2015 Group Project 3: Difference between revisions
(→Causes) |
|||
| Line 18: | Line 18: | ||
====Animal Models==== | ====Animal Models==== | ||
Studies conducted on female rhesus monkeys have supported evidence for the foetal origins of the clinical features of PCOS <ref name=five><pubmed>18406243</pubmed></ref>. It has been shown that in human females, an excess of androgen exposure at any stage from the development of the ovaries to the onset of puberty manifests as characteristic features of PCOS, such as resistance to insulin and Luteinising Hormone (LH) hypersecretion. After exposure to levels of testosterone matching those of fetal males, in utero, female rhesus monkeys were found to display the clinical and biochemical features typical of PCOS such as hypersecretion of LH and abnormal insulin action. These results correlated to those of similar studies conducted on sheep, where the pregnant ewe was exposed to excessive levels of testosterone and LH secretion and abnormality in ovarian cycles were again prominent features. (need footnote) While these studies indicate that similar biochemical and clinical manifestations in humans originate from excess androgen exposure of the female fetus, it must be noted that it is very unlikely for any excess androgen production to be passed across the placenta from the mother to her daughter. This is because mechanisms such as androgen binding proteins and placental metabolism of androgen prevent excess androgen entering the circulation of the fetus <ref name=six><pubmed>12098657</pubmed></ref>. These findings were followed by several studies investigating the source of excess androgen through cell culture models and human studies. | Studies conducted on female rhesus monkeys have supported evidence for the foetal origins of the clinical features of PCOS <ref name=five><pubmed>18406243</pubmed></ref>. It has been shown that in human females, an excess of androgen exposure at any stage from the development of the ovaries to the onset of puberty manifests as characteristic features of PCOS, such as resistance to insulin and Luteinising Hormone (LH) hypersecretion. After exposure to levels of testosterone matching those of fetal males, in utero, female rhesus monkeys were found to display the clinical and biochemical features typical of PCOS such as hypersecretion of LH and abnormal insulin action. These results correlated to those of similar studies conducted on sheep, where the pregnant ewe was exposed to excessive levels of testosterone and LH secretion and abnormality in ovarian cycles were again prominent features. (need footnote) While these studies indicate that similar biochemical and clinical manifestations in humans originate from excess androgen exposure of the female fetus, it must be noted that it is very unlikely for any excess androgen production to be passed across the placenta from the mother to her daughter. This is because mechanisms such as androgen binding proteins and placental metabolism of androgen prevent excess androgen entering the circulation of the fetus <ref name=six><pubmed>12098657</pubmed></ref>. These findings were followed by several studies investigating the source of excess androgen through cell culture models and human studies which also implicated the involvement of genes responsible for hyperandrogenism. | ||
===Genetic Factors=== | ===Genetic Factors=== | ||
Revision as of 21:53, 4 October 2015
| 2015 Student Projects | ||||
|---|---|---|---|---|
| 2015 Projects: Three Person Embryos | Ovarian Hyper-stimulation Syndrome | Polycystic Ovarian Syndrome | Male Infertility | Oncofertility | Preimplantation Genetic Diagnosis | Students | ||||
| 2015 Group Project Topic - Assisted Reproductive Technology | ||||
| This page is an undergraduate science embryology student and may contain inaccuracies in either description or acknowledgements. | ||||
Female Infertility
Female Infertility refers to the failure to conceive after one year of regular unprotected intercourse in females [1]. In 2010, infertility affected 48.5 million couples world wide and the areas of highest prevalence included North Africa and Middle East, South Asia, Central/Eastern Europe and Central Asia and Sub-Saharan Africa [2]. As there are several causes of female infertility, we will be focusing on infertility that is caused by Polycystic Ovarian Syndrome (PCOS), the most common cause of infertility that is medically treatable [1].
Polycystic Ovarian Syndrome
Polycystic Ovarian Syndrome is a common endocrine disorder, affecting up to 20% of reproductive aged women [3] It is the most common cause of anovulatory infertility [1] which refers to infertility caused by the the absence of ovulation.
Causes
PCOS is a disorder of heterogeneous origin with unknown aetiology [4].
