2014 Group Project 3: Difference between revisions
| Line 222: | Line 222: | ||
If the cloaca ruptures before completely partitioning, it can lead to extrophy. Although extremely rare with only occuring in one in 200 000-400 000 births, it requires immediate surgical attention postnatal <ref name="PMID12651743"><pubmed>12651743</pubmed></ref>. It arises from the failure of the caudal fold to close. Cloacal extrophy results in a child being born with with many inner-abdominal structures exposed. A portion of the large intestine lies outside of the body, and on either side of it are the two halves of the bladder. To diagnose this, MRI Imaging is usually performed during development to detect the 'elephant trunk sign' of cloacal extrophy<ref name="PMID22878705 "><pubmed>22878705</pubmed></ref> | If the cloaca ruptures before completely partitioning, it can lead to extrophy. Although extremely rare with only occuring in one in 200 000-400 000 births, it requires immediate surgical attention postnatal <ref name="PMID12651743"><pubmed>12651743</pubmed></ref>. It arises from the failure of the caudal fold to close. Cloacal extrophy results in a child being born with with many inner-abdominal structures exposed. A portion of the large intestine lies outside of the body, and on either side of it are the two halves of the bladder. To diagnose this, MRI Imaging is usually performed during development to detect the 'elephant trunk sign' of cloacal extrophy<ref name="PMID22878705 "><pubmed>22878705</pubmed></ref> | ||
[[File:383 2012 3133 Fig2 HTML.jpg]] | |||
MRI of Cloacal extrophy during fetal development showing omphalocele (a), neural tube defect (b) and bladder exstrophy (c) | |||
Revision as of 11:26, 8 October 2014
| 2014 Student Projects | ||||
|---|---|---|---|---|
| 2014 Student Projects: Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | Group 6 | Group 7 | Group 8 | ||||
| The Group assessment for 2014 will be an online project on Fetal Development of a specific System.
This page is an undergraduate science embryology student and may contain inaccuracies in either description or acknowledgements. | ||||
Gastrointestinal System
--Mark Hill (talk) 15:12, 26 August 2014 (EST) No sub-headings yet, get moving on your project.
--Mark Hill (talk) 15:56, 6 September 2014 (EST) While you have divided the GIT into sections, there is no feral specific development described or media yet.
GIT system Overview
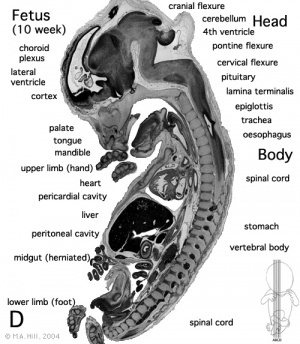
The GIT system is a complicated system that extends from the Esophagus of the mouth to the anal canal of the hind-gut. Its function is to ultimately turn food that is eaten into energy. GIT (Gastrointestinal Track) consist of the Fore-gut, Mid-gut and Hind-gut. Majority of the organs are located in the fore-gut. This includes the stomach, duodenum, Liver, pancreas and the spleen. The mid-gut begins below the hepato-pancreatic ampulla and consist of the lower Duodenum, Jejunum, Ileum, Cecum, Appendix and the Ascending colon as well as the first two third of the transverse colon. In fetal development after the rotation and fixation of the mid-gut is complete, it starts to herniate at beginning of week 6 and continues to do so till week 10. Hind-gut begins from the left third of the transverse colon and ends to the cloaca (rectum). It consists of the left third of transverse colon, descending colon, sigmoid colon, rectum and anal canal. There is no rotation occurring in the hind-gut instead it gets pushed to the left side by mid-gut during development.
Timeline
Foregut
Week 4:
- Ventral outgrowth (hepatic diverticulum) of liver, gallbladder and bile duct
Week 6:
- Liver obtains a bright reddish appearance due to hematopoiesis
Week 7:
- 90 degrees clockwise rotation of the stomach
Week 8:
- Occluded lumen and vacuoles appear in the oesophagus [[1]]
- The outer and inner muscle layers of the oesophagus development [[2]]
- Due to the stomach rotation, the duodenum is pulled into a "C" shaped position
Week 9:
- Liver is 10% of the total fetus weight
Week12:
- Bile formation via hepatic cells begin
Week 13:
- Bile entering via bile duct into the duodenum gives the intestinal contents a dark green colour (meconium)
Midgut
Week 6: -Embryonic Rotations[[3]]
week 9:
-Interstitial cells of Cajal present in the small intestine[[4]]
week 10:
-Midgut Herniation[[5]]
-Interstitial cells of Cajal present in the large intestine[[6]]
week 11:
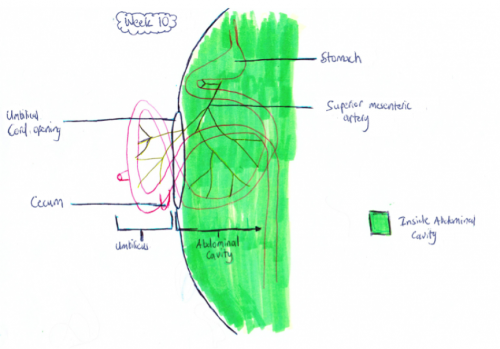
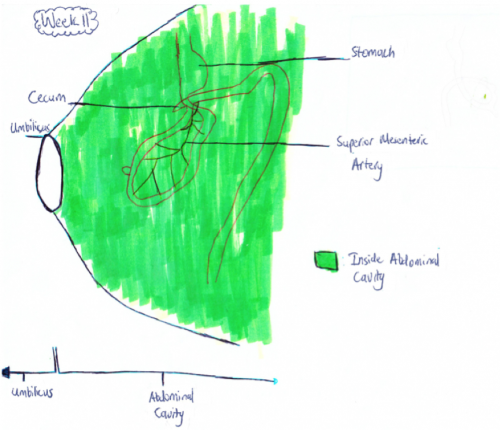
-Retraction of Midgut[[7]]
week 19:
-Peyers patches formed in small intestine[[8]]
Hindgut
Week6-
• Cecum diverticulum appears
Week9-
• A shallow pit known as the anal pit it is formed due to the proliferation of mesenchyme located around the anal membrane giving rise to the surrounding ectoderm`
Recent findings
http://www.ncbi.nlm.nih.gov/pubmed/16369776
Recent Findings on Omphalocele:
In a recent research experiment, the Hh hedge-hog signalling pathway was believed to be a possible causative factor of omphalocele formation. The TM -inducible gene recombination system was used to show association of Hh signalling with omphalocele. Omphalocele was prominently observed in embryos from dams treated with the higher dose of TM (2 mg/40 g bw) but not with the lower dose (1 mg/40 g bw). The study observed ectopic Hh signal activity in the ventral wall region through del5-LacZ staining and a gain of function mutants of Hh signalling expressed defects in the body wall, therefore results suggested ectopically induced Hh signalling was a potential causative agent in a dose dependant formation of omphacele.
<pubmed>3024424</pubmed>
Foregut
The foregut consists of the following organs [1] :
- Oesophagus
- Stomach
- Liver
- Gallbladder and Bile Duct
- Pancreas
- Duodenum
Oesophagus
[2] The primordium of the oesophagus is a portion between the respiratory diverticulum and stomach dilation. The oesophagus is a short tube initially but elongates significantly over time as the fetus grows. Oesophagus has an endoderm derived epithelial lining which proliferates. The epithelial lining also undergoes a series of transformations. Occluded lumen appears by week eight as well as vacuoles. With time the lumen is recanalized and the vacuoles combine. The epithelium of the oesophagus becomes stratified squamous during the fourth month. The development of muscle layers consisting of outer and inner layers, are recognised by eight weeks. The oesophagus contains smooth (splanchnic mesoderm derivative) and skeletal muscle fibres.
Stomach
Dorsal and ventral mesenteries anchor the developing stomach to the body walls. In the 7th week, the stomach undergoes a 90 degrees clockwise rotation about a longitudinal axis. The rotation is such that the right side moves dorsally and left side moves ventrally. The vagus nerve follows this stomach rotation such that the anterior surface becomes left vagus nerve and the right vagus nerve becomes the posterior surface. Since the left vagus nerve is located on the anterior surface and right vagus nerve on the posterior surface, they are renamed as anterior vagal trunk and posterior vagal trunk respectively. The growth of the dorsal wall of the stomach is faster than the ventral wall. [3] The greater and lesser curvatures of the stomach are established via the different growth on the left and right sides. Pylorus is tipped superiorly by cranio-caudal rotation. Pyloric sphincter is formed by the proliferation of the mesoderm derived smooth muscle. This smooth muscle lies in the caudal end of the stomach. The stomach is pulled up by the rotation of the stomach and duodenum about a ventrodorsal (A-P) axis. In the 8th week, due to these rotations, the duodenum is pulled into a C-shaped position. Hence the postnatal position for the stomach and duodenum is achieved.
