Talk:Hearing - Outer Ear Development
| About Discussion Pages |
|---|
On this website the Discussion Tab or "talk pages" for a topic has been used for several purposes:
Glossary Links
Cite this page: Hill, M.A. (2026, March 1) Embryology Hearing - Outer Ear Development. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Talk:Hearing_-_Outer_Ear_Development |
2016
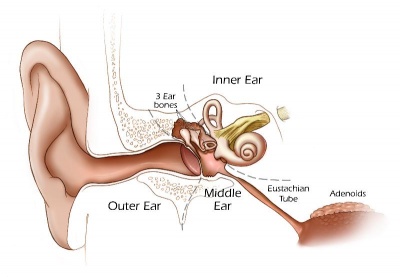
The development of the mammalian outer and middle ear
J Anat. 2016 Feb;228(2):217-32. doi: 10.1111/joa.12344. Epub 2015 Jul 30.
Anthwal N1, Thompson H1.
Abstract
The mammalian ear is a complex structure divided into three main parts: the outer; middle; and inner ear. These parts are formed from all three germ layers and neural crest cells, which have to integrate successfully in order to form a fully functioning organ of hearing. Any defect in development of the outer and middle ear leads to conductive hearing loss, while defects in the inner ear can lead to sensorineural hearing loss. This review focuses on the development of the parts of the ear involved with sound transduction into the inner ear, and the parts largely ignored in the world of hearing research: the outer and middle ear. The published data on the embryonic origin, signalling, genetic control, development and timing of the mammalian middle and outer ear are reviewed here along with new data showing the Eustachian tube cartilage is of dual embryonic origin. The embryonic origin of some of these structures has only recently been uncovered (Science, 339, 2013, 1453; Development, 140, 2013, 4386), while the molecular mechanisms controlling the growth, structure and integration of many outer and middle ear components are hardly known. The genetic analysis of outer and middle ear development is rather limited, with a small number of genes often affecting either more than one part of the ear or having only very small effects on development. This review therefore highlights the necessity for further research into the development of outer and middle ear structures, which will be important for the understanding and treatment of conductive hearing loss. KEYWORDS: Eustachian tube cartilage; development; embryonic origin; external auditory meatus; middle ear; ossicles; outer ear; tympanic membrane
PMID: 26227955 PMCID: PMC4718165 DOI: 10.1111/joa.12344
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4718165/
Developmental mechanisms of the tympanic membrane in mammals and non-mammalian amniotes
Congenit Anom (Kyoto). 2016 Jan;56(1):12-7. doi: 10.1111/cga.12132.
Takechi M1, Kitazawa T2,3, Hirasawa T4, Hirai T4, Iseki S1, Kurihara H2,5,3, Kuratani S4.
Abstract
The tympanic membrane is a thin layer that originates from the ectoderm, endoderm, and mesenchyme. Molecular-genetic investigations have revealed that interaction between epithelial and mesenchymal cells in the pharyngeal arches is essential for development of the tympanic membrane. We have recently reported that developmental mechanisms underlying the tympanic membrane seem to be different between mouse and chicken, suggesting that the tympanic membrane evolved independently in mammals and non-mammalian amniotes. In this review, we summarize previous studies of tympanic membrane formation in the mouse. We also discuss its formation in amniotes from an evolutionary point of view. © 2015 Japanese Teratology Society. KEYWORDS: Goosecoid; middle ear; morphological evolution; pharyngeal arch; tympanic membrane
PMID 26754466
2015
Developmental genetic bases behind the independent origin of the tympanic membrane in mammals and diapsids
Nat Commun. 2015 Apr 22;6:6853. doi: 10.1038/ncomms7853.
Kitazawa T1, Takechi M2, Hirasawa T3, Adachi N3, Narboux-Nême N4, Kume H5, Maeda K6, Hirai T3, Miyagawa-Tomita S6, Kurihara Y1, Hitomi J7, Levi G4, Kuratani S3, Kurihara H8.
Abstract
The amniote middle ear is a classical example of the evolutionary novelty. Although paleontological evidence supports the view that mammals and diapsids (modern reptiles and birds) independently acquired the middle ear after divergence from their common ancestor, the developmental bases of these transformations remain unknown. Here we show that lower-to-upper jaw transformation induced by inactivation of the Endothelin1-Dlx5/6 cascade involving Goosecoid results in loss of the tympanic membrane in mouse, but causes duplication of the tympanic membrane in chicken. Detailed anatomical analysis indicates that the relative positions of the primary jaw joint and first pharyngeal pouch led to the coupling of tympanic membrane formation with the lower jaw in mammals, but with the upper jaw in diapsids. We propose that differences in connection and release by various pharyngeal skeletal elements resulted in structural diversity, leading to the acquisition of the tympanic membrane in two distinct manners during amniote evolution.
