
The age is believed to be approximately 44 postovulatory days.
External Form
(figs. 18-1 to 18-3) From external form alone it is not always possible to distinguish between less-advanced specimens of stage 18 and more-advanced ones of stage 17. In drawing an arbitrary borderline between the two groups, as has been done in this study, one must rely on selected structural characters of the internal organs that are revealed in sections. Indeed, in several instances, it has been found necessary to change the placement of certain embryos from a provisional one based on their external form to one in an adjacent group, based on the study of the same embryos after they had been cut in serial sections.
To gauge accurately the level of development of an embryo, its internal structure must be taken into account. There are many things, however, to be learned from the external form. One can see, for instance, that the group of embryos shown in figure 18-2 is more advanced than the similar group of the preceding stage, shown in figure 17-2. The embryo of stage 18 is larger and has advanced in the coalescence of its body regions, which have now become more closely integrated in a common cuboidal bulk, no longer consisting of individual and obviously separable parts.
The hands in all specimens have distinct finger rays and interdigital notches on the rims. In the more advanced half of the group, one can begin to speak of an elbow. The feet in some specimens exhibit toe rays, but the rim is not definitely notched. Even in some of the more advanced members one can still find a trace of somitic elevations in the sacral region.
All the embryos illustrated in figures 18-1 and 18-2 have been serially sectioned and studied histologically, and all of them conform microscopically to the criteria arbitrarily set for stage 18. The two embryos in figure 18-1 are typical specimens taken respectively from among less-advanced and more-advanced members of the group. The embryos in figure 18-2 illustrate such variations as are incident to differences in photography, methods of fixation, and variations in the degree of development encountered within this stage. The most advanced specimen (fig. 18-2,I) is on the borderline of the next stage.
Face
Above and below the eye one sees in more-advanced specimens of this stage the early rudiments of the eyelids (more marked in stage 19: Pearson, 1980) and the grooves initiating the conjunctival sacs. The thin outer lamina of the retina now contains considerable pigment, causing the retinal rim to be conspicuous. This is particularly true in formalin-preserved specimens, where the tissues are translucent. In embryos of stage 18 this pigmented rim of the optic cup is characteristically polygonal, partly because of the forward projection of the upper margin of the retina and partly because of the retinal fissure. At the same time, white opaque zones of mesenchymal condensation spread progressively forward over the pigmented area, especially above and lateral to it. These opaque thickenings mark the laying down of the sclera and its muscular attachments.
In profile views of the nasal region one can begin to recognize the tip of the nose and the frontonasal angle, from which point the future bridge of the nose is to extend “forward.” To see the face satisfactorily one must study either decapitated specimens or reconstructions of the facial region made from serial sections. Such a model of a representative embryo of stage 18 is illustrated in figure 18-3. The nose, nostrils, nasal tip or apex, nasal wings, and nasal septum (columella nasi) can be identified clearly. The nose at this time can be thought of as a raised window awning; later, as the bridge of the nose forms, the awning will be let down. What is now in a vertical plane will then be horizontal. The upper lip is not differentiated as a separate structure. It will be formed jointly and in segments by the premaxillary and maxillary centers of the two sides. In this same figure the auricular hillocks are visible, although they are seen better in profile views (figs. 18-1 and 18-2). These hillocks were prominent elevations in the preceding stage. Here they are merging with one another and the adjacent surfaces, thereby forming the primordia of definite parts of the auricle. Those rostral to the auditory cleft are more advanced than those caudal to it. Thus the two dorsal hillocks (Nos. 2 and 3) of the mandibular arch are losing their individuality in the process of fusing to form the crus helicis. In more-advanced specimens of the group, the two dorsal hillocks (Nos. 4 and 5) caudal to the cleft have similarly merged to become the helix. Hillocks 1 and 6 persist and become, respectively, the tragus and antitragus, with the lobule pendent from the latter.
Nasal Passages
The nostrils (fig. 18-4) are the rims of the right and left nasal depressions, that is, lines of junction of nasal disc and skin ectoderm. The depressed center of each disc takes the form of a deep pocket, and, as a result of the growth of its own epithelium and the enveloping effect of the proliferating surrounding tissues, it becomes a supplementary air passage. This passage at first ends blindly, but in the more advanced members of this stage its blind end opens through what had been the temporary bucconasal membrane and participates in the formation of the funnel-like transition into the pharynx. A communication between the respiratory system and the surface of the face is in this way provided. It bypasses the mouth, leaving the latter free to perform its own special functions.