The greater and lesser omenta are formed by the dorsal and ventral mesenteries of the stomach respectively. Also the ventral mesentery is attached to the developing liver. Distinct spaces of the peritoneal cavity is produced by the rotations of the forgut structures and the development of the omenta. The space posterior to the stomach is called the lesser sac or omental bursa. The space anterior to the stomach is called the greater sac. The greater sac is anteriorly inferior to the stomach. A small opening is located near the liver’s hilum which is called the epiploic foramen. The greater and lesser sacs communicate through the epiploic foramen. From 4 layers of peritoneum, a thick sheet is forms via the anterior and posterior folds of the greater omentum fusing.
Liver, Gallbladder and Bile Duct
Early in the fourth week of the foregut development, the liver, biliary duct system and gallbladder are seen as ventral outgrowth (hepatic diverticulum). This ventral outgrowth is from the caudal/distal part of the foregut. Hepatic diverticulum is formed via the interaction between bipotential cells and FGF’s which is secreted by the developing heart. There is a mass of splanchnic mesoderm between the midgut and the developing heart. This mass is called septum transversum which is an extension of diverticulum. The ventral mesentry for this region is then formed by the septum transversum.
As the hepatic diverticulum grows in between the layers of ventral mesogastrium, it divides into two parts. Primordium of the liver is the larger cranial part of the hepatic diverticulum. The kupffer cells, fibrous and hematopoietic tissues of the liver are all derived by mesenchyme in the septum transversum.
From the 5th to 10th weeks, the liver grows rapidly and fills the upper abdominal cavity largely. The development and segmentation of the liver is determined by the amount of oxygenated blood flowing from the umbilical vein and into the liver itself. At first, both right and left lobe are of the same size but eventually the right lobe becomes larger. During the 6th week, hematopoiesis begins which gives liver a bright reddish appearance. The liver will account for 10% of the total fetus weight by the ninth week. The bile formation begins during the 12th week. Bile is formed by hepatic cells.
The gall bladder is formed by the small caudal part of the hepatic diverticulum. The cystic duct is formed by the stalk of the diverticulum. The connection between the hepatic and cystic ducts via stalk to the duodenum becomes the bile duct. Initially the bile duct is to the ventral aspect of the duodenum but as the duodenum grows and rotates, the bile duct entrance is carried to the dorsal aspect of the duodenum. After the 13th week, the bile entering the duodenum via bile duct gives the meconium (intestinal contents) a dark green colour.
Ventral mesentery is a thin double layered membrane which gives rise to:
- The lesser omentum passing from the liver to the lesser curvature of the stomach (hepatogastric ligament) and from liver to duodenum (hepatoduodenal ligament)
- Extending from the liver to the ventral abdominal wall is the falciform ligament.
From the umbilical cord to the liver, the umbilical vein passes on the border of falciform ligament. Also the visceral peritoneum of the liver is derived from ventral mesentery which is derived from mesogastrum. The whole liver is covered with peritoneum except the bare area which is in direct contact with the diaphragm.
Midgut
Blood Supply: superior mesenteric artery[7].
Midgut Rotations (Embryonic)
Development of the midgut in the embryonic period is characterized by rapid elongation of the gut and its mesentery. The loops of the week 10 intestine position as shown in the hand drawn illustration below is a result of embryonic rotations.
Midgut Herniation
Herniation of the midgut usually begins around week 5 of the embryonic period. The herniation occurs as a result of the intestine (particularly the ileum) growing faster than the abdominal cavity during this embryonic period. As seen in the table below it is thought that all midgut herniation’s of the fetus should occur by weeks 9-10 [8].
| Week of Gestation | Percentage of Foetuses Herniated |
|---|---|
| 8 | 64% (note: embryo) |
| 9 | 100% |
| 10 | 100% |
| 11 | 25% |
| 12 | 0% |
Table 1.1: Percentage of Herniated midguts during weeks 8-12[9].
Week 10: The herniation occurs because of the lack of space in the intra-abdominal cavity mainly due to the large liver and kidneys. As a result the rapidly growing intestinal loops of the midgut are herniated at the umbilicus to accommodate for this lack of space.
Week 10 Herniated Midgut[10]
Retraction of Midgut
Week 11: The intestinal loops that have migrated to the umbilicus usually return to the abdominal cavity between 8 and 12 weeks of gestation[11]. This is shown in figure 1.b as now all the intestine is back in the abdominal cavity in comparison to the week 10 fetus whose midgut was still herniated. Although it is not exactly known why the midgut goes back into the abdominal cavity it is thought that the following factors play a major role:
-growth and expansion of the abdominal cavity to cover the herniated midgut
-regression of mesonephric kidney for more space
-reduced growth of the liver for more space[12]
Week 11 Midgut[10]
Features of Midgut
Peyer's Patches Peyer's patches are organised lymphoid nodules. By week 30 of gestation the fetal small intestine contains on average 60 peyer's patches[13]. During weeks 15-16 there is a rapid spurt in which the development and maturation of lymphoid follicles of T and B cells allows the continual development of the foci of peyers patches at a continual rate within the small intestine[14]. At week 19 these aggregations mature into recognisable peyer's patches. In week 24 of gestation the patches become macroscopically visible[13].
