Seminal Vesicle Development
| Embryology - 19 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Introduction
In humans, the male accessory glands are the seminal vesicles, prostate, and the bulbourethral glands. The male gonad, the testis, differentiates embryonically initially under the influence of the Y chromosome. Later under the influence the gonad-derived fetal testosterone acting through androgen receptors, a region of the urogenital sinus (UGS) mesenchyme differentiates to form the primordial prostate buds. The buds then signal back to the overlying epithelium, inducing duct formation, this was one of the early studied (1970's) example of an mesenchymal-epithelial interaction in development. Interestingly, the female equivalent gland originally called Skene's gland, then paraurethral gland has now also been renamed the female prostate.
- The seminal vesicles were incorrectly named as they were thought to be the storage site for spermatozoa.
Historic article: Watson EM. The development of the seminal vesicles in man. (1918) Amer. J Anat. 24(4): 395 - 439.
There are also currently separate pages describing prostate and puberty.
Some Recent Findings
|
| More recent papers |
|---|
|
This table allows an automated computer search of the external PubMed database using the listed "Search term" text link.
More? References | Discussion Page | Journal Searches | 2019 References | 2020 References Search term: Seminal Vesicles Embryology | Seminal Vesicles Development | Bulbourethral Embryology | Bulbourethral Development |
Textbooks
- Human Embryology (2nd ed.) Larson Chapter 10 p261-306
- The Developing Human: Clinically Oriented Embryology (6th ed.) Moore and Persaud Chapter 13 p303-346
- Before We Are Born (5th ed.) Moore and Persaud Chapter 14 p289-326
- Essentials of Human Embryology, Larson Chapter 10 p173-205
- Human Embryology, Fitzgerald and Fitzgerald Chapter 21-22 p134-152
- Developmental Biology (6th ed.) Gilbert Chapter 14 Intermediate Mesoderm
Seminal Vesicle Timeline
| Week | Fetal CRL mm | Event |
|---|---|---|
| 13 | 80 | seminal vesicles appear as lateral evaginations or out-pocketings from the lower portion of the mesonephric ducts (Wolffian ducts). At this stage their lateral diameter is always greater than the anteroposterior length. |
| 14 | 100 | sacculations or diverticula of the vesicles as evaginations of the walls of the vesicles. At this time 3 small but distinct sacculations may be counted at a given level in each vesicle. |
| 19 | 170 | dilatation of the mesonephric ducts in their lower portion, to form the ampullae of the deferent ducts. Sacculations of the ampullae in the form of slight but definite irregularities of their lumina. Each vesicle at this stage shows from 3 to 8 distinct sacculations at a given level. The lateral and antero—posterior diameters are now about equal. |
| 21 | 180 | sacculations of the mesonephric ducts have become well developed and the dilatation of the ducts to form the ampullae have assumed very nearly their proportions at birth. |
| 25 | 220 | vesicles and ampullae have assumed practically their adult form as regards their general topography and arrangement of sacculations. At a single level may be counted from~nine to twelve separate diverticula. |
| 25 to 31 | 220 to 275 | prostatic utricle opens into the posterior urethra. At the latter date has a bifid lumen showing the union of two partially fused tubes. At this stage 5 to 7 distinct sacculations in each vesicle at a given level. |
| Birth | vesicles show at a given level 7 distinct sacculations at one time. At this stage many of the diverticula are traversed by a network of fine trabeculae. | |
| Data[3] Links: seminal vesicle | timeline | ||
Genital Development Overview
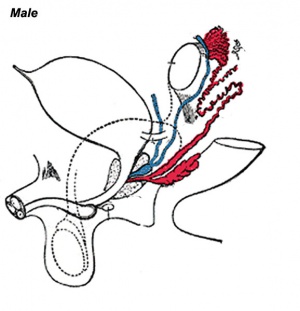
Three main stages during development, mesonephric/paramesonephric duct changes are one of the first male/female differences that occur in development, while external genitaila remain indeterminate in appearance for quite a while.
