File:Thalidomide- hydrolyzed metabolites.jpg: Difference between revisions
From Embryology
mNo edit summary |
mNo edit summary |
||
| Line 1: | Line 1: | ||
===Hydrolyzed | ===Hydrolyzed Metabolites of Thalidomide=== | ||
Putative hydrolyzed metabolites of thalidomide were prepared and characterized, and their inhibitory activity on tumor necrosis factor (TNF)-alpha production in the human monocytic leukemia cell line THP-1 was evaluated. Alpha-(2-Carboxybenzamido)glutarimide was a more potent TNF-alpha production inhibitor than thalidomide. | Putative hydrolyzed metabolites of thalidomide were prepared and characterized, and their inhibitory activity on tumor necrosis factor (TNF)-alpha production in the human monocytic leukemia cell line THP-1 was evaluated. Alpha-(2-Carboxybenzamido)glutarimide was a more potent TNF-alpha production inhibitor than thalidomide. | ||
:"Thalidomide was developed in the 1950s as a nontoxic sedative/hypnotic drug, but was withdrawn from the market in the early 1960s because of its teratogenicity.However, it was subsequently identified as an effective agent for the treatment of multiple myeloma (MM), AIDS, Hansen’s disease, and various cancers. The US Food and Drug Administration (FDA) approved it first for the treatment of erythema nodosum in Hansen’s disease in 1998, and then, in combination with dexamethasone, for the treatment of MM in 2006." | :"Thalidomide was developed in the 1950s as a nontoxic sedative/hypnotic drug, but was withdrawn from the market in the early 1960s because of its teratogenicity.However, it was subsequently identified as an effective agent for the treatment of multiple myeloma (MM), AIDS, Hansen’s disease, and various cancers. The US Food and Drug Administration (FDA) approved it first for the treatment of erythema nodosum in Hansen’s disease in 1998, and then, in combination with dexamethasone, for the treatment of MM in 2006." | ||
:Links: | |||
===Reference=== | ===Reference=== | ||
Revision as of 13:41, 17 June 2016
Hydrolyzed Metabolites of Thalidomide
Putative hydrolyzed metabolites of thalidomide were prepared and characterized, and their inhibitory activity on tumor necrosis factor (TNF)-alpha production in the human monocytic leukemia cell line THP-1 was evaluated. Alpha-(2-Carboxybenzamido)glutarimide was a more potent TNF-alpha production inhibitor than thalidomide.
- "Thalidomide was developed in the 1950s as a nontoxic sedative/hypnotic drug, but was withdrawn from the market in the early 1960s because of its teratogenicity.However, it was subsequently identified as an effective agent for the treatment of multiple myeloma (MM), AIDS, Hansen’s disease, and various cancers. The US Food and Drug Administration (FDA) approved it first for the treatment of erythema nodosum in Hansen’s disease in 1998, and then, in combination with dexamethasone, for the treatment of MM in 2006."
- Links:
Reference
<pubmed>17409565</pubmed>| Chem Pharm Bull (Tokyo).PDF
Cite this page: Hill, M.A. (2024, April 30) Embryology Thalidomide- hydrolyzed metabolites.jpg. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/File:Thalidomide-_hydrolyzed_metabolites.jpg
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G
File history
Click on a date/time to view the file as it appeared at that time.
| Date/Time | Thumbnail | Dimensions | User | Comment | |
|---|---|---|---|---|---|
| current | 15:03, 9 September 2010 | 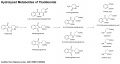 | 1,000 × 535 (57 KB) | S8600021 (talk | contribs) | ===Hydrolyzed metabolites of thalidomide.=== Putative hydrolyzed metabolites of thalidomide were prepared and characterized, and their inhibitory activity on tumor necrosis factor (TNF)-alpha production in the human monocytic leukemia cell line THP-1 was |
You cannot overwrite this file.
File usage
The following page uses this file: