1987 Developmental Stages In Human Embryos - Stage 13
| Embryology - 26 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
O'Rahilly R. and Müller F. Developmental Stages in Human Embryos. Contrib. Embryol., Carnegie Inst. Wash. 637 (1987).
| Online Editor Note |
|---|
| O'Rahilly R. and Müller F. Developmental Stages in Human Embryos. Contrib. Embryol., Carnegie Inst. Wash. 637 (1987).
The original 1987 publication text, figures and tables have been altered in formatting, addition of internal online links, and links to PubMed. Original Document - Copyright © 1987 Carnegie Institution of Washington.
|
- 1987 Stages: Introduction | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | References | Appendix 1 | Appendix 2 | Historic Papers | Embryonic Development
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
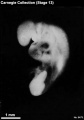
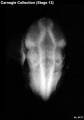
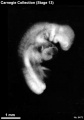
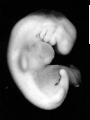
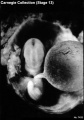
Stage 13
- Approximately 4–6 mm
- Approximately 28 postovulatory days
- Characteristic feature: 30 or more pairs of somites
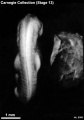
Stage 13 (6473)
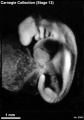
Stage 13 (6473)
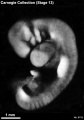
Stage 13 (6473)
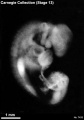
Stage 13 (6469)
Stage 13 (8066)
Stage 13 (8066)
Stage 13 (8119)
Stage 13 (7433)
Stage 13 (7433)
Summary
External: all four limb buds are usually visible; the otic vesicle is closed; the lens disc is generally not yet indented.
Internal: septum primum and foramen primum appear in the heart; right and left lung buds are recognizable, and the trachea begins its development; retinal and lens discs appear; the endolymphatic appendage becomes distinguishable by the end of the stage.
Size and Age
The overall size of the chorion in stage 13 commonly ranges between 19 and 30 mm (eleven specimens out of nineteen from which accurate measurements were available). The mean of largest and smallest diameters in the same eleven specimens ranges from 16.5 to 22.5 mm. The smallest diameter is less easily measured and introduces various inaccuracies. If we accept the greatest diameter as the most practicable measurement, we can then say that in the majority of specimens of this group it is between 20 and 30 mm. There is a minority group (four specimens) having somewhat smaller chorions 17–19 mm in largest diameter. In addition, two exceptionally small specimens and three exceptionally large ones have been noted.
The length of the embryos as given in Appendix I is based on their greatest length, measured in formalin solution or in some instances in 80 percent alcohol following fixation in another fluid. In the latter cases an 0.8 percent allowance was made for the shrinkage after alcohol. This allowance is based on observed shrinkage of similar material in similar fixatives. The measurements given are the corrected ones (i.e., greatest length after fixation). Of the 26 specimens, half are 4.5–5.8 mm long. About one-third of the 26 specimens are smaller and range from 3.9 to 4.3 mm in length. Thus 80 percent (21) of the specimens are found to be about 4–5 mm long. The remainder comprises two poorly preserved specimens of 3.0 and 3–5 mm, and three exceptionally large and borderline specimens each of 6 mm.
The age of embryos of stage 13 is believed to be approximately 28 postovulatory days.
External Form
(figs. 13-1 to 13-3, and 15-3) After stage 12 the number of pairs of somites becomes increasingly difficult to determine and is no longer used in staging. Various numbers have been given for certain embryos. For example, No. 836 has sometimes been stated to possess 30 pairs, whereas the present authors believe that 32 pairs are present.
This is the stage when one can first see both upper and lower limb buds. As was true in stage 12, the central nervous system seems to be the chief factor in determining the form of the embryo, because the external contour of the neural tube is the same as that of the embryo. Otherwise the most prominent surface characteristics of the group are supplied by the series of bulging condensed masses, the pharyngeal arches, and the similarly appearing whitish opaque limb buds. To the rear of the hyoid mass lies the glossopharyngeal arch, and through it courses the large third aortic arch. Caudal to it is the depressed triangular area where the surface ectoderm sinks in to come in contact with the pharyngeal endoderm. As the surrounding parts increase in size, the floor of this triangular area becomes partly covered over, and the depression is known as the cervical sinus. In stage 13 the floor is still plainly visible. It is less so in the next stage. This is the region through which a new blood channel is forming, to become the fourth aortic arch. Also underlying this area is the telopharyngeal body.
