Paper - The development of the human maxilla, vomer, and paraseptal cartilages (1911)
| Embryology - 27 May 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Fawcett E. The development of the human maxilla, vomer, and paraseptal cartilages. (1911) J Anat. Physiol. 45(4): 378-405.
| Online Editor |
|---|
| This historic 1911 paper describes the development of the human maxilla, vomer, and paraseptal cartilages.
See also:
|
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
The Development of the Human Maxilla, Vomer, and Paraseptal Cartilages
By Professor Fawcett, M.D.,
University of Bristol.
Introduction
The usually accepted descriptions of the development of the maxilla of man state that it arises by a number of separate centres—the number varying somewhat with the authority, likewise the situation of these centres,
No description of the maxilla can be considered complete unless at the same time notice is taken of the manner of development of the premaxilla, which, of course, forms the anterior segment of the adult bone as usually interpreted. But the consideration of the development of the premaxilla may be left until that of the maxilla has been fully dealt with. Before breaking new ground, it may be well to state what are the usual statements with reference to the ossification of the maxilla.
These statements are apparently for the most part based on Work done by Callender, Toldt, Rambaud and Renault, and Bland Sutton, so far as concerns human anatomy. A
More recently Franklin Mall has given his views on the subject in the American Journal of Anatomy, views based on observation of specimens treated by the “clearing” method of Schulze. So far as they go, these statements are in harmony with my own notions, which I have for several years now taught.
A very precise account is given in Cunningham’s Textbook of Anatomy.
The maxilla is there stated to be developed in the connective tissue around the oral cavity of the embryo from centres which are not preceded by cartilage, of uncertain number, as early fusion takes place between them. They appear during the second month of foetal life, shortly after the clavicle has begun to ossify, and by the sixth month are so united that their independent character is obscured.
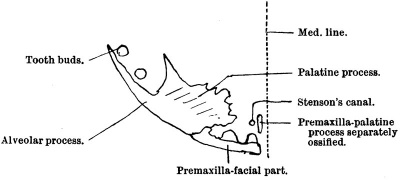
Five centres are described, viz. :—An external or malarr, which forms the bone to the outer side of the infra-orbital canal; an inner or orbitanasal, from which is developed the inner part of the floor of the orbit, the frontal process, and the wall of the antrum; a palatine, for the posterior three-fourths of the palatine process; a nasal, situated between the frontal process and the canine tooth. Then an incisive centre is added to form the premaxilla; finally mention is made of a centre described by Rambaud and Renault, which is wedged in between the incisive and palatine elements beneath the vomer, explaining the Y-shaped arrangement of the foramina of Stenson which open into the anterior palatine canal. This is the sub-vomerian centre of Rambaud and Renault. or the centre of Huschke, and, it will later be seen, belongs really to the premaxilla.
The account given in Gray’s Anatomy varies a little from the foregoing.
The malar centre is described as giving origin to that part of the bone external to the infraorbital canal and to the zygomatic process; the orbitanasal forms that part of the body internal to the infraorbital canal, including the inner part of the floor of the orbit and the alter wall of the nasal fossa ; the palatine gives rise to the palatine process behind Stenson’s canal, and to the adjoining part of the nasal wall; the premawillary forms the front part of the alveolus which carries the incisor teeth; the nasal gives rise to the frontal process and the portion above the canine tooth; the infravomerine lies between the palatine and premaxillary centres and beneath the vomer. These various centres appear about the eighth week, and by the tenth week the bone consists of two portions, one the maxilla proper, and the other the premaxilla. The suture between the two portions persists on the palate till middle life, but is not to be seen on the facial surface because, according to Callender, the front wall of the sockets of the incisor teeth is not formed by the premaxillary bone, but by an outgrowth from the facial part of the maxilla proper.
The view adopted in Quain is similar to the above of Gray, Callender and Sutton being quoted in evidence. Gaupp, in Hertwig’s H andbuch, describes the maxilla as arising from five centres, and quotes Toldt as finding five centres which appear at the end of the second month and the beginning of the third. These towards the end of the fourth month blend together and with the os incisivum.