PMID 25902370
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4423235/
2012
Movement of the external ear in human embryo
Head Face Med. 2012 Feb 1;8:2.
Kagurasho M, Yamada S, Uwabe C, Kose K, Takakuwa T. Source Human Health Science, Graduate School of Medicine, Kyoto University, 606-8507, Shogoin Kawahara-cyo 53, Kyoto, Japan.
Abstract
INTRODUCTION: External ears, one of the major face components, show an interesting movement during craniofacial morphogenesis in human embryo. The present study was performed to see if movement of the external ears in a human embryo could be explained by differential growth. METHODS: In all, 171 samples between Carnegie stage (CS) 17 and CS 23 were selected from MR image datasets of human embryos obtained from the Kyoto Collection of Human Embryos. The three-dimensional absolute position of 13 representative anatomical landmarks, including external and internal ears, from MRI data was traced to evaluate the movement between the different stages with identical magnification. Two different sets of reference axes were selected for evaluation and comparison of the movements. RESULTS: When the pituitary gland and the first cervical vertebra were selected as a reference axis, the 13 anatomical landmarks of the face spread out within the same region as the embryo enlarged and changed shape. The external ear did move mainly laterally, but not cranially. The distance between the external and internal ear stayed approximately constant. Three-dimensionally, the external ear located in the caudal ventral parts of the internal ear in CS 17, moved mainly laterally until CS 23. When surface landmarks eyes and mouth were selected as a reference axis, external ears moved from the caudal lateral ventral region to the position between eyes and mouth during development. CONCLUSION: The results indicate that movement of all anatomical landmarks, including external and internal ears, can be explained by differential growth. Also, when the external ear is recognized as one of the facial landmarks and having a relative position to other landmarks such as the eyes and mouth, the external ears seem to move cranially.
© 2012 Kagurasho et al; licensee BioMed Central Ltd.
PMID 22296782
2011
A missense mutation in PPARD causes a major QTL effect on ear size in pigs
PLoS Genet. 2011 May;7(5):e1002043. Epub 2011 May 5.
Ren J, Duan Y, Qiao R, Yao F, Zhang Z, Yang B, Guo Y, Xiao S, Wei R, Ouyang Z, Ding N, Ai H, Huang L. Source Key Laboratory for Animal Biotechnology of Jiangxi Province and the Ministry of Agriculture of China, Jiangxi Agricultural University, Nanchang, China.
Abstract
Chinese Erhualian is the most prolific pig breed in the world. The breed exhibits exceptionally large and floppy ears. To identify genes underlying this typical feature, we previously performed a genome scan in a large scale White Duroc × Erhualian cross and mapped a major QTL for ear size to a 2-cM region on chromosome 7. We herein performed an identical-by-descent analysis that defined the QTL within a 750-kb region. Historically, the large-ear feature has been selected for the ancient sacrificial culture in Erhualian pigs. By using a selective sweep analysis, we then refined the critical region to a 630-kb interval containing 9 annotated genes. Four of the 9 genes are expressed in ear tissues of piglets. Of the 4 genes, PPARD stood out as the strongest candidate gene for its established role in skin homeostasis, cartilage development, and fat metabolism. No differential expression of PPARD was found in ear tissues at different growth stages between large-eared Erhualian and small-eared Duroc pigs. We further screened coding sequence variants in the PPARD gene and identified only one missense mutation (G32E) in a conserved functionally important domain. The protein-altering mutation showed perfect concordance (100%) with the QTL genotypes of all 19 founder animals segregating in the White Duroc × Erhualian cross and occurred at high frequencies exclusively in Chinese large-eared breeds. Moreover, the mutation is of functional significance; it mediates down-regulation of β-catenin and its target gene expression that is crucial for fat deposition in skin. Furthermore, the mutation was significantly associated with ear size across the experimental cross and diverse outbred populations. A worldwide survey of haplotype diversity revealed that the mutation event is of Chinese origin, likely after domestication. Taken together, we provide evidence that PPARD G32E is the variation underlying this major QTL.
PMID 21573137
2010
Ear reconstruction through tissue engineering
Adv Otorhinolaryngol. 2010;68:108-19. Epub 2010 May 3.
Haisch A.
Department of Otorhinolaryngology, Head and Neck Surgery Campus Benjamin Franklin, Charité University Medicine Berlin, Berlin, Germany. andreas.haisch@charite.de Abstract For decades, reconstructive surgery of the auricle has presented a challenge to surgeons. An immense number of publications now document the efforts to develop and improve techniques designed to provide reasonable shape and functionality. Since the early 1990s, tissue engineering has become increasingly popular in the field of reconstructive surgery. In particular, when an in-vitro-manufactured auricular-shaped cartilage implant was implanted on the back of a nude mouse, reconstructive surgeons were intrigued and patients' expectations were raised. However, almost 20 years after tissue engineering was defined by Langer and Vacanti [Science 1993;260:920-926] as: 'an interdisciplinary field that applies the principles of engineering and life sciences toward the development of biological substitutes that restore, maintain, or improve tissue function or a whole organ', only single case reports have been published. These reports detail the clinical application of in-vitro-manufactured cartilage for reconstructive procedures in the head and neck. The present article describes the fundamentals and potential of tissue engineering in reconstructive surgery of the auricle, and highlights the limitations that prevent its current clinical application.
Copyright 2010 S. Karger AG, Basel. PMID: 20442565
Combined reconstruction of congenital auricular atresia and severe microtia
Adv Otorhinolaryngol. 2010;68:95-107. Epub 2010 May 3.
Siegert R.
Department of Oto-Rhino-Laryngology, Head and Neck Surgery, Prosper Hospital, Ruhr University Bochum, Recklinghausen, Germany. profsiegert@web.de Abstract OBJECTIVES: Due to their embryological development, auricular atresia and severe microtia are, in most cases, combined malformations. The aims of this study were firstly to develop a surgical technique for combined esthetic and functional reconstruction with a minimum of operations and secondly to evaluate its results.
STUDY DESIGN: Prospective clinical evaluation.
PATIENTS AND METHODS: Fifty-two patients with third-degree microtia and congenital aural atresia with a sound-conducting block of about 50 dB were treated. In the first operation, autogenous cartilage was harvested, and the auricular framework was fabricated and implanted. In addition, the tympanic membrane and the external ear canal were prefabricated, and stored in a subcutaneous pocket. In the second step, the elevation of the new framework was combined with the operation for atresia, utilizing the prefabricated tympanic membrane and external ear canal. In the third step, the cavum concha was deepened, and the external ear canal was opened and covered with a skin graft.
RESULTS: In total, 76% of the patients had a final conductive hearing loss of 30 dB or less. No restenosis of the new external ear canal was observed. The esthetic results of the constructed auricles are shown in this report.
CONCLUSION: With this combination of plastic surgery for the auricle and functional surgery for the middle ear, no additional operations are necessary and the prefabrication of the external ear canal and the tympanic membrane gives stable and reliable results. This combined technique offers the best chance of optimal esthetic and functional rehabilitation for patients with these malformations.
Copyright 2010 S. Karger AG, Basel. PMID: 20442564
Morphology of the human tympanic membrane annulus
Otolaryngol Head Neck Surg. 2010 May;142(5):682-7.
Kassem F, Ophir D, Bernheim J, Berger G.
Department of Otolaryngology-Head and Neck Surgery, Meir Medical Center, Kfar Saba, Israel. firas67@hotmail.com Abstract OBJECTIVE: To study the full panoramic view with figuring of the morphology and topography of the human tympanic annulus.
STUDY DESIGN: Postmortem material analysis.
SETTING: University-affiliated hospital.
SUBJECTS AND METHODS: Twenty-three single, normal human adult tympanic membranes were completely extracted from formalin-fixed temporal bones. They were faced medially and placed at the same level of a graph paper mounted on a board. High-quality images of the tissue preparations were taken, and computer-aided measurements of the annular caliber were calculated at nine reference points. The 6 o'clock direction served as a midpoint, and another four reference points were set anteriorly and posteriorly in clockwise and counterclockwise directions.
RESULTS: The annulus has a horseshoe-like shape with a small part absent above the neck of the malleus. The maximal mean caliber at the manubrial axis (6 o'clock direction) was 748 +/- 201 mum. The annulus gradually thins out almost symmetrically anteriorly and posteriorly, until it reaches about 15 percent of the maximal caliber at its end points (152 +/- 87 and 113 +/- 42 mum, respectively). Significant differences were found between adjacent reference points on both anterior and posterior sides.
CONCLUSIONS: The annulus has a horseshoe-like shape and gradually thins out almost symmetrically, reaching anteriorly and posteriorly about 15 percent of the maximal caliber at the manubrial axis. These new data may provide guidance in transcanal middle ear exploration and suggest the possibility of varied functions attributable to the annulus regarding middle ear sound transmission and TM vibratory properties. The data may contribute to understanding the development of marginal perforations and posterior superior retraction pockets.