In stage 12 there is either no upper limb bud at all, or in the oldest members of the group a representative of it in the form of a slight elevation. In stage 13 the upper limb buds consist of definite ridges, and there are also beginning lower limb buds. The cardiac chambers are now distended with fluid, making them and the heart as a whole more prominent. The hepatic mass is also larger. The combined volume of the heart and liver has become about equal to that of the overlying head. The communication between the umbilical vesicle and the gut cavity has been transformed from a wide space or, in some cases, a compressed slit-like passage to a slender, vascularized stalk. Its junction with the gut endoderm is sharply marked. The transformation from a spacious communication between the gut and umbilical vesicle to a slender stalk combines actual decrease in the transverse diameters with elongation of the structure: i.e., it is brought about by adaptation in shape, and no tissue appears to be wasted in the process. As this takes place, the amnion closes in and ensheathes the connecting stalk, the coelomic cavity, and the stalk of the umbilical vesicle. This marks the beginning of the umbilical cord.
In formalin-fixed specimens the major outlines of the brain and optic vesicle can be seen. The otocyst is completely closed off from the surface. In front of the otocyst is the vestibulofacial ganglion streaming down to the dorsal segment of the hyoid arch. The large trigeminal ganglion, connecting the maxillary and mandibular processes with the lateral pontine angle of the neural wall, is conspicuous, both in dorsal and profile views.
Four pairs of occipital somites are found in the human embryo at stage 13 (O'Rahilly and Müller, 1984b).
Cardiovascular System
(figs. 13-3 to 13-5) Even in the less advanced members of this group the blood circulation is well established. The current flows out through the arterial system supplying, notably, the massive central nervous system through capillaries that invest it as a surface network and from which drainage vessels lead back into the venous system. Numerous small arteries are also present throughout the mesoderm that will later constitute the walls of the lungs and intestine. At the rapidly extending caudal end of the embryo is a vascular plexus in which the channels progressively shift in adaptation to successive segments laid down by the active caudal bud. Out of this plexus stem the umbilical arteries, which join through the connecting stalk with the chorionic plexus and end in the terminal capillaries that are forming in situ in the chorionic villi. A moderate amount of plasma and numerous blood cells are seen throughout the villous network. The structural basis is now established for interchange between the maternal blood of the intervillous space and the embryonic blood in the capillaries of the villi. The venous return through the connecting stalk is made through a coarse venous plexus, which on reaching the body of the embryo terminates symmetrically in the right and left umbilical veins; these in turn empty into the sinus venosus at the base of the heart. In addition, the specialized umbilical vesicle and the associated sinusoidal plexus of the liver possess a circulation. The communications between the vitelline plexus and the blood vessels of the body proper become limited to a vitelline artery arising in plexiform manner from the splanchnic branches of the aorta, and the right and left vitelline veins, which terminate in the vascular plexus that enmeshes the epithelial trabeculae of the liver (fig. 13-5). The hepatic plexus in turn empties into the sinus venosus, and thus this umbilical vesicle–hepatic circulation terminates at the heart. Mention will be made later of the special vascular communications that characterize the liver in the period before the organization of the intestinal circulation and the portal vein.
The form and relations of the vascular pattern outlined can be best shown by drawings (see figs. 13-3 and 13-4). In figure 13-4 are illustrated the principal arteries and their distribution with reference to the alimentary canal. Only the larger arteries are shown. It is to be understood that these give off smaller arteries that terminate in various capillary networks; these in turn are drained into the venous system, and the blood is thereby brought back to the heart. The main channels of the venous return are shown in figure 13-3. In such a right profile view one sees how the sinus venosus serves as a common antechamber to the heart, into which empty the cardinal system of veins draining the main axis of the embryo, the umbilical veins from the connecting stalk and chorionic villi, and the hepatic system from the umbilical vesicle. It is to be noted that, in addition to the blood from the umbilical vesicle and hepatic plexus, the portal system at this time includes a large channel (hepatocardiac vein) that projects into the coelom. Overlying this, the coelomic covering is specialized in a manner that makes it easily permeable to the coelomic fluid. The venous pattern is in general bilaterally symmetrical. The sinus venosus, however, is already asymmetrical, and the hepatic plexus becomes more and more so in adaptation to the establishment of the one-sided pattern of the definitive portal system.