Interstitial cells of cajal; Interstitial cells of Cajal (ICC) are specialised network-forming cells that play important roles in the control of digestive motility. In the small intestine c-kit immunoreactive (c-kit IR) cells identifiable as interstitial cells of Cajal appear in week 9. ICC cells then appear in between weeks 10-12 in the large intestine[15]. The ICC cells are only present in the proximal duodenum at the end of the embryonic period in the form of a wide belt of tightly packed cells around the inception of the myenteric plexus ganglia. The ICC cells emerge in the distal duodenum at the beginning of the fetal period in the arrangement of thin rows of pleomorphic cells at the level of the myenteric plexus[16].
Midgut Structures
Caecum The caecum is located at the right bottom corner at the beginning of the fetal period. Towards the end of the fetal life it descends towards the right iliac fossa. This transition is heavily related to the ascending colon during this time.It is in the shape of a long tube during this fetal time and similar tot hat of a succule, the right side is larger[17].
Appendix The vermiform appendix increases markedly in length during the fetal period and is closely related to the growth of the caecum, so that at birth it becomes a worm-shaped tube arising from the distal end of the caecum[17].
Hindgut
Cloaca partitioning
By the end of week seven, the urorectal septum, a coronal ridge of mesenchyme has formed down the angle between the allantois and hindgut and fuses with the cloacal membrane. This forms an anterior urogenital sinus and a posterior anorectal canal. The anterior portion of the membrane that forms the urogenital membrane is larger than its posterior anal membrane. In Week 9, there is proliferation of the the esenchyme around the anal membrane raising the surrounding ectoderm, forming the proctodeum (a shallow pit). There are swelling around this pit that are refered to as anal folds. The rectal membrane at the base of the anal pit, soon ruptures to create the anal canal which connects the GIT from the rectum to the amniotic cavity.
The junction between the anal pit ectoderm and hindgut endoderm Is designated by the anatomic anorectal or pectinate (dentate) line, the former site of the anal membrane. This area is where the epithelium changes from columnar to stratified squamous epithelium. This squamous tissue begins to keratinise and becomes continueous with the perineum. Within the hindgut portion, the anal canal is supplied by the inferior mesenteric artery, whereas the anal pit is spuulied by the internal pudenal branch of the internal iliac artery
Anorectal deformities
There are 3 major types of anorectal deformities that can be found clinically
- imperforate anus (abscent): the rectal membrane doesn’t rupture leaving Gastrintestinal tract to be completely or incompletely walled off from the outside. All varieties of this deformity require immediate surgical intervention at birth
- Insufficient anus leads to problems of meconium evacuation and should be treated without delay
- Ectopic sinus: does allow some intestinal transport, but is usually functionally insufficient
Cloacal Extrophy
If the cloaca ruptures before completely partitioning, it can lead to extrophy. Although extremely rare with only occuring in one in 200 000-400 000 births, it requires immediate surgical attention postnatal [18]. It arises from the failure of the caudal fold to close. Cloacal extrophy results in a child being born with with many inner-abdominal structures exposed. A portion of the large intestine lies outside of the body, and on either side of it are the two halves of the bladder. To diagnose this, MRI Imaging is usually performed during development to detect the 'elephant trunk sign' of cloacal extrophy[19]
File:383 2012 3133 Fig2 HTML.jpg MRI of Cloacal extrophy during fetal development showing omphalocele (a), neural tube defect (b) and bladder exstrophy (c)
Developmental problems
Superficial deformities;
• Anal agenesis or insufficient anus (with or without fistula): • Membranous atresia or covered anus (with or without fistula • Anorectal agenesis with or without fistula
Deep deformities:
• Pure rectal atresia: complete failure of the formation of the inferior part of the rectum and anal canal • Rectal atresia with fistula: always insufficient. The length and degree of anastomosis differentiate the various types of anomaly
Mixed deformities include all forms of ectopic anus
• May involve abnormal anastomoses of the anus to the perineum and reflect both perineal and cloacal abnormalities
1.<pubmed>10716947</pubmed> 2.<pubmed>12171973</pubmed> 3.<pubmed>23073994</pubmed>
Deformities
Gastroschisis
Definition:
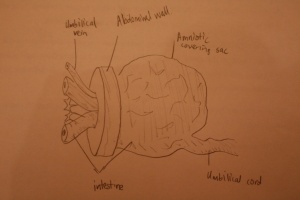
Gastroschisis is a congenital birth defect which can be diagnosed early in fetal development. It is the most common neonatal abdominal wall defect which occurs equally in males and females. Studies suggest white population is more affected by this abnormality in comparison to Hispanics and dark skinned people.[20] It is a defect of the anterior abdominal wall, usually to the right of the umbilical cord and the peritoneal membrane that normally covers the bowel is absent. The defect involves all layers of the abdominal wall and in this abnormality, an infant’s intestine (small and large intestine) protrude out and into the amniotic fluid. Other organs such as the stomach and liver can also bulge out from the hole and no membrane covers these organs in this abnormality.