Copyright 2010 American Academy of Otolaryngology-Head and Neck Surgery Foundation. Published by Mosby, Inc. All rights reserved. PMID: 20416456
2009
Craniofacial Microsomia Overview
Heike CL, Hing AV. In: Pagon RA, Bird TC, Dolan CR, Stephens K, editors. GeneReviews [Internet]. Seattle (WA): University of Washington, Seattle; 1993-. 2009 Mar 19.
Excerpt Disease characteristics. Craniofacial microsomia (CFM) includes a spectrum of malformations primarily involving structures derived from the first and second branchial arches. Findings include facial asymmetry resulting from maxillary and/or mandibular hypoplasia; preauricular or facial tags; ear malformations that can include microtia (hypoplasia of the external ear), anotia (absence of the external ear), or aural atresia (absence of the external ear canal); and hearing loss. Severity can range from subtle facial asymmetry with a small skin tag in front of an otherwise normal-appearing ear to bilateral involvement (typically asymmetric), microtia/anotia with atresia of the ear canals, microphthalmia, and respiratory compromise from severe mandibular hypoplasia. Other craniofacial malformations including cleft lip and/or palate can be seen. Non-craniofacial malformations, especially vertebral, cardiac, and limb, can be seen. Diagnosis/testing. The diagnosis of CFM is based on clinical findings. Genetic counseling. CFM most frequently occurs as a simplex case (i.e., occurrence in a single individual in a family) with unknown etiology; recurrence risks are empiric. If an individual with CFM is found to have an inherited or de novo chromosome abnormality, genetic counseling for that condition is indicated. Occasional autosomal dominant or autosomal recessive inheritance is observed. If a proband has CFM and no reported family history of CFM, the risk to sibs is two to three percent; this may be an underestimate because of the difficulty of obtaining an accurate family history for some of the subtle features of CFM. Management. Treatment of manifestations: For optimal outcome children with CFM require timely and coordinated assessments and interventions. Ideally, children should be managed by an experienced multidisciplinary craniofacial team. The goals of treatment for CFM are to assure adequate respiratory support and feeding in infants with severe facial malformations, maximize hearing and communication, improve facial symmetry, and optimize dental occlusion. Treatment is age-dependent, with time-sensitive interventions at appropriate stages of craniofacial growth and development. Copyright © 1993-2010, University of Washington, Seattle. All rights reserved.
PMID: 20301754 http://www.ncbi.nlm.nih.gov/pubmed/20301754
Congenital upper auricular detachment: Report of two unusual cases
Indian J Plast Surg. 2009 Jul;42(2):265-8.
Agarwal P.
Plastic Surgery Unit, Department of Surgery, Netaji Subhash Chandra Bose Government Medical College, Jabalpur-482 003, MP, India. Abstract Two unusual cases of congenital bilateral ear deformity have been presented. The deformity is characterized by upper auricular detachment on the right side with anotia on the left side in the first case and upper auricular detachment on the left side with normal ear on the right side in the second case. An attempt has been made to correlate the presented deformity with the embryological - foetal development of the auricle. Satisfactory correction can be obtained by repositioning the auricle back in to its normal position.
PMID: 20368874 http://www.ncbi.nlm.nih.gov/pubmed/20368874
The kidney and ear: emerging parallel functions
Annu Rev Med. 2009;60:339-53.
Torban E, Goodyer P.
Departments of Medicine, Faculty of Medicine, McGill University, Montreal, Quebec, Canada. Abstract The association between renal dysplasia and minor malformations of the external ear is weak. However, there is a remarkable list of syndromes that link the kidney to the inner ear. To organize these seemingly disparate syndromes, we cluster representative examples into three groups: (a) syndromes that share pathways regulating development; (b) syndromes involving dysfunction of the primary cilium, which normally provides critical information to epithelial cells about the fluid in which they are bathed; (c) syndromes arising from dysfunction of specialized proteins that transport ions and drugs in and out of the extracellular fluid or provide structural support.
PMID: 18976115
2008
Mutation in the human homeobox gene NKX5-3 causes an oculo-auricular syndrome
Am J Hum Genet. 2008 May;82(5):1178-84.
Schorderet DF, Nichini O, Boisset G, Polok B, Tiab L, Mayeur H, Raji B, de la Houssaye G, Abitbol MM, Munier FL.
