Talk:Lymph Node Development: Difference between revisions
(→1996) |
(→1975) |
||
| Line 53: | Line 53: | ||
PMID: 1167215 | PMID: 1167215 | ||
http://www.ncbi.nlm.nih.gov/pubmed/1167215 | http://www.ncbi.nlm.nih.gov/pubmed/1167215 | ||
===Light and electron microscopic studies of postcapillary venules in developing human fetal lymph nodes=== | |||
Am J Anat. 1975 May;143(1):43-58. | |||
Bailey RP, Weiss L. | |||
Abstract | |||
Developing lymph nodes from 30 human fetuses with crownrump lengths (CRL) of 38 mm (8.7 wk) to 245 mm (26 wk) were studied by light and electron microscopy. Blood vessels that appear to be postcapillary venules (PCV) are present in nodes of 47 mm CRL and older fetuses. These venules first appear in nodes whehn the nodal population of lymphocytes is sparse. In these early nodes PCV are distributed randomly and consist of a low endothelium, underlying basal lamina and incomplete pericyte sheath. Early nodal PCV are distinguised from other nodal blood vessels by the presence of lymphocyte diapedesis and several luminal lymphocytes. In the late stages of nodal development PCV are the more common non-capillary blood vessel and appear in the parenchyma near the periphery of the node. Late nodal PCV are generally characterized by a cuboidal endothelium that is rich in Golgi apparatus, lysosomes and Weibel-Palade bodies. The lumen and wall of late nodal PCV contain lymphocytes. The relationship between the development of the parenchyma of fetal nodes and the appearance and activity of PCV, the passage of lymphocytes through the PCV wall and the fine structure of developing PCV are described. It is suggested that the lymphocytes that first appear in developing nodes, and the majority of the lymphocytes found in late nodes, migrate to the node via the blood vascular system and enter the nodal parenchyma by passing across PCV endothelium. | |||
PMID: 165702 | |||
http://www.ncbi.nlm.nih.gov/pubmed/165702 | |||
==Lymph Nodes== | ==Lymph Nodes== | ||
Revision as of 15:31, 16 April 2011
| About Discussion Pages |
|---|
On this website the Discussion Tab or "talk pages" for a topic has been used for several purposes:
Glossary Links
Cite this page: Hill, M.A. (2024, May 7) Embryology Lymph Node Development. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Talk:Lymph_Node_Development |
2009
Lymph sacs are not required for the initiation of lymph node formation
Vondenhoff MF, van de Pavert SA, Dillard ME, Greuter M, Goverse G, Oliver G, Mebius RE.
Development. 2009 Jan;136(1):29-34.
The lymphatic vasculature drains lymph fluid from the tissue spaces of most organs and returns it to the blood vasculature for recirculation. Before reaching the circulatory system, antigens and pathogens transported by the lymph are trapped by the lymph nodes. As proposed by Florence Sabin more than a century ago and recently validated, the mammalian lymphatic vasculature has a venous origin and is derived from primitive lymph sacs scattered along the embryonic body axis. Also as proposed by Sabin, it has been generally accepted that lymph nodes originate from those embryonic primitive lymph sacs. However, we now demonstrate that the initiation of lymph node development does not require lymph sacs. We show that lymph node formation is initiated normally in E14.5 Prox1-null mouse embryos devoid of lymph sacs and lymphatic vasculature, and in E17.5 Prox1 conditional mutant embryos, which have defective lymph sacs. However, subsequent clustering of hematopoietic cells within these developing lymph nodes is less efficient.
PMID: 19060331 http://www.ncbi.nlm.nih.gov/pubmed/19060331
2008
Structure and function of rat lymph nodes
Arch Histol Cytol. 2008 Sep;71(2):69-76.
Ohtani O, Ohtani Y.