Hyperandrogenism
Animal Models
Studies conducted on female rhesus monkeys have supported evidence for the foetal origins of the clinical features of PCOS [5]. It has been shown that in human females, an excess of androgen exposure at any stage from the development of the ovaries to the onset of puberty manifests as characteristic features of PCOS, such as resistance to insulin and Luteinising Hormone (LH) hypersecretion. After exposure to levels of testosterone matching those of fetal males, in utero, female rhesus monkeys were found to display the clinical and biochemical features typical of PCOS such as hypersecretion of LH and abnormal insulin action. These results correlated to those of similar studies conducted on sheep, where the pregnant ewe was exposed to excessive levels of testosterone and LH secretion and abnormality in ovarian cycles were again prominent features. (need footnote) While these studies indicate that similar biochemical and clinical manifestations in humans originate from excess androgen exposure of the female fetus, it must be noted that it is very unlikely for any excess androgen production to be passed across the placenta from the mother to her daughter. This is because mechanisms such as androgen binding proteins and placental metabolism of androgen prevent excess androgen entering the circulation of the fetus [6]. These findings were followed by several studies investigating the source of excess androgen through cell culture models and human studies which also implicated the involvement of genes responsible for hyperandrogenism.
Genetic Factors
Despite the lack of information regarding its aetiology, there is increasing evidence for a genetic involvement in the endocrine disorder. Experimental observations during cell culture and human studies show that genes play an important role in contributing towards the clinical and biochemical features of the disease [4].
Cell Culture Models
While studies have shown that the adrenal could be a source of excess androgen such as the adrenal [7], they were followed by various studies using cell culture models which demonstrated that the ovary was the major source of excess androgen [8] [9]. In vitro studies have shown that the production of steroids is abnormal in theca cells [6]. In women affected by PCOS, stimulation by human chorionic gonadotrophin (hCG) is followed by an increase in thecal steroid production after suppression of LH by a GnRH analogue [9]. Theca cells cultured from polycystic ovaries produce 20 times as much androstenedione as those from normal ovaries [8] with increased expression of mRNA for enzymes responsible for making steroids also reported in following studies. [10] These results have prompted many studies on genes encoding these steroidogeneic enzymes. There has been evidence linking the gene CYP11a with hyperandrogenism in women with PCOS. [11] While it doesn't isolate this gene as the sole cause of PCOS, variation of the genotype at this locus contributes to production of excess androgen [6].
Human Studies
Other studies have also indicated strong evidence for the genetic involvement in the aetiology of PCOS. This evidence includes the family clustering of cases, greater unanimity between monozygotic twins than heterozygotic twins and the ability to inherit endocrine and metabolic features of PCOS [6].
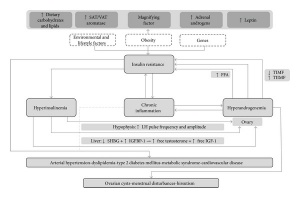
Pathogenesis
The pathogenesis of polycystic ovarian syndrome is poorly understood, however it is believed that insulin resistance and excess androgens such as testosterone play a fundamental role. Underlying factors such as obesity and genetic predispositions likely contribute to the onset of polycystic ovarian syndrome through the development of a combination of hyperinsulinemia and hyperandrogenemia.[9]
Hyperinsulinemia
Hyperinsulinemia is a term that defines the presence of excessive amounts of insulin in the blood relative to the amount of glucose. [12] This phenomenon is typically associated with obesity and excess adipose tissue, both of which are associated with insulin resistance. In order to overcome this resistance, insulin secretion is increased in an attempt to normalise blood glucose levels, thereby leading to hyperinsulineamia. [13] Excessive levels of insulin typically cause disruption to the hypothalamus-hypophysis-ovary axis whilst insulin resistance in ovarian tissues leads to decreased metabolic signalling. However, steroidogenic activity is largely unaffected by the impaired metabolic signalling, thereby paving the way androgens to become more effect and paving the way towards hyperandrogenemia. [14]
Hyperandrogenemia
Hyperandrogenemia is a hallmark of the development of polycystic ovarian syndrome, with 80% of women presenting with excess androgen also having polycystic ovaries. [15]
The Genetics of Infertility: Current Status of the Field
<pubmed>PMC3885174</pubmed> This article attempted to determine the role that genetics plays in female infertility. It was noted that several prominent causes of female infertility such as Galactosemia and Primary Ovarian Failure (POF) were associated with specific genes, with the GALT gene contributing to the former condition and the FMR1 gene contributing to the latter.