Institut de Recherche en Ophtalmologie, 1950 Sion, Switzerland. daniel.schorderet@irovision.ch Abstract Several dysmorphic syndromes affect the development of both the eye and the ear, but only a few are restricted to the eye and the external ear. We describe a developmental defect affecting the eye and the external ear in three members of a consanguineous family. This syndrome is characterized by ophthalmic anomalies (microcornea, microphthalmia, anterior-segment dysgenesis, cataract, coloboma of various parts of the eye, abnormalities of the retinal pigment epithelium, and rod-cone dystrophy) and a particular cleft ear lobule. Linkage analysis and mutation screening revealed in the first exon of the NKX5-3 gene a homozygous 26 nucleotide deletion, generating a truncating protein that lacked the complete homeodomain. Morpholino knockdown expression of the zebrafish nkx5-3 induced microphthalmia and disorganization of the developing retina, thus confirming that this gene represents an additional member implicated in axial patterning of the retina.
PMID: 18423520
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2427260
http://www.elsevier.com/wps/find/authorsview.authors/supplementalterms1.0
<pubmed>18261212</pubmed>| PMC2267455 | Head Face Med.
2007
Human ears grow throughout the entire lifetime according to complicated and sexually dimorphic patterns--conclusions from a cross-sectional analysis
Anthropol Anz. 2007 Dec;65(4):391-413.
Niemitz C, Nibbrig M, Zacher V.
Institute for Human Biology and Physical Anthropology, Freie Universität Berlin, Germany. cniemitz@zedat.fu-berlin.de Abstract In most of its anatomical constituents, e.g. in the Helix, etc., the external human ear is homologous to that of all Primates and Scandentia (tree shrews). Thus, its genetic basis is largely older than 60 Mio yrs. Based upon the observation of lifelong growth of the ear (e.g. Montacer-Kuhssary 1959), we aimed to elucidate the growth of the human ear in a more detailed way throughout life and in both sexes. On standardized photographical material collected randomly in Berlin (Germany), we measured N = 1448 ears from neonate children to volunteers of 92 yrs in age. 10 longitudinal measurements and 5 further anatomical parameters yielded a data set of roughly 19,000 data in total. Based upon our cross-section analysis, we quantified several sexual dimorphisms. Furthermore, we deduced ontogenetic developments and, partially, corrected their proportions for secular acceleration and body height shrinking with age. At the time of birth, in proportion to the body, the external ear was even bigger than the large head and continued growing rather linearly throughout life, reaching the highest average lengths in the volunteers aged over 85 yrs. The large yearly increases during childhood began to diminish at as early an age as 8 or 10 yrs. In all parameters where post adult growth was observed, female ears showed a lesser increase than those of men. The greatest ear length in females was 52 mm (SD +/- 4.3 mm) at birth, 61 mm (SD +/- 3.9 mm) at around 20 yrs of age and 72 mm (SD +/- 4.6 mm) in women older than 70 yrs. For the male subjects, these three values were: 52 mm (SD +/- 4.1 mm), 65 mm (SD +/- 4.0 mm) and 78 mm (SD +/- 4.8 mm), respectively. In spite of extreme premature growth of the auricle and its further lifelong growth, three anatomical features of the ear did practically not grow at all after birth: the width of the Concha auriculae and of the Incisura intertragica, as well as the diameter of the helical brim of the auricle. The problems arising concerning the functions and selective values of all these very unusual proportions and growths are discussed. The ontogenetic development of one or more pretragal skin folds could be used as a contribution to age estimations in forensic anthropology.
PMID 18196763
1992
The embryologic development of the human external auditory meatus. Preliminary report
Acta Otolaryngol. 1992;112(3):496-503.
Nishimura Y, Kumoi T. Source Department of Otorhinolaryngology, Hyogo College of Medicine, Nishinomiya, Japan.
Abstract
During the final period of embryogenesis, a funnel-shaped tube continues medially into the mesenchymal tissue forming a curved path. Although this may sound simple, the development occurring during early fetal life is in fact very complex. At first, ectodermal cells proliferate to fill the lumen of the meatus, forming the meatal plug, and then at 10 weeks the bottom of the plug extends in a disc-like fashion, so that in the horizontal plane the meatus is boot-shaped with a narrow neck and the sole of the meatal plug spreading widely to form the future tympanic membrane medially. At the same time, the plug in the proximal portion of the neck starts to be resorbed. In the 13-week fetus, the disc-like plug begins to show signs of its final destiny; the innermost surface of the plug in contact with the anlage of the malleus is ready to contribute to the formation of the tympanic membrane. In the 15-week fetus, the innermost portion of the disc-like plug splits, leaving a thin ectodermal cell layer of immature tympanic membrane. The neck of the boot forms the border between the primary and secondary meatus, and is the last part to split. In the 16.5-week fetus, the meatus is fully patent throughout its entire length, although the lumen is still narrow and curved. In the 18-week fetus, the meatus is already fully expanded to its complete form.
PMID 1441991