Transverse serial sections through the nasal passages of two embryos belonging to this stage, one slightly less advanced than the other, are shown in figure 18-4. In the first one (No. 6524) it can already be seen that the walls of the passage are differentiated into regions, and that the ethmoidal epithelium (upper part of medial wall) is becoming distinct from the maxillary territory of the lateral wall. The shallow fold in the lower part of the medial wall marks the vomeronasal organ (fig. 18-14). These regional demarcations are still more evident in the second embryo (No. 4430). The epithelium, like that of the gut, is characterized by mosaic-like organ-forming regions, the boundaries of which are evident very early.
In these serial sections one can trace the fate of the nasal fin. In stage 17 it appeared as a plate of epithelium that maintains a connection between the nasal disc and the ordinary surface epithelium, in line with the groove between the maxillary and premaxillary growth centers. In transverse sections the nasal fin has the appearance of a ventral stem for the nasal passage. In the present stage, in the more rostral sections, it becomes disconnected and is taken up by the overlying nasal epithelium. Caudally, this fin cleaves open to form part of the choanae. In doing so it becomes stretched to form the bucconasal membrane, and this in turn is detached and absorbed in the wall of the choana, leaving a free respiratory passage. The topographical relations of the nasal passage are further clarified by sagittal sections.
Two embryos thus sectioned, a less advanced (No. 6525) and a more advanced one (No. 144), are shown in figure 18-4. Although in the more advanced of these two the respiratory bypass is an open channel, its mature relations are not yet established. These will be attained through differential growth which is accompanied by a relative shifting forward of the tongue and lower jaw.
The initiation of the nasolacrimal duct is shown in figure 18-4 (No. 6524 b and c, and No. 4430 a and b). An irregular lamina or strand of epithelium is seen sprouting from the under-surface of the nasomaxillary groove. This epithelial strand descends through the mesenchyme, and later joins the lateral wall of the inferior meatus of the nose. The transformation of this strand into a duct and the junction of its upper end by two small canals with the conjunctival sac occurs later in prenatal life. Politzer (1936) disagreed with the usual account of the early development of the nasolacrimal duct.
Heart
(figs. 18-5 and 18-6) An important feature is the appearance of septum secundum and hence the beginning of the foramen ovale at stages 18–21.
The heart is now a composite, four-chambered organ with separation of the pulmonary and aortic blood streams. In the more advanced members of the group the secondary interventricular foramen may already have closed, producing the future membranous part of the interventricular septum. The complete closure of the secondary interventricular foramen takes place during stages 18–21 (i.e., separating the cavity of the left ventricle from that of the right). The final boundaries of the foramen are the conal septum (particularly the right conal ridge) and the fused atrioventricular cushions (Wenink, 1971).
The aortic and pulmonary valves have acquired increased definition and are becoming cup-shaped. The tricuspid and mitral valves are later in their differentiation, but one can now recognize the ridges of condensed mesenchyme that are to form them. It is probable that they already have some valvular function.
Drawings of selected sagittal sections of a typical heart from one of the more advanced members of the group are shown in figure 18-5. In the condensing mesenchyme of the primary cardiac tube one sees, in such sections, sheets or areas of more-condensed tissue, shown in the figure by hatched lines, which underlie the endothelium and mark the proximity of cleavage and of adjustment of the walls.
From a coronal series of sections of another embryo at a similar level of development, a transparent reconstruction was made of the heart. Five study slabs (A–E) are illustrated in figure 18-6. The location of the front surface of each slab is indicated on the profile view of the heart, shown in the lower right corner. In this figure, as in the preceding one, the ventricular musculature is shown in solid black to distinguish it as a specialized tissue from the condensed mesenchyme of the primary cardiac tube and the venous type of muscular wall of the atria. In slab A one sees the nature of the future membranous part of the interventricular septum separating the right and left ventricles. In slab B, directly behind it, is shown the fusion of the wall of the primary cardiac tube, which thereby separates the tricuspid canal from the aortic corner of the left ventricle. In slab C is shown the complete separation of the aorta and pulmonary trunk, as far as the endothelium is concerned. Adjustments, however, are still in progress in the muscular and connective-tissue coats. The tricuspid and mitral passages are indicated. In slab D the sinus venosus is shown as it opens into the right atrium, immediately to the rear of the inferior vena cava. The two freely open at the same time, through the foramen ovale into the left atrium. In slab E is shown the manner of entrance of the superior vena cava. It is thus clear that from these three sources (sinus venosus, inferior vena cava, and superior vena cava) practically the whole of the incoming blood now arrives at the entrance of the foramen ovale.