- Differentiation of gonad (Sex determination)
- Differentiation of internal genital organs
- Differentiation of external genital organs
The 2nd and 3rd stages dependent on endocrine gonad. Reproductive development has a long maturation timecourse, begining in the embryo and finishing in puberty. (More? Puberty Development)
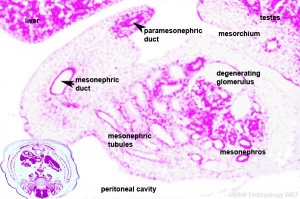
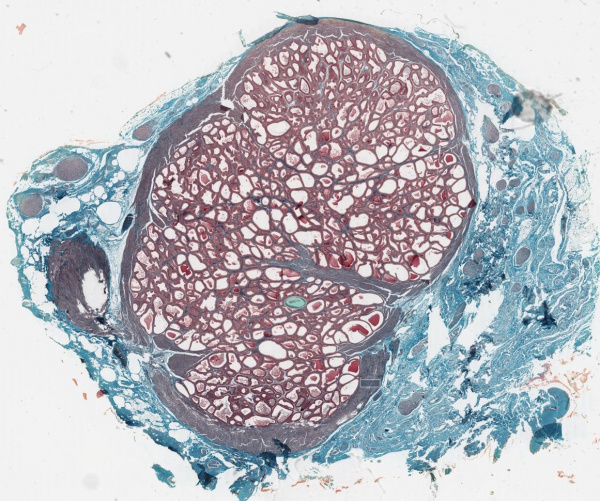
Seminal Vesicle Histology
Secretory Glands
Seminal Vesicle Proteins
Mouse has seven major proteins (SVS1-7) involved in seminal vesicle secretion
- SVS2 - both a capacitation inhibitor and a decapacitation factor. Binds ganglioside GM1 on the spermatozoa membrane.
- SVS3 and SVS4 - facilitates SVS2 effect on sperm capacitation.[4]
- SVS4 - a capacitation inhibitor.
Signaling agents in seminal fluid include prostaglandins and transforming growth factor-β. These and other factors may also aid a "female response".[1]
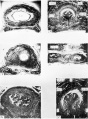
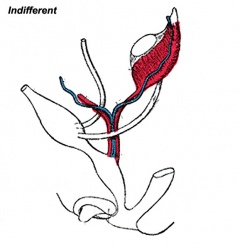
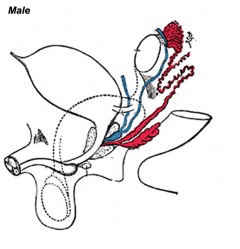
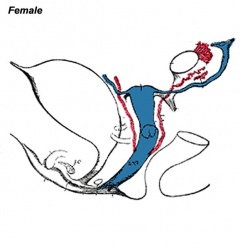
Historic Images of Genital Changes
|
|
|
| Urogenital indifferent | Urogenital male | Urogenital female |
Historic Gray's Anatomy - Seminal Vesicle
Anatomy
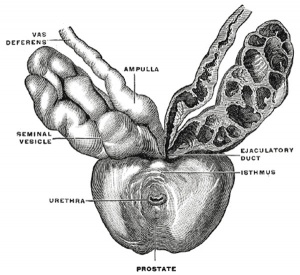
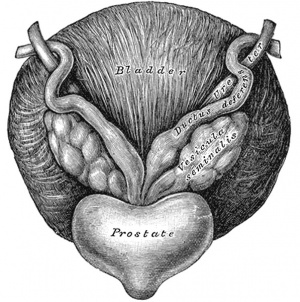
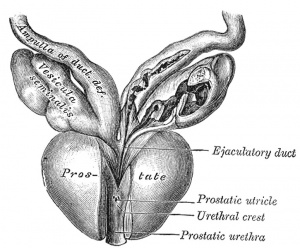
The vesiculæ seminales (Fig. 1152) are two lobulated membranous pouches, placed between the fundus of the bladder and the rectum, serving as reservoirs for the semen, and secreting a fluid to be added to the secretion of the testes. Each sac is somewhat pyramidal in form, the broad end being directed backward, upward and lateralward. It is usually about 7.5 cm. long, but varies in size, not only in different individuals, but also in the same individual on the two sides. The anterior surface is in contact with the fundus of the bladder, extending from near the termination of the ureter to the base of the prostate. The posterior surface rests upon the rectum, from which it is separated by the rectovesical fascia. The upper extremities of the two vesicles diverge from each other, and are in relation with the ductus deferentes and the terminations of the ureters, and are partly covered by peritoneum. The lower extremities are pointed, and converge toward the base of the prostate, where each joins with the corresponding ductus deferens to form the ejaculatory duct. Along the medial margin of each vesicle runs the ampulla of the ductus deferens.
Each vesicle consists of a single tube, coiled upon itself, and giving off several irregular cecal diverticula; the separate coils, as well as the diverticula, are connected together by fibrous tissue. When uncoiled, the tube is about the diameter of a quill, and varies in length from 10 to 15 cm.; it ends posteriorly in a cul-de-sac; its anterior extremity becomes constricted into a narrow straight duct, which joins with the corresponding ductus deferens to form the ejaculatory duct.