Department of Anatomy, Faculty of Medicine and Graduate School of Medicine and Pharmaceutical Sciences, University of Toyama, Toyama, Japan. osmotani@med.u-toyama.ac.jp
Abstract
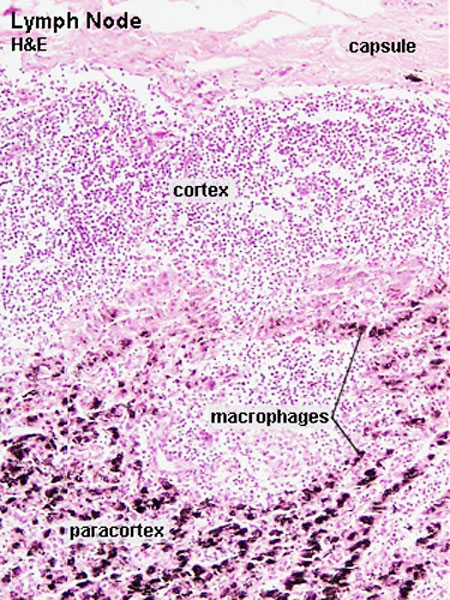
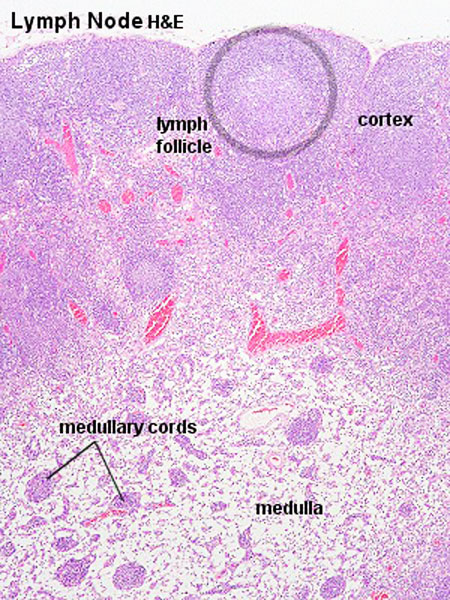
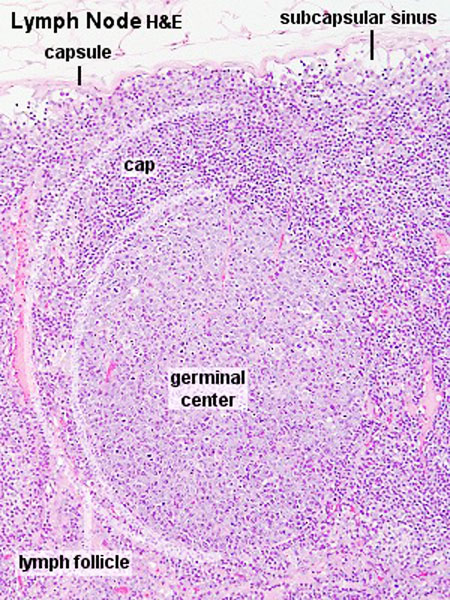
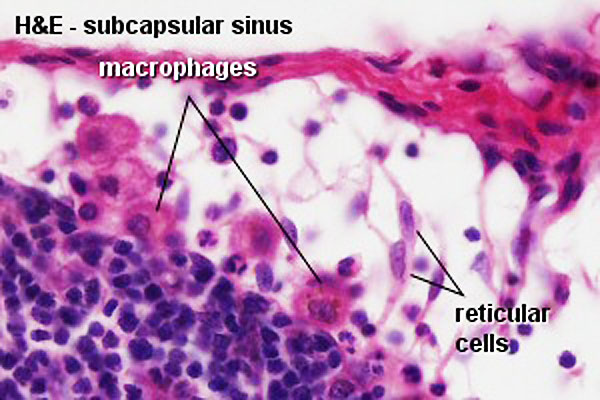
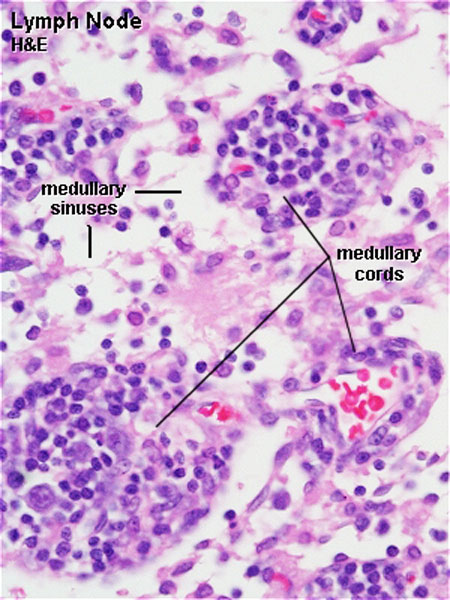
The lymph node comprises a critical crossroad for encounters between antigen presenting cells, antigens from lymph, and lymphocytes recruited into lymph nodes from the blood. The node consists of spaces lined with lymphatic endothelial cells and parenchyma. The former spaces can be divided into the subcapsular sinuses, lymphatic labyrinths in the deep cortex, intermediate sinuses, and medullary sinuses. The sponge-like framework of the node parenchyma is composed of collagen fibers invested with reticular cells. The parenchyma can be divided into the cortex, deep cortex, and medullary cord. Lymphocytes migrate from the node parenchyma into the lymphatic labyrinths in the deep cortex. Close to the labyrinths are high endothelial venules (HEVs), through which circulating lymphocytes enter the node parenchyma. HEVs strongly express Aquaporin-1, suggesting that HEVs are involved in the net absorption of water, but not protein, from lymph coming through afferent lymphatics. Many LYVE-1 positive sinus reticular cells (i.e., lymphatic endothelial cells) with attached macrophages form a network within the lumen of the medullary sinuses. Fluids and migrating cells arriving at the node preferentially flow through the subcapsular sinuses, intermediate sinuses, and medullary sinuses in this order. Fluids and migrating cells may also enter the cortex through gaps in the floor of the subcapsular sinuses. PMID: 18974599
1996