Aortic arches 4 and 6 develop (Congdon, 1922), and a fifth aortic arch can be found in at least some instances.
Many other, isolated features have been described at this important stage but the sources are difficult to locate. It is difficult to believe that in the foreseeable future it will be possible to enter in a computer such a question as “What does the venous system at stage 13 look like?” and be given the answer “A reconstruction of No. 588 was published by C. F. W. McClure and E. G. Butler in fig. 2, p. 339 of the American Journal of Anatomy, 35, 1925!”
Heart
(fig. 13-5) It is convenient to follow Streeter's restriction of the term primary cardiac tube to the arterial part of the heart (i.e., distal to the atrioventricular junction). It should be appreciated, however, that the cardiac jelly is not confined to this tube but extends partly into the atria (de Vries and Saunders, 1962).
The four principal subdivisions of the primary cardiac tube are visible: (1) and (2) the trabeculated portions of the left and right ventricles, respectively, (3) the conus cordis, and (4) the truncus arteriosus (O'Rahilly, 1971, fig. 1). The last two (3 and 4) compose the outflow tract. Together with the sinus venosus and the atria, “these parts of the developing heart should not be simplistically identified with the components of the full-term heart” (Los, 1978).
The conus cordis is believed to give rise later to the conus arteriosus (of the right ventricle) and the aortic vestibule of the left ventricle. The truncus arteriosus is a feature of normal development and should not be confused with the anomaly known as truncus arteriosus communis. Los (1978) maintains that the truncus arteriosus “participates in the development of the outflow tracts of the ventricles ... but not of the ascending aorta and the pulmonary trunk,” both of which are stated to be derivatives of the aortic arches.
The first steps in the partitioning of separate right and left circulations begin already in stage 12, and “functional septation begins . . . in stage 13,” whereby “the ventricular pumps now operate in parallel” instead of in series (McBride, Moore, and Hutchins, 1981). The “ejection stream from the left ventricle is dorsal to that of the right,” a relationship that, according to deVries and Saunders (1962), accounts for clockwise spiralling streams.
Other features found at stage 13 are the left venous valve, septum primum and foramen primum (stages 12 and 13), the first indication of the common pulmonary vein (Neill, 1956, fig. 2), rostroventral and caudodorsal atrioventricular cushions (fig. 13-6), and the atrioventricular bundle (stages 13–16: Magovern, Moore, and Hutchins, 1986).
The motive force that propels the blood through the various channels is provided by a heart that still lacks true valves. The heart at stage 13 is shown in simplified form in figure 13-6. It will be seen that a considerable contractile force can be provided by the rapidly differentiating atrial and ventricular walls (myocardium), each having its own characteristics. From observations on early stages in animals, it is known that the rhythmic contractions of the myocardium and their coordination in contractile waves that progress from the sinus through the atrium, ventricle, and outflow tract to the aortic sac is a function of the myocardial cells. This myogenic period of the cardiac pulsations extends to the time of invasion by nerves. The establishment of neurogenic control probably occurs subsequent to stages 13 and 14, and it does not take place until after an active circulation is well in operation.
A summary of the histogenesis of the wall of the heart in stages 11–14 is shown in figure 13-7. In the least developed specimen the visceral coelomic wall is not distinguishable from the primordium of the myocardium. The latter should be regarded as a specialized part of the former, and its histological character can be seen in the photomicrograph. In a similar way, that part of the opposite parietal coelomic wall is producing the cells that are to become the framework and blood vessels of the liver. The coelomic primordium of the myocardium is separated from the partially distended endocardium by the myoendocardial interval throughout the whole length of the cardiac tube. This gap, at first with few or no reticular cells, is filled with a homogeneous transparent jelly which intervenes between the myocardium and the endocardium. This gelatinous layer is an incompressible elastic cushion by means of which the contractions of the myocardium produce obliteration of the lumen of the endocardium.