Reconstructions of the heart at stage 18 have been illustrated by Kramer (1942, fig. 9) and by Vernall (1962)[1].
Digestive System
The esophagus shows distinct muscular and submucous coats. The fundus of the stomach begins to develop at about stages 18 and 19.
Respiratory System
The vomeronasal organ is represented by a groove in the medial wall of the nasal cavity (fig. 18-14). Choanae are being produced by the breakdown of the bucconasal membrane. The larynx, which became definable in the previous stage, is now undergoing specialization (O'Rahilly and Tucker, 1973)[2].
The trachea possesses a dense connective-tissue coat (fig. 15-7D) and is markedly different from the esophagus in both its epithelium and its supporting wall.
The pattern of the bronchial tree is shown in figure 18-14. The segmental bronchi are well defined (Wells and Boyden, 1954, plate 2), and a few subsegmental buds appear. In the embryonic period proper the left lung is said to lag behind the right lung in size and degree of development.
Urinary System
Collecting tubules develop from the calices at stages 17 and 18 (fig. 18-14). They are surrounded by sharply outlined condensed primordia in the process of forming secretory tubules. Renal corpuscules are not yet present. By stage 18 the mesonephric duct and the ureter open almost independently into the vesico-urethral canal (Shikinami, 1926): i.e., the common excretory duct is disappearing. The cloacal membrane is ready to rupture.
Reproductive System
In stage 15 the primordial germ cells are found sparsely distributed among the proliferating cells of the coelomic epithelium (fig. 18-9). The coelomic epithelium provides the framework for the gonad. In growth of the epithelium in stage 16 is sufficiently advanced to produce a crescentic strip of gonadal tissue that is fairly well demarcated from the mesonephros. In stage 17 the gonad has increased in size and is beginning to acquire an oval form. Its surface is still irregular. By stage 18 the gonad has become an elongated oval, distinctly set off from the mesonephros. The surface of the gonad is now smooth. Angiogenesis is commencing. The gonadal framework may begin to show sexual distinction by the presence of testicular cords. The presence of testicular cords in the gonadal blastema at stage 18 and of a tunica albuginea at stage 19 makes possible the determination of sexual differentiation in the male embryo.
A precise area of the coelomic epithelium, at the rostral end of the mesonephros, becomes thicker and invaginated to form the paramesonephric duct. The site of its infolding becomes the abdominal ostium of the uterine tube. In stage 18 the paramesonephric duct makes the principal part of its way down the front of the mesonephros, as a lateral companion to the mesonephric duct (fig. 18-7). The origin and rate of growth and elongation are precisely regulated, so that an additional clue to the level of development is provided. The duct tends to be straight, and its length can be calculated by multiplying the number of transverse sections by the thickness of each section. Alternatively, the length can be obtained by superimposing outlines of sagittal or coronal sections.
The paramesonephric ducts are present in all embryos of stage 18; their length was used by Streeter as an index of development within the stage. Of 35 specimens, a less advanced group of nineteen in which the length of the paramesonephric duct was 0.6 mm or less was distinguished from a more advanced group of sixteen specimens in which it was more than 0.6 mm. Most of the embryos in the first group range from 13 to 16.5 mm, whereas most of those in the second group are 14–17.2 mm in length.
In 34 embryos of stage 18, Streeter found that the length of the paramesonephric duct could be correlated with the level of development of other parts of the embryo. For example, in embryos in which only one semicircular duct had been pinched off in the membranous labyrinth, the paramesonephric duct ranged from 0.2 to 0.4 mm in length. Where two semicircular ducts were present, the range was 0.4–0.7 mm. Where three semicircular ducts were present, the range, with but a few exceptions, was 0.8–1.1 mm in length (fig. 18-8). Hence, from the status of the paramesonephric duct, the degree of development of an organ as remote as the internal ear can be estimated.