A developmental switch in lymphocyte homing receptor and endothelial vascular addressin expression regulates lymphocyte homing and permits CD4+ CD3- cells to colonize lymph nodes
Proc Natl Acad Sci U S A. 1996 Oct 1;93(20):11019-24.
Mebius RE, Streeter PR, Michie S, Butcher EC, Weissman IL. Department of Pathology, Stanford University School of Medicine, CA 94305, USA.
Abstract
IN adult mice, the dominant adhesion molecules involved in homing to lymph nodes are L-selectin homing receptors on lymphocytes and the peripheral lymph node addressins on specialized high endothelial venules. Here we show that, from fetal life through the first 24 hr of life, the dominant adhesion molecules are the mucosal addressin MAdCAM-1 on lymph node high endothelial venules and its counterreceptor, the Peyer's patch homing receptor, integrin alpha 4 beta 7 on circulating cells. Before birth, 40-70% of peripheral blood leukocytes are L-selectin-positive, while only 1-2% expresses alpha 4 beta 7. However, the fetal lymph nodes preferentially attract alpha 4 beta 7-expressing cells, and this can be blocked by fetal administration of anti-MAdCAM-1 antibodies. During fetal and early neonatal life, when only MAdCAM-1 is expressed on high endothelial venules, an unusual subset of CD4 + CD3- cells, exclusively expressing alpha 4 beta 7 as homing receptors, enters the lymph nodes. Beginning 24 hr after birth a developmental switch occurs, and the peripheral node addressins are upregulated on high endothelial venules in peripheral and mesenteric lymph nodes. This switch in addressin expression facilitates tissue-selective lymphocyte migration and mediates a sequential entry of different cell populations into the lymph nodes. PMID: 8855301
1975
Ontogeny of human fetal lymph nodes
Am J Anat. 1975 Jan;142(1):15-27.
Bailey RP, Weiss L.