(1) An independent centre for the lateral part, together with the lateral half of the orbital surface and the lateral wall of the alveoli of the molar teeth. (2) A second for the medial hinder part of the body and the medial half of the orbital surface. (3) A third for the facial surface over the canine tooth and the frontal process. (4) A fourth for the palatine process, the medial. lamella of the alveolar process, and the anterior part of the nasal surface of the body. ' (5) A fifth (doubtful) for the region of the sulcus and the crista lacrymalis. Gaupp then states that, according to Mihalkovics, the upper jaw incorporates into itself the paranasal process of the nasal caps-ule, and that also during the fourth to fifth month small cartilaginous nuclei appear in the alveolar part without any connection with the nasal capsule and later becomes incorporate with the upper jaw. Keibel and Mall incline to the view that the maxilla proper develops from one centre only, and adduce in support Hertwig’s model from an 80 mm. embryo. (This statement is clearly based on Mall’s paper in the American Journal of Anatomy, which has been before alluded to, and perhaps one here might state generally what Mall found. He states that the maxilla in his specimen arises from two centres, one to form the maxillary bone proper and one to form the premaxilla. In one of the youngest specimens, 16 mm., the maxilla is marked by a mass of granules, together §~ mm. in diameter, lying spread out just beneath the eye and 1 mm. from the middle line. In another specimen, of 18 mm., the maxilla is absent, but the premaxilla, measuring 9; mm., is present. In another specimen of the forty—second day both centres are present. At a later stage the bones are denser, and both have parallel processes which no doubt are to form the frontal process. At the fifty-sixth day the two bones are found united along the alveolar border, but the processes are separate for a long time. At no time are more than two centres present, and these unite in the very beginning of the third month. The numerous centres described by Rambaud and Renault, Mall attributes to breaks in preparing the specimens.)
In a footnote in Keibel and Mall’s Embryology, Le Double (1906) is quoted as giving the number of centres as described by various authors including the incisoria as: One—Camper, Rousseau, Cleland; two—Jamain; three—Serres, Meckel, Cruveilhier; five—Béclard, Sappey, Leidy, Poirier, W. Krause; five or six—Portal; six—Rambaud and Renault; seven —Weber.
Humphry (1858), who is very accurate in his statements concerning ossification, doubts the real existence of more than one centre for the maxilla proper. In a footnote he states: “There are many fissures which extend to some depth in the foetal and young bone; but we must not think that these are necessarily the indications of its having been developed from several centres.” (The italics are mine.)
J. Bland Sutton describes the maxilla as consisting of four distinct portions. a. The premaazillary region in relation with the ethmo-vomerine cartilage; b, a prepalatine portion forming a platform for the support of the anterior end of the vomer; c, a maxillary centre situate to the inner side of the superior maxillary division of the fifth nerve; d, the malar piece, lying outside this nerve and supporting the malar bone. Three figures are given in illustration of these statements. In another -page by the same author, ‘an extraordinary figure is given of a three months’ embryo, in which a bar of cartilage is represented as passing from the malleus region to the inner side of the maxilla. How such figure was arrived at, it is diflicult to say.
From the above statements it is clear, first, that there is a doubt as to the manner of ossification of the maxilla, secondly, that we are divided between a one-centred and a many-centred origin for the maxilla proper.
The object of this part of this communication is to show that the maxilla proper arises from one centre of ossification only, unless, as appears to be the case, the paranasal process be regarded as ossified independently.
The conclusions which I have arrived at are based on exhaustive examination of sections with the microscope, models reconstructed from these sections, specimens cleared by the Schulze method, and dry specimens of various ages.
Personal Observations
The maxilla proper commences as a membrane bone on the outer side of the nasal capsule, and above the canine-tooth germ. In point of time one may say that, so far as the microscope reveals, it commences to ossify about the 18-mm. stage. (Mall, who claims greater delicacy for the Schulze method, shows in his table that several embryos of 15-mm. length have an ossified maxilla. I have never observed it at that stage.) So far as my experience goes, it is preceded in ossification by both the clavicle and the mandible. But the maxilla is not wholly a membrane bone; it is added to, as Mihalkovics has stated, by periosteal cartilage along the alveolar wall—in the rabbit, Fuchs has observed a hyaline cartilaginous nucleus at the hinder part of the palatine process near the inner alveolar wall—and by incorporation of that cartilaginous offshoot of the lateral nasal wall which Mihalkovics has termed the paranasal process. However, these cartilages play but a small part in the general growth of the bone, and we may return to that question later.