Causes of Sterility in Bosnia-Herzegovina Population
<pubmed>PMC4499307</pubmed> The study conducted as laid out in this article examined the causes of female sterility in the Bosnia-Herzegovina population. Married participants were arranged into various groups based upon their age. The experiment came to the conclusion that in approximately 42% of infertile married couples, female sterility was the primary cause. The two primary causes of female infertility were tubal deficiencies (31% of cases) and Diminished Ovarian Reserves (38% of cases)
Epidemiology, diagnosis, and management of polycystic ovary syndrome
<pubmed>PMC3872139</pubmed> This research article examines the causes of polycystic ovary syndrome (POS), a common cause of infertility in women. The article concluded that 50-70% of women suffer from insulin resistance secondary to Type 2 Diabetes and in many cases obseity, which may contribute partially to POS. Furthermore, 85-90% of women with oligomenorrhea also had POS, suggesting that it is an underlying cause. However, the exact pathophysiology of POS was not determined.
Signs and Symptoms
Diagnosis
MR imaging of disorders associated with female infertility: use in diagnosis, treatment, and management.[16]
MR imaging has extended its use to evaluating and diagnosing disorders related to female infertility, including ovulatory, fallopian tube, and uterine disorders, and also pelvic enometiosis. It is used in a variety of clinical settings by providing a clear vizualization of the pelvic region and is now useful in conjunction with other diagnostic techniques like laparoscopy, hysteroscopy, and hysterosalpinogography.
PMID 14615553
Comparison of diagnostic accuracy of laparoscopy, hysteroscopy, and hysterosalpingography in evaluation of female infertility. [17]
In a group of 77 women evaluated, Laparoscopy confirmed 84% evidence of tubal disease and hysteroscopy confirmed 69% radiographic evidence of intrauterine abnormalities. IN contrast HS gave a false-positive rate of 16% and 31% respectively. Data suggests that laparoscopy is best detecting previously unsuspected tubal disease, and hysteroscopy with information of managing abnormalities. But for optimum evaluation, a combined approach of all three procedures is best.
PMID 6232154
Diagnosis and Management of Female Infertility. [18]
Earlier assessment is best in women who have history of oligomenorrhea/amenorrhea, with key objective of assessment and diagnosis to rule out azoospermia, anovulation, or tubal obstruction. In order to do so an assesment of ovulatory function, uterine cavity, and tubal patency by HSG/laparoscopyis needed. Assessments like post coital tests, endometrial biopsies, and basal bdy temperature records are no longer a routine investigation.
PMID 14519712
Prevention
Impact of physical activity on ovarian reserve markers in normal, overweight and obese reproductive age women
<pubmed>25509968</pubmed> This study focuses on the effect of physical activity on fertility in three groups of women at reproductive age; normal, overweight and obese. The results from the study suggest that there was a marked improvement in fertility as shown by the ovarian reserve markers for all 3 groups, however it was most significant in the overweight and obese groups. This study is relevant to our project as it gives an insight into the preventative measures that can be taken for infertility.
Risk factors of polycystic ovarian syndrome among Li People
<pubmed>26276294</pubmed> This study examined the risk factors entailed in Polycystic Ovarian Syndrdome (PCOS) amoung Li people. Using the method of a case control study, questionnaires were given to female Li people with and without PCOS. Analysis of the questionnaires showed that family history of diabetes, family history of infertility, bad mood, lack of physical exercise are all high risk factors of PCOS. As a result, management of these risk factors can be taken into consideration when preventing infertility through PCOS.
Vitamin D and female fertility
<pubmed>24717915</pubmed> This article is a review focusing on research regarding Vitamin D and fertility over the past year. The review found that the levels of Vitamin D is crucial for women undergoing in-vitro fertilisation. It was also found that Vitamin D was beneficial for women with PCOS and carried a protective effect against endometriosis. These observations suggest that having sufficient Vitamin D in your body can be preventative for problems associated with fertility.
Treatment/Prevention
The Effect of a Complex Multi-modality Ayurvedic Treatment in a Case of Unknown Female Infertility
This article documented a 38 year old woman's journey to giving birth, after she was advised that she was infertile (of unknown cause). The researchers detail the various modern day medical treatments she underwent to become pregnant, all of which were unsuccessful. She then decided to try holistic medicine in the form of Ayurvedic treatment, which consisted of meditation, a controlled diet and yoga. To the researchers' surprise, she became pregnant soon after, and gave birth to a healthy baby boy in 2012. The study comes to the conclusion that Ayurvedic medicine and successful birth rates do not show a strong correlation, and thus should not be favoured over standard medical treatments. However, they did state that holistic medicine could improve the overall wellbeing of the mother (in terms of stress and diet), which in turn increases the chances of becoming pregnant.