In stage 12 the myocardium has become three or four cells thick. One can distinguish the coelomic surface cells as the germinal basis from which more-specialized cells are crowding toward the endocardium as definitive myocardial cells. The residual cells at the surface will perhaps form the epicardium, but the latter is not defined until the delamination of myocardial cells is completed. Reticular cells now appear in the myoendocardial interval, and one may speak of a gelatinous reticulum or cardiac mesenchyme. The coelomic channel surrounding the heart is reduced to a thin space, outside which is a simple single-layered parietal coelomic membrane covered in turn by a thin surface ectoderm.
In stage 13 the cardiac chambers are distended with plasma and blood cells. It will be seen that those myocardial cells most advanced in their differentiation lie toward the cavity of the heart. Loops and strands of these cells extend inward, indenting and interlocking with the endocardium and thereby producing the trabecular character that so early distinguishes the ventricle from the atria. The trabecular arrangement of the wall requires a corresponding growth and elaboration of the endocardium.
In stage 14, the most advanced in the series illustrated, the wall is considerably thicker; passing from the surface to the cavity of the heart one can recognize the successive steps in the differentiation of the myocardial cells. Outermost is a compact wall or cortex of less-differentiated cells. Extending inward is the trabecular zone, consisting of loops or columns of elongating cells; these are the ones that first acquire fibrillae and striations. On the surface toward the narrow coelomic cleft, one can begin to recognize a thin layer of residual cells that appear to constitute the epicardium.
By this time the cardiac wall is actively pulsating with rhythmic and coordinated contractions, progressing from the sinus to the outflow tract. The propulsion of the contained blood toward the aorta is in part aided by the virtually valve-like character of the cardiac tube at critical places. First of all, the fold of the atrial wall at the narrow opening of the sinus venosus into the atrium, the sinu-atrial foramen, serves as a valvular lip which, when the atrium contracts, tends to prevent the blood from escaping backward into the sinus (fig. 13- 6). The next check to regurgitation is at the atrioventricular canal, where the so-called endocardial cushions reduce the passage from the atria to the ventricle to a narrow canal. Because of the gelatinous nature of the tissue of the cushions, the canal can be effectively closed by myocardial contraction. The third site of constriction of the cardiac tube is at the outflow tract and along the aortic trunk, stopping abruptly at the aortic sac. This prevents regurgitation from the aortic arches. Also along this site there is found a zone or cushion of gelatinous reticulum, similar in character to the endocardial cushion at the atrioventricular canal. Both of these regions are to be regarded as parts of the cardiac tube where the myoendocardial interval with its gelatinous reticulum persists and where a valve-like function is being performed. Instead of extending the whole length of the tube, this cardiac mesenchyme is now limited to the particular areas where support against backflow is needed (fig. 13-6). Even in the absence of true valves, such a heart is capable of actively propelling the blood to the chorionic villi and back, through the umbilical vesicle and throughout the tissues of the embryo.
Blood Vessels of the Liver
In stages 11 and 12 the sinus venosus and the atria, if they are not enlarged specialized parts of the vitelline plexus, are at least intimately related to it. Multiple drainage channels from the plexus (fig. 13-5) converge at the caudal end of the heart and empty into the venous antechamber which lies rostrally athwart the umbilical vesicle near its junction with the intestine. This antechamber soon resolves itself into the right and left atria and the sinus venosus (fig. 13-3). In that transformation the atria become more specialized and are soon incorporated in the heart, whereas the sinus venosus retains its venous character and serves as the place of terminal confluence of all the large veins of the body, namely, the cardinal veins from the whole trunk, the umbilical veins from the connecting stalk and chorionic villi, and the vitelline veins from the umbilical vesicle. This is the vascular pattern one finds in the hepatic region at stage 13.
The section in figure 13-8 passes transversely through the central part of the liver and shows the typical appearance under low magnification of the epithelial trabeculae spreading outward and enmeshing the hepatic plexus, as is characteristic of this stage. This figure should be compared with figure 13-3, which is at the same magnification. One thereby recognizes the increase in size of the liver and its advance in complexity. Growth is particularly active dorsalward, producing right and left horns in which are found the hepatocardiac veins. From this time on, the area pre-empted by the vascular plexus in such a section of the liver increases in proportion to the amount of epithelial parenchyma. In more-advanced specimens of this group they are present in about equal proportion. The hepatic epithelium finally spreads close to the coelomic surface of the cardiac chamber, leaving but a thin rim to represent the location of the future diaphragm.