Abstract
Developing lymph nodes from 30 human embryos and fetuses with crown-rump lengths (CRL) of 18 mm (5.6 wk) to 245 mm (26 wk) were examined by light microscopy. The nodes were embedded in araldite, and the sections examined were approximately 1 mu in thickness. The development of nodes was divided into three stages: 1. the lymphatic plexus and connective tissue invagination (30 mm to 67 mm CRL); 2. the early fetal lymph node (43 mm to ,5 mm CRL); and 3. the late fetal lymph node (CRL greater than 75 mm). The lymphatic plexus was formed by connective tissue invaginations and bridges which divided a lymph sac into a meshwork of channels and spaces. Connective tissue invaginations were endothelially-lined and were surrounded by lymphatic space. Reticular cells, macrophages, and blood vessels were found in these invaginations. Early fetal lymph nodes were formed from invaginations when the cellular density and lymphocyte content increased. The lymphatic space surrounding the early node was the developing subcapsular sinus. With further development the early node became packed with lymphocytes, increasing the cellular density and size of the node. The connective tissue surrounding the subcapsular sinus condensed to form the capsule. Afferent lymphatic vessels pierced the capsule. Capillaries, veins, postcapillary venules, and occasional arteries were found in early and late nodes.
PMID: 1167215 http://www.ncbi.nlm.nih.gov/pubmed/1167215
Light and electron microscopic studies of postcapillary venules in developing human fetal lymph nodes
Am J Anat. 1975 May;143(1):43-58.
Bailey RP, Weiss L.
Abstract
Developing lymph nodes from 30 human fetuses with crownrump lengths (CRL) of 38 mm (8.7 wk) to 245 mm (26 wk) were studied by light and electron microscopy. Blood vessels that appear to be postcapillary venules (PCV) are present in nodes of 47 mm CRL and older fetuses. These venules first appear in nodes whehn the nodal population of lymphocytes is sparse. In these early nodes PCV are distributed randomly and consist of a low endothelium, underlying basal lamina and incomplete pericyte sheath. Early nodal PCV are distinguised from other nodal blood vessels by the presence of lymphocyte diapedesis and several luminal lymphocytes. In the late stages of nodal development PCV are the more common non-capillary blood vessel and appear in the parenchyma near the periphery of the node. Late nodal PCV are generally characterized by a cuboidal endothelium that is rich in Golgi apparatus, lysosomes and Weibel-Palade bodies. The lumen and wall of late nodal PCV contain lymphocytes. The relationship between the development of the parenchyma of fetal nodes and the appearance and activity of PCV, the passage of lymphocytes through the PCV wall and the fine structure of developing PCV are described. It is suggested that the lymphocytes that first appear in developing nodes, and the majority of the lymphocytes found in late nodes, migrate to the node via the blood vascular system and enter the nodal parenchyma by passing across PCV endothelium.
PMID: 165702 http://www.ncbi.nlm.nih.gov/pubmed/165702
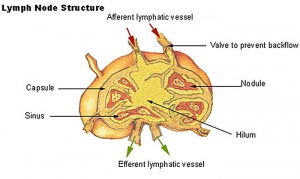
Lymph Nodes
Immunobiology - Figure 1.8. Organization of a lymph node
MBoC Figure 24-16. A simplified drawing of a human lymph node
- Encapsulated organ (1 mm - 2 cm)
- In lymph vessel pathways “filter”
- Afferent- towards node
- Efferent- away from node
- Location throughout the entire body - Concentrated in axilla, groin, mesenteries
- Antigen transformed lymphocytes from the blood
Lymph Node Structure
- Capsule - dense connective tissue
- Trabeculae - dense connective tissue
- Reticular Tissue - Reticular cells and fibers, supporting meshwork
Lymph
- enters the node through afferent vessels
- filters through the sinuses
- leaves through efferent vessels
Subcapsular sinus = marginal sinus
Continuation of trabecular sinus
Lymphocyte (T and B) Traffic
Links: Immunobiology - Figure 1.8. Organization of a lymph node | Blue Histology - Lymph Nodes