Proceeding with the process of ossification in membrane we observe that, from the centre above described, rapid growth in certain directions takes place; thus the bone grows backwards underneath and at some distance from the infraorbital nerve. Splitting in the region of the giving off of the anterior dental nerve to allow of the passage of that nerve through it on its way to the incisor teeth, the two extremities unite later behind the anterior dental nerve, the outer limb of the fork forms the malar process, the inner one the inner orbital margin, bridges connecting the two in front of and behind the anterior dental nerve from the very small triangular floor of the orbit.’ T
At the same time the bone grows downwards to the outer side of the tooth germs to form the outer alveolar wall, it also shoots upwards by the side of the nasal capsule to form a part at least of the frontal process. If a coronal section of the 19 mm. stage be now examined, the section passing through the frontal process and the outer alveolar wall, the maxilla in section appears as a bent rod, convex inwards; the part above the angle is the frontal process, that below the outer alveolar process. From the inwardly directed angle, bone is seen to be projected inwards. This is the anlage of the palatine process, which is clearly not an independent formation but an outgrowth from the main mass.
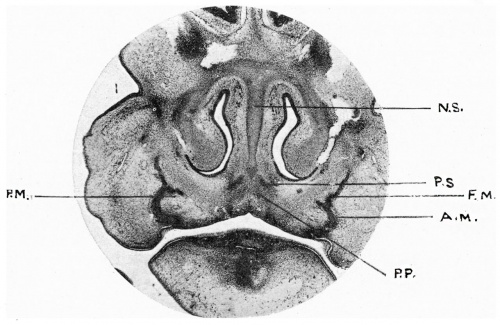
Fig. 1. Coronal section 19 mm Minot embryo.
N .S , nasal septum ; P.S., paraseptal cartilage anlage; F.M., frontal process of maxilla ; A.M., alveolar process of maxilla; P.M., palatine process of maxilla; P.P., anlage of palatine centre of premaxilla.
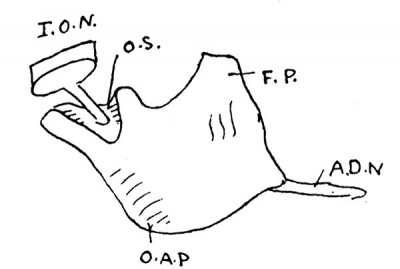
Fig. 2. Model of 19 mm maxilla, Minot embryo, from outer side.
I.O.N., infraorbital nerve; 0.S.,orbital surface of maxilla; F.P., frontal process; O.A.P.,outer alveolar process; A.D.N.,anterior dental nerve.
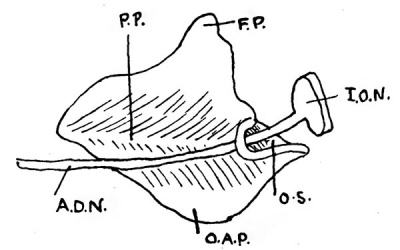
Fig. 3. Inner view of 19 mm model, Minot embryo.
P.P., palatine process; F.P., frontal process; I.O.N., infraorbital nerve; A.D.N., anterior dental nerve; 0.8., orbital surface; O.A.P., buter alveolar process.
In a later stage, 35 mm much advance has been made; the malar process has grown greatly, and is separated from the outer alveolar wall a by a relatively wide bar of bone, and at its outer side shows signs of periosteal chondrification. The palatine process, too, has thickened enormously near its origin from the main mass. This is especially well seen in the section of a 37 mm. embryo kindly lent by Professor Minot. It will be noted here that the palatine process is a great spongework of bone, which in section is triangular in shape, whose apex has not yet reached the middle line. When at a later stage, 42 mm. (fig. 5), the palatine process, is examined inohorizontal sections, it will be seen to have the form of a right-angled triangular plate, the base of the triangle being forwards and attached by one angle to the main mass of the maxilla, the apex backwards and separated by a wide gap from the maxilla, a gap which will be bridged over later by spicules of bone, which form the septa of the alveoli.