Structure
The vesiculæ seminales are composed of three coats: an external or areolar coat; a middle or muscular coat thinner than in the ductus deferens and arranged in two layers, an outer longitudinal and inner circular; an internal or mucous coat, which is pale, of a whitish brown color, and presents a delicate reticular structure. The epithelium is columnar, and in the diverticula goblet cells are present, the secretion of which increases the bulk of the seminal fluid.
Vessels and Nerves
The arteries supplying the vesiculæ seminales are derived from the middle and inferior vesical and middle hemorrhoidal. The veins and lymphatics accompany the arteries. The nerves are derived from the pelvic plexuses.
Gray H. Anatomy of the human body. (1918) Philadelphia: Lea & Febiger.
Abnormalities
Single Seminal Vesicle
A very rare congenital malformation, that can be associated with associated with unilateral kidney agenesis.[5]
Seminal Vesicle Cyst
References
- ↑ 1.0 1.1 Schjenken JE & Robertson SA. (2020). The Female Response to Seminal Fluid. Physiol. Rev. , 100, 1077-1117. PMID: 31999507 DOI.
- ↑ Araki N, Kawano N, Kang W, Miyado K, Yoshida K & Yoshida M. (2016). Seminal vesicle proteins SVS3 and SVS4 facilitate SVS2 effect on sperm capacitation. Reproduction , 152, 313-21. PMID: 27486266 DOI.
- ↑ Watson EM. The development of the seminal vesicles in man. (1918) Amer. J Anat. 24(4): 395 - 439.
- ↑ Araki N, Kawano N, Kang W, Miyado K, Yoshida K & Yoshida M. (2016). Seminal vesicle proteins SVS3 and SVS4 facilitate SVS2 effect on sperm capacitation. Reproduction , 152, 313-21. PMID: 27486266 DOI.
- ↑ Mischianu D, Ilie CP, Pacu O & Rusu F. (2008). Very rare congenital malformation: single seminal vesicle, associated with unilateral kidney agenesis: first case report. J Med Life , 1, 228-32. PMID: 20108470
Reviews
Kim B, Kawashima A, Ryu JA, Takahashi N, Hartman RP & King BF. (2009). Imaging of the seminal vesicle and vas deferens. Radiographics , 29, 1105-21. PMID: 19605659 DOI.
Articles
Hajji F, Hammoune N, Miloudi M & Belasri S. (2017). Unusual variant of inverted Y ureteral duplication with an ipsilateral seminal vesicle cyst and renal dysgenesis. Ann R Coll Surg Engl , 99, e19-e21. PMID: 27659383 DOI.
Batur A, Karakose S & Karalezli G. (2014). Congenital seminal vesicle cyst accompanying ipsilateral renal and ureteral agenesis. Urol J , 11, 1203. PMID: 24595924
Arora SS, Breiman RS, Webb EM, Westphalen AC, Yeh BM & Coakley FV. (2007). CT and MRI of congenital anomalies of the seminal vesicles. AJR Am J Roentgenol , 189, 130-5. PMID: 17579162 DOI.
Thomson AA & Marker PC. (2006). Branching morphogenesis in the prostate gland and seminal vesicles. Differentiation , 74, 382-92. PMID: 16916376 DOI.
Settle S, Marker P, Gurley K, Sinha A, Thacker A, Wang Y, Higgins K, Cunha G & Kingsley DM. (2001). The BMP family member Gdf7 is required for seminal vesicle growth, branching morphogenesis, and cytodifferentiation. Dev. Biol. , 234, 138-50. PMID: 11356025 DOI.
Search PubMed
Search Pubmed: seminal vesicle Embryology | seminal vesicle Development
Additional Images
Historic
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
Watson EM. The development of the seminal vesicles in man. (1918) Amer. J Anat. 24(4): 395 - 439.
Terms
- 5-α-reductase - enzyme that converts testosterone to dihydrotestosterone.
- androgen receptor - (AR)
- benign prostatic hyperplasia
- mesenchyme - embryonic connective tissue
- paraurethral gland - (Skene's gland) - female prostate gland is the correct nomenclature
- prostate gland - Greek, prostates = "one who stands before", "protector", a female prostate gland exists
- prostate cancer
- UGE - urogenital epithelium
- UGS - urogenital sinus
- Zinner syndrome - rare abnormality of unilateral renal agenesis, ipsilateral seminal vesicle cyst, and ejaculatory duct obstruction. Named after the first report of this syndrome (Zinner A. Ein fall von intravesikaler Samenblasenzyste. Wien Med Wochenschr. 1914;64:605).
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2024, April 19) Embryology Seminal Vesicle Development. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Seminal_Vesicle_Development
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G