Possible cause:
The cause of Gastroschisis is not completely clear however a potential mechanism include tearing at the base of umbilical cord before the umbilical ring closes or the failure of one or more folds in the abdominal wall to fuse completely and in the correct manner. Due to the failure of the closing as stated above, organs protrude out and the eviscerated bowl is exposed to amniotic fluid for a long period of time, causing mucosal and muscular injury. The organs that remain out of the body wall are also exposed and vulnerable to infection in the open air as an infant is born. In more uncommon cases, chromosomal syndromes such as trisomy 18, 13, or 21 or sex chromosome anomalies have been found in association with Gastroschisis. In recent findings Gastroschisis has also been seen to be a result from vascular events causing disruption of fetal abdominal wall. For example Maternal vascular under perfusion is a possible underlying cause of intermittent ischemia to the abdominal wall.
Omphalocele
Definition/overview
Omphalocele is a common midline abdominal wall defect of variable size affecting 2-3 infants per 10,000 births worldwide. [21] It is similar to Gastroschisis however, it is characterised with the absence of fascia, muscle, and skin and occurs due to a defect in the development of the muscles in the abdominal wall.[21] A fetus with omphalocele tends to have herniation of abdominal contents into the base of the umbilical chord. A membranous sac that has amnion and peritoneum covers these contents lying within the umbilical chord. Omphalocele in the later stage of fetal development (approximately week 11) occurs after normal infolding of the embryo therefore having formed an abdominal cavity. When the umbilical ring does not close around the umbilical cord then this small defect occurs containing only the intestine.[21]
<pubmed>4075420<pubmed/>
<pubmed>23553304<pubmed/>
<pubmed>2960962<pubmed/>
Abnormalities that can occur in GIT system during fetal development
List of research/articles:
1.<pubmed>22777173</pubmed> 2.<pubmed>3832654</pubmed> 3.[22]
References
- ↑ <pubmed>19575677</pubmed>
- ↑ <pubmed>22750256</pubmed>
- ↑ <pubmed>9664826</pubmed>
- ↑ <pubmed>16052677</pubmed>
- ↑ <pubmed>23799566</pubmed>
- ↑ <pubmed>23720330</pubmed>
- ↑ <pubmed>24891783</pubmed>
- ↑ <pubmed>14745932</pubmed>
- ↑ <pubmed>2528908</pubmed>
- ↑ 10.0 10.1 <pubmed>2908440</pubmed>
- ↑ <pubmed>9203209</pubmed>
- ↑ <pubmed>8345550</pubmed>
- ↑ 13.0 13.1 <pubmed>18668776</pubmed>
- ↑ <pubmed>2276071</pubmed>
- ↑ <pubmed> 24414177</pubmed>
- ↑ <pubmed> 21352475</pubmed>
- ↑ 17.0 17.1 <pubmed>11316934</pubmed>
- ↑ <pubmed>12651743</pubmed>
- ↑ <pubmed>22878705</pubmed>
- ↑ <pubmed>11778986</pubmed>
- ↑ 21.0 21.1 21.2 4075420<pubmed>4075420</pubmed> Cite error: Invalid
<ref>tag; name 'PMID' defined multiple times with different content Cite error: Invalid<ref>tag; name 'PMID' defined multiple times with different content - ↑ Charles D. Bluestone M.D., Roy Kerry M.D. andWilliam K. Sieber M.D,2009,January,Congenital esophageal stenosis†‡,The Laryngoscope,volume79,issue 6,1095–1104,http://onlinelibrary.wiley.com.wwwproxy0.library.unsw.edu.au/doi/10.1288/00005537-196906000-00004/pdf}}