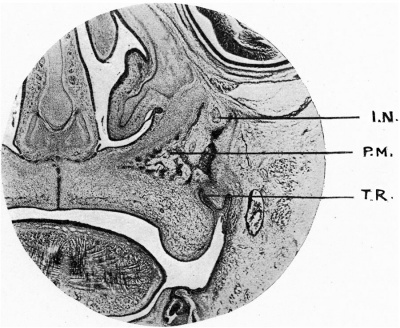
Fig. 4. Coronal section Minot 37 mm embryo. I.N., supraorbltal nerve; P.M., palatine process of maxilla; T.R., tooth ridge.
About this time, too, there grows downwards from the outer edge of the palatine process the inner alveolar wall, but it is better seen at, say, the 65 mm. stage (fig. 6).
FIG. 5.—Scheme of 42 mm. maxilla from above.
From this stage onwards until the 100 mm. stage there is little of note to be recorded save a general enlargement of the bone in all directions except that of height of the body. This remains small till a later period, so small that the tooth sockets are only separated by what appears as a thin, long lamella, at once the floor of the orbit and the roof of the tooth sockets from the orbital cavity
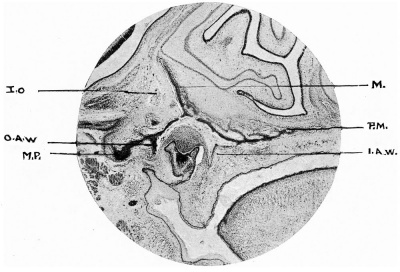
FIG. 6.—Coronal section 65 mm. embryo.
M., inner orbital wall of maxilla; P.M., palatine process ; I.A.W., inner alveolar wall; O.A.W., outer alveolar wall; M.P., malar process; I.O., infraorbital nerve.
Although before this period the antrum of Highmore has appeared, which will eventually, by pushing outwards between the floor of the orbit and the roof of the tooth sockets, raise the bone to its permanent height, yet at this time it is so small as to have no effect at all on the height of the maxilla.
At the 100 mm. stage a new ossific element becomes evident, which will later become incorporated in the jaw. This is the paranasal process of Mihalkovics. This offshoot of the nasal capsule, which runs forward on the outer side of the lacrymal duct, is evident as early as the 30 mm. stage (fig. 7), but it is not until the 100 mm. stage that it becomes ossified. It undergoes both perichondral and entochondral ossification, as will ‘be seen in fig. 8 — which is an oblique, horizontal section of the head of a 100 mm. embryo—and at a later stage becomes incorporated with the maxilla.
This, perhaps, may be regarded as a second centre of ossification of the maxilla, as it seems to ossify quite independently of the rest of the bone.
Apart from this point, it will be abundantly clear that the maxilla - consisting of body, malar process, frontal, alveolar, and palatine processes —is ossified so far as the membranous part is concerned from one centre, and that its various parts are all apparent at a very early period of embryonic life. But there are marked differences between the foetal bone and that of the adult; for example, the shallowness of the young bone is one feature, the small size of the orbital surface is another, the absence of an infraorbital groove—apart from the tunnel—is another. The infraorbital groove is only formed when the maxilla is expanded by the antrum of Highmore, and the infraorbital tunnel, which at first alone exists, is only visible at the 30 mm. stage; the uprising of the walls of the groove, in which the infraorbital nerve lies, later converts this very short groove into a tunnel, and until the antrum is well developed the infraorbital groove behind the tunnel so well shown in the adult is absent. The maxilla at this time is exactly like, in this respect, the maxilla of a quadruped. (This was alluded to by Wright some time ago at a meeting of the Anatomical Society.) A
The Premaxilla
The statements which I have been able to make regarding the development of the maxilla are precise, and, I hope, conclusive. It is very different with regard to the premaxilla, which has for long been an object of interest, seeing that it is the bone involved in the production of alveolar cleft palate of various kinds. Many different statements have been made in times past concerning its origin, and Albrecht, dealing with the matter from the point of view of explanation of cleft palate and its varieties, has derived the bone from two centres, a median and a. lateral, entitled respectively endognathion and mesognathion. Others have denied that the premaxilla, as said to be seen on the face, is existent as a separate element. Franklin Mall, in his paper in the American Journal of Anatomy, describes it very precisely. Callender was. in a difliculty concerning it because, not being able to see any suture on the face between it and the maxilla, he described the premaxilla as being hidden from view by a forward growth of the maxilla, “the maxillary clip.” None of these accounts take into account the presence of a palatine process to the premaxilla.