The large channels marked right and left hepatocardiac veins in figure 13-8 empty into the sinus venosus (compare fig. 13-5). It is through these that the main drainage of the hepatic plexus flows into the sinus venosus, at first through both right and left veins. Later the right vein enlarges and the left closes off (Ingalis, 1908, fig. 12). The right is finally transformed to become the terminal segment of the inferior vena cava. But in the present group, both of the hepatocardiac veins appear to be functioning chiefly in connection with the specialized coelomic walls separating them from the coelomic fluid. This relation is shown in figure 13-8. It can be seen that the permeable character of the tissue overlying the hepatocardiac veins would facilitate the passage of fluid from the coelomic cavity to these large veins just as they are about to enter the heart. At this time the chorionic circulation is but poorly established, and therefore the main source of water and food substances for the embryonic tissues must still be the fluid circulating in the coelomic channel. The areas covering the hepatocardiac veins shown in figure 13-5 appear to be the histological expression of that activity. A similar specialization of the coelomic surface is found in the region of the connecting stalk and on the inner surface of the body wall over the saclike distention of the umbilical veins. Certainly such areas would be more permeable than those surfaces of the body covered with surface ectoderm.
For the purpose of determining the communications of the hepatic vessels, the view shown in figure 13-5 was made from reconstructions and serial tracings of one of the less developed embryos of this group. It will be seen that the hepatic plexus intervenes as a filtration, or perhaps an absorption, network between that of the umbilical vesicle and the general blood stream (sinus venosus). It foreshadows the portal circulation of later development, when the mesenteric blood from the digestive canal is passed through the hepatic parenchyma before reaching the heart. At any rate, in stage 13 all the blood from the umbilical vesicle passes through the hepatic plexus, and it is this blood alone that the liver receives. This is a temporary arrangement that coexists with the maximum development of the umbilical vesicle, and one might speak of it as a preportal system, pending further information on its functional activities.
It will be noted that the blood from the chorionic villi coming in through the umbilical veins bypasses the liver and is poured into the sinus venosus directly. Subsequently the terminal section of the umbilical veins becomes closed off, and the blood is rerouted through an anastomosis that is established between the left umbilical vein and some of the peripheral loops of the hepatic plexus. In this way a new channel, the ductus venosus, is established ventral to the liver, carrying the blood from the placenta directly to the base of the heart.
It can be concluded, first, that the blood vessels of the liver include the intrinsic hepatic plexus that arises in situ from cells of the coelomic mesoderm, apparently stimulated by the epithelium of the hepatic diverticulum. Secondly, the plexus of the umbilical vesicle empties into this intrinsic network through the vitelline veins. Thirdly, the left umbilical vein anastomoses with some of the peripheral loops of the hepatic plexus to form a direct channel, the ductus venosus, across the base of the liver, emptying into the inferior vena cava. Finally, there are the large hepatocardiac veins that drain the hepatic plexus into the sinus venosus, and that also appear to serve temporarily as permeable organs by means of which coelomic fluid gains ready access to the blood stream where it enters the heart.
Digestive System
(fig. 13-9) In stage 12 it can be seen that certain endodermal areas are at that time definitely allotted to the lung bud, dorsal pancreas, and liver. In stage 11 it is possible to recognize the pulmonary primordium and hepatic area. It can be concluded that long before that time (i.e., in the presomitic period), definitive areas of the endoderm are limited in their potentialities to the making of specific primary organs, although their outlines are not yet recognizable. It is also in those early periods that the primary induction of axial relationships with bilateral symmetries and asymmetries occurs (O'Rahilly, 1970). Although at first undetermined and uniform in their potentialities, subsequent proliferation of the endodermal cells provides them with a mechanism for segregation into groups having diverse constitutions. The consequent arrangement in organ territories is thus understandable, especially when account is taken of the directive influences of the overlying embryonic disc and its primitive node and notochordal process. These diverse fields of endoderm become the dominant tissues in the organogenesis of the structures composing the digestive system.