<pubmed>26278074</pubmed>
* * *
Pregnancy Rate after Controlled Ovarian Hyperstimulation and Intrauterine Insemination for the Treatment of Endometriosis following Surgery
This study investigated various treatments available for women with endometriosis, and the rate of successful pregnancies and births in patients following their treatment. The researchers came to the conclusion that a woman's chances of becoming pregnant increased following laparoscopic surgery, particularly in the first six months following the procedure. If pregnancy does not occur, controlled ovarian hyperstimulation and intrauterine insemination (COH-IU) should be the next option, due to its success rates.
<pubmed>26247014</pubmed>
* * *
Time-limited Hydrotubation Combined with Clomiphene Citrate Treatment for Unexplained Infertility
In this experiment, 80 random patients with "unexplained inferility" were selected and treated with hydotrubation and clomiphene citrate (CC). Of the 80 patients, 15 became pregnant, with the researchers concluding that combined hydrotubation and CC treatment increased a woman's chance of becoming pregnant to a greater degree than just CC alone. Further tests need to be completed to strengthen the correlation between the treatment and outcome.
<pubmed>26152000</pubmed>
* * *
Frequency and Outcome of Treatment in Polycystic Ovaries Related Infertility
This study examined treatments available to women with Polycystic Ovarian Syndrome (PCOS). It came to the conclusion that in overweight women with PCOS, weight loss, exercise and better lifestyle choices are the best treatments as they significantly increases pregnancy rates. Furthermore, clomiphene citrate (CC) and metformine combined treatments are highly effective in PCOS women, and should be one of the first options offered to a patient.
<pubmed>26150870</pubmed>
Polycystic Ovarian Syndrome
'TREATMENT'
<pubmed>11034687</pubmed>
<pubmed>26289303</pubmed>
<pubmed>26267328</pubmed>
<pubmed>26194691</pubmed>
<pubmed>26177098</pubmed>
References
- ↑ 1.0 1.1 1.2 <pubmed>26150870</pubmed>
- ↑ <pubmed>23271957</pubmed>
- ↑ <pubmed>199103210</pubmed>
- ↑ 4.0 4.1 <pubmed>21896560</pubmed>
- ↑ <pubmed>18406243</pubmed>
- ↑ 6.0 6.1 6.2 6.3 <pubmed>12098657</pubmed>
- ↑ <pubmed>7962325</pubmed>
- ↑ 8.0 8.1 <pubmed>7962289</pubmed>
- ↑ 9.0 9.1 9.2 <pubmed>9302378</pubmed> Cite error: Invalid
<ref>tag; name 'nine' defined multiple times with different content - ↑ <pubmed>10852468</pubmed>
- ↑ <pubmed>9147642</pubmed>
- ↑ <pubmed>PMC4114053</pubmed>
- ↑ <pubmed>PMC2782313</pubmed>
- ↑ <pubmed>PMC4334071</pubmed>
- ↑ <pubmed>PMC3872139</pubmed>
- ↑ <pubmed>14615553</pubmed>
- ↑ <pubmed>6232154 </pubmed>
- ↑ <pubmed>14519712</pubmed>
SIGN IN
--Z3462166 (talk) 13:43, 21 August 2015 (AEST)
--Z3460352 (talk) 13:44, 21 August 2015 (AEST)
--Z3459224 (talk) 13:52, 21 August 2015 (AEST)
--Z3416054 (talk) 13:56, 21 August 2015 (AEST)
--Z3460352 (talk) 13:51, 28 August 2015 (AEST)
--Z3462166 (talk) 13:51, 28 August 2015 (AEST)
| 2015 Student Projects | ||||
|---|---|---|---|---|
| 2015 Projects: Three Person Embryos | Ovarian Hyper-stimulation Syndrome | Polycystic Ovarian Syndrome | Male Infertility | Oncofertility | Preimplantation Genetic Diagnosis | Students | ||||
| 2015 Group Project Topic - Assisted Reproductive Technology | ||||
| This page is an undergraduate science embryology student and may contain inaccuracies in either description or acknowledgements. | ||||