Nervous System
(fig. 18-10)
The most developed area of the brain is the rhombencephalon. The motor nuclei are better organized than the sensory. In the more advanced embryos the choroid plexus of the fourth ventricle begins to be identifiable by the presence of some villi. In the cerebellum, in addition to the inner cerebellar bulge present already in stage 17, an outer swelling appears and represents the future flocculus. Vestibulocerebellar fibers are present in great number at its surface. A clear destination between auditory and optic colliculi in the midbrain as represented by Streeter has not been confirmed. In the diencephalon the neurohypophysis has folded walls. The adenohypophysis, open to the pharynx in stage 17, is now closed off from the pharyngeal cavity. An epithelial stalk, containing a faint lumen, is connected tothe pharyngeal epithelium. The epiphysis, representing the “anterior lobe,” is illustrated in figure 18-11. Sections of two specimens (A and B) belonging to the middle third of the embryos of stage 18 are shown. A pineal recess is forming for the first time, and a follicular arrangement of the cells may be encountered in some embryos. This “anterior lobe” of the epiphysis corresponds to Stadium III of Turkewitsch (O'Rahilly, 1968). The rostral part of the diencephalic roof is richly vascularized, and some ingrowth of the epithelial lamina at the level of the telencephalon indicates the first signs of a choroid plexus of the lateral ventricles. Approximately half of the length of the cerebral hemispheres now extends more rostrally than the lamina terminalis. A slight groove is developing in the corpus striatum, which has grown considerably and now reaches as far caudally as the preoptic sulcus. The olfactory bulb is better delimited and, in some embryos, contains an olfactory ventricle.
Eye
(figs. 18-12C.D, 18-13, and 18-14)
Mesenchyme invades the interval between the lens epithelium and the surface ectoderm (as may have already begun in the previous stage), and possibly the posterior epithelium of the cornea (the mesothelium of the anterior chamber) is forming. The cavity of the lens vesicle is becoming obliterated by primary lens fibers.
Reconstructions of the optic vesicle/optic cup at stages 13–18 are shown in figure 18-13.
Ear
(fig. 18-14) The semicircular ducts form from thick epithelial areas of the membranous labyrinth. Adjacent epithelial layers fuse, lose their basement membrane, and disappear (O'Rahilly, 1963)[3]. From one to three semicircular ducts are formed during this stage, and the order is anterior, posterior, and lateral. The crus commune is evident from the beginning. The cochlear duct is L-shaped. The mesenchymal stapes (containing the stapedial artery) and the stapedius can be identified, and the bars of pharyngeal arches 1 and 2 may begin to chondrify.
References
| Online Editor Note |
|---|
| O'Rahilly R. and Müller F. Developmental Stages in Human Embryos. Contrib. Embryol., Carnegie Inst. Wash. 637 (1987).
The original 1987 publication text, figures and tables have been altered in formatting, addition of internal online links, and links to PubMed. Original Document - Copyright © 1987 Carnegie Institution of Washington.
|
- ↑ VERNALL DG. (1962). The human embryonic heart in the seventh week. Am. J. Anat. , 111, 17-24. PMID: 14037955 DOI.
- ↑ O'Rahilly R & Tucker JA. (1973). The early development of the larynx in staged human embryos. I. Embryos of the first five weeks (to stage 15). Ann. Otol. Rhinol. Laryngol. , 82, 1-27. PMID: 4746614 DOI.
- ↑ O'RAHILLY R. (1963). THE EARLY DEVELOPMENT OF THE OTIC VESICLE IN STAGED HUMAN EMBRYOS. J Embryol Exp Morphol , 11, 741-55. PMID: 14081992
See also Müller F & O'Rahilly R. (1990). The human brain at stages 18-20, including the choroid plexuses and the amygdaloid and septal nuclei. Anat. Embryol. , 182, 285-306. PMID: 2268071
Search Pubmed
- 1987 Stages: Introduction | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | References | Appendix 1 | Appendix 2 | Historic Papers | Embryonic Development
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
Cite this page: Hill, M.A. (2024, April 26) Embryology 1987 Developmental Stages In Human Embryos - Stage 18. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/1987_Developmental_Stages_In_Human_Embryos_-_Stage_18
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G