Some of the component organs of the digestive system are acquiring definite outlines, at least in their primary tissue, the epithelium (fig. 13-9). The roof of the pharyngeal region shows little activity, but the cellular proliferation of the floor and lateral margins is producing characteristic plates and folds that foreshadow the various derivatives that originate in this region. The bilobed median thyroid primordium and the telopharyngeal body are conspicuous (Weller, 1933). In addition, the thymic primordium of pharyngeal pouch 3 becomes distinguishable (ibid.). It will be noted that one of the factors in producing the pharyngeal pouches is the necessity of sparing spaces for the passage of large communicating channels from the aortic sac to the large dorsal aortae (figs. 13-4 and 13-9). These channels serve as functioning elements for the maintenance of the embryo. For each of these aortic arches there is produced a ridge on the surface and an indentation of the lateral margin of the pharynx. The surface ridges, or pharyngeal arches, are further conditioned by the precocious mesenchymal masses of the mandibular, hyoid, and other primordia. All these are to be considered in addition to the proliferative parts of the pharyngeal epithelium.
A Born reconstruction of the pharynx of No. 836 has been illustrated by Weller (1933, fig. 1).
The stomach now is definitely spindle-shaped. The dorsal pancreas is beginning its visible constriction from the intestinal tube. The ventral pancreas may perhaps be distinguishable. The epithelial trabeculae of the liver have spread almost to the ventral periphery of this organ. The stalk of the umbilical vesicle has become slender and longer, and retains its sharp demarcation from the intestinal epithelium. The intestinal system is larger, as is shown by comparison of figure 13-9 with figure 12-4, both drawn to the same scale. Further elongation of the intestine and the corresponding body axis is by interstitial growth.
The beginning of the omental bursa may be visible at stage 12 and is constant by stage 13 (McBride, Moore, and Hutchins, 1981). Pleuroperitoneal canals are becoming defined and the diaphragmatic primordium can be followed (Wells, 1954).
A “splenic primordium” has been identified as early as stage 13 (Gasser, 1975).
Respiratory System
(figs. 13-9 and 15-6) The right and left lung buds with their characteristic diverging axial growth are the index of the advancement of growth of the lung. In figure 13-9 is shown the range included in this stage. A cross in each instance marks the level below which the respiratory diverticulum possesses an independent lumen. A trachea is present in the more advanced specimens. With few exceptions, the right primary bronchus, as soon as it acquires appreciable length, is directed more caudally, whereas the left bronchus is more nearly transverse. In this, the bilateral bronchial asymmetry existing in the maturer state is anticipated.
Urinary System
Glomeruli begin to develop in the mesonephros, and nephric tubules become S-shaped (Shikinami, 1926). A ureteric bud may possibly be present in some specimens (Wells, 1954, fig. 4), although further confirmation is needed. The mesonephric duct, which becomes separated from the surface ectoderm except in its caudal portion, is fused to the cloaca, into which it may open (fig. 13-9). A urorectal cleavage line is apparent.
Reproductive System
The primordial germ cells migrate from the hindgut to the mesonephric ridges, and several hundred of them are present in the embryo (Witschi, 1948).
Nervous System
(fig. 13-10) The central nervous system is still a tubular structure, and from the midbrain region caudalward it presents the classic arrangement of basal and alar plates, demarcated by a sulcus limitans. Transverse sections through the cervical levels of the tube show a narrow marginal layer. The remaining wall is made up of the undifferentiated ependymal zone, except for a beginning mantle zone in the region that is to form the ventral horn of gray matter. This nuclear zone in more-advanced specimens of the group shows early neurons, the processes of which can be seen emerging as ventral roots. Ventral root fibers are present as far caudally as the upper thoracic segments. The dorsal roots of the spinal nerves consist of a few fibers at the more rostral levels.
A marginal layer begins to appear also in the rhombencephalon and the mesencephalon in the area ventral to the sulcus limitans. The most differentiated part of the brain is still the rhombencephalon. Rhombomere 1 can be distinguished, and hence eight rhombomeres can be counted. In addition to the lateral bulges of the areas of the trigeminal and facial nerves, there are also dips in the floor in the median plane at corresponding levels. The trigeminal and facial dips are constantly present in this stage and constitute reliable landmarks. From the single sheet of motor cells present already in stage 12, two columns of motor nuclei begin to form in the midbrain and hindbrain: the ventromedial column contains the cells for nerves 4, 5, 7/8, 9, 10, and 11, the ventrolateral column the nuclei for nerves 3, 6, and 12. The motor nuclei of the spinal nerves are in continuity with the hypoglossal nucleus. The motor roots for nerves 5, 6, 9, 10, and 11 are present in most embryos. The hypoglossal nerve consists of several roots. The geniculate and vestibular ganglia form a unity; in the more advanced embryos, nerve fibers begin to develop in the vestibular part. The sulcus limitans terminates at the rostral end of the mesencephalon and hence does not continue into the diencephalon. Several afferent tracts are appearing in the hindbrain, as detailed by O'Rahilly et al. (1984). The cervical flexure is appearing.
Two segments can still be distinguished in the midbrain. The tegmental area is indicated by an internal swelling. The term synencephalon is sometimes used for an area that is transitional between midbrain and forebrain. Two segments are still identifiable in the diencephalon. Segment 2 contains the future thalamus. Segment 1 gave rise to the optic evagination. The telencephalon medium (impar) is adjacent to the nasal plate.
Outline drawings of reconstructions of typical brains from stages 11–13 are shown in figure 13-10. All are at the same enlargement. A less advanced and a more advanced specimen of stage 11 are illustrated to cover the transformation incident to the closure of the rostral neuropore.
Eye
(fig. 13-11) In embryos belonging to stage 13 the optic evagination has grown and advanced in differentiation sufficiently to enable one to outline its parts, namely, optic part of retina, future pigmented layer of retina, and optic stalk. Four examples showing the range in development are shown in figure 13-11. These were selected from embryos all sectioned coronally. In such sections the evagination appears less rounded than when it is cut through its long axis, as in transverse series, and appears smaller. It is the better plane, however, in which to see the outline of the retina. The latter is still largely a flat disc, and its proliferating cells cause it to bulge inward. On the outer surface of the retinal disc, a marginal zone free of nuclei can be distinguished. This is the beginning of the framework through which the retinal fibers will pass on their way to the optic nerve. An abrupt transition occurs from the retinal disc to the thinner adjoining segment of the evagination, the pan that is to become the pigmented layer of the retina. This latter, in turn, is indistinctly set off from the part that is to form the stalk—a thick, short segment at the junction with the brain wall. The optic vesicle is covered by a basement membrane, and the surface ectoderm is lined by a basement membrane (O'Rahilly, 1966, fig. 10). In the mesoderm adjoining the optic evagination, early stages of angiogenesis can be noted.
The retinal disc presses outward and is in contact with the overlying surface ectoderm, with little or no mesoderm between them. In response to this contact the mesoderm proliferates and forms the lens disc. This has been shown to be an inductive phenomenon. Although when the lens plate begins to invaginate the specimen is generally allotted to the next stage, the beginning of an optic cup can be seen in a few embryos of stage 13 (O'Rahilly, 1966, fig, 22).
Ear
(fig. 13-12) For illustration of the range of development of the otic vesicle in stage 13, four specimens were selected in which the serial sections pass approximately through the long axis of the vesicle. Drawings of these, arranged in order of development, are shown in figure 13-12. It is to be pointed out that they are now all closed vesicles (Anson and Black, 1934)[1]. In the less developed specimens of the group a stalk of tissue may still attach the vesicle to the surface ectoderm, or the stalk may be detached from the vesicle but still be present as a tag or thickening of the overlying ectoderm. The cells included in this temporary stalk appear to be surface ectoderm stimulated to this activity by the adjacent neural ectoderm. At any rate, the stalk phenomenon has disappeared completely in the more advanced members of the group. Apparently the cells spread out in adaptation to the surface and resume their activity as ordinary surface ectoderm.
The otic vesicle is surrounded by the basement membrane of the otic disc (O'Rahilly, 1963).
Stage 13 covers the period of demarcation of the endolymphatic appendage. In the less developed specimens one can begin to recognize the segment of the vesicular wall that is going to form it. The cells of the appropriate region are characterized by a relatively greater amount of cytoplasm, meaning that this region is slightly further advanced in its differentiation. In macerated specimens these cells are more stable than those in the other parts of the vesicular wall, and they retain their form when the latter may have become completely disassociated. All the evidence points to a very early specialization of the potentialities of the several parts of the vesicular wall. In the more advanced specimens of the group the appendage projects dorsalward as a definite recess. By that time, when the tissues are sufficiently transparent, one can see it in a gross specimen as a distinct part of the vesicle.
This stage is further characterized by the reaction of the surrounding mesoderm to the growing vesicle. The first notable reaction is angiogenesis among the cells near the vesicle, producing a capillary network that closely invests it. This is followed by a proliferation of mesenchymal cells, which is greatest in the regions ventral and lateral to the vesicle. The vestibular part of the vestibulocochlear ganglion and vestibular nerve fibers can be distinguished.
Specimens of Stage 13 Already Described
- His embryo (a), 4 mm. Described in detail by His (1880).
- Fischel embryo, 4.2 mm, Hochstetter Atlas. This embryo is sharply and spirally curved, and the length given is approximate. Its place in stage 13 is verified by the form of the limb buds.
- Keibel embryo No. 112, 5.3 mm. Described in the Keibel and Elze Normentafeln (1908). The lens is a flat, slightly thickened disc. The otic vesicle is detached, but a remnant of the stalk is still present; there is some indication of the endolymphatic appendage. There are 36 pairs of somites.
- R. Meyer, 5-mm embryo, No. 318, Anatomisches Institut, Zurich. This embryo is a more advanced example of stage 13. The epithelium of the lens disc begins to indent. There are 38 pairs of somites. The embryo is referred to in the Keibel and Elze Normentafeln.
- C. Rabl, 4-mm embryo. The specimen closely resembles the Fol 5.6-mm embryo and the Hertwig G 31 embryo. It was used by Rabl in his study of the face (1902).
- Broman, embryo Lf., 3 mm, Anatomisches Institut, Lund. This embryo was described systematically by Broman (1896). He made revisions for the Keibel and Elze Normentafeln. Broman reports that it has 30 pairs of somites. Regarding its relatively small size, it is to be noted that it was fixed in absolute alcohol and then preserved for two years in weak spirits. This embryo is a less advanced example of stage 13.
- Carnegie No. 148, 4.3 mm. A monographic description of this embryo was published by Gage (1905).
- Fol, 5.6-mm embryo. This well-preserved embryo is a more advanced example. It was carefully described and illustrated by Fol (1884).
- Hertwig, 4.9-mm embryo, G31 Anatomisches-biologisches Institut, Berlin. This well-preserved embryo, a typical representative of stage 13, was first described in a study of the development of the pancreas by Jankelowitz (1895). An excellent systematic study followed later (Ingalis, 1907, 1908). The embryo was considered also in the Keibel and Elze Normentafeln. There are 35 pairs of somites.
- 4-mm and 5-mm twin embryos, University of Basel. A graphic reconstruction of the normal specimen was Issued by Müller and O'Rahilly (1980a), and reconstructions, with particular reference to the nervous system, were published by Müller and O'Rahilly (1984), who described the cerebral dysraphia (future anencephaly) present in one twin.
- Free Hospital for Women, Brookline, Massachusetts, No. 5, 5 mm. The histochemistry of this embryo was studied by McKay et al. (1955).
References
| Online Editor Note |
|---|
| O'Rahilly R. and Müller F. Developmental Stages in Human Embryos. Contrib. Embryol., Carnegie Inst. Wash. 637 (1987).
The original 1987 publication text, figures and tables have been altered in formatting, addition of internal online links, and links to PubMed. Original Document - Copyright © 1987 Carnegie Institution of Washington.
|
- ↑ Anson, B. J., and Black, W. T. 1934. The early relation of the auditory vesicle to the ectoderm in human embryos. Anat. Rec, 58, 127-137.
See also Müller F & O'Rahilly R. (1988). The development of the human brain from a closed neural tube at stage 13. Anat. Embryol. , 177, 203-24. PMID: 3354839
Search Pubmed
- 1987 Stages: Introduction | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | References | Appendix 1 | Appendix 2 | Historic Papers | Embryonic Development
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
Cite this page: Hill, M.A. (2024, April 26) Embryology 1987 Developmental Stages In Human Embryos - Stage 13. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/1987_Developmental_Stages_In_Human_Embryos_-_Stage_13
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G