FIG. 7 . Coronal seotion, 37 mm. Minot embryo.
P.N.P., paranasal process.
FIG. 8. Obliquely horizontal section, 100 mm. embryo. P.N.P., paranasal process; M., maxilla; L.N.C., lateral nasal capsule; L.D.. laryngeal duct.
FIG. 9. Coronal section, 19 mm. Minot embryo.
N ;S., nasal septum; P.S., paraseptal cartilage anlage; F.M., frontal process of maxilla ; A.M., alveolar process of maxilla; P.M., palatine process of maxilla; P.P., anlage of palatine centre of premaxilla.
Gaupp, in Hertwig’s Handbnoh, quoting Schwink, states that in Insectivora, Chiroptera, Rodentia, Carnivora, and Artiodactyla the premaxilla is as a rule single, that its body first ossifies, and that from that centre the processes thereof are derived ; that Schwink in one case observed independent origin of the medial palatine process, and that similar independence of origin of this process has been observed in the sheep, pig, and man, by Biondi; that Rambaud and Renault have described for the latter a subvomerian centre which forms the elevated part of the nasal crest, a part of the incisive canal, and later blends with the premaxilla to form the medial palatine process of that bone; that Ktilliker maintains single origin for the whole bone. In monotremata the processus extranasalis (frontal or nasal process) arises independently and maintains the condition for a long time. This is an excellent summary of our knowledge of the position of affairs, and I have therefore quoted it bodily. I think the general conception of the form of the premaxilla is that it consists of a facial part which holds the two incisor teeth and which gives off’ two processes, one which is directed upwards to help to form the frontal process of the completed maxilla, and another which grows backwards on the medial side of Stenson’s canal—the palatine,—to lie finally under and support the anterior pointed extremities of the vomer. When the young maxilla is viewed from the inner side and below, certain fissures seem to give countenance to the view that there is or was a free premaxilla reaching backwards almost to the canine tooth, and that this premaxilla may have had, so far as its facial part is concerned, a double origin, as a fissure can be traced from behind forwards into the region behind the interval between the two incisor teeth.
Fig. 10. Coronal section, 65 mm embryo.
N .S., nasal septum; M., maxilla; P.S., paraseptal cartilage; M.P.S., median ventral process of paraseptal cartilage. Note the brush of periosteal fibres turning upwards from the premaxilla (B).
Fig. 11. Coronal section of 65 mm embryo.
N.S., nasal septum; P.S.C., paraseptal cartilage; P.P.P., centre for palatine process of. premaxilla; S.D., Stenson’s duct.
Personally I have, over a large series of young embryos of about 19 mm. in length, never been able to observe an independent facial part‘ of the premaxilla either by examining sections serially with the microscope—in which, of course, one may err—or by making reconstructions from such sections. But Mall, as has been before stated, is very positive on the point as the result of examination of cleared specimens even in the table given, dating its appearance as early as the 15 mm. stage, and he states that ossification centres can be detected in this way much earlier than in stained specimens. In one specimen of his the premaxilla is ossified before the maxilla; in others it is shown to be independent of’that bone; and in another a frontal process of it is visible. My own experience of cleared specimens is not sufficient to enable me to confirm or dispute these conclusions.
Speaking, however, of the medial process, my models and sections show clearly that it has an independent origin in the connective tissue placed between the two Stenson’s canals and between the two paraseptal cartilages (fig. 9). At 19 mm. two cellular anlagen are visible between the anlagen of the paraseptal cartilages; these later become invaded by the periosteal tissue of the bone in front, which, we may assume, is the facial part of the premaxilla, and in coronal section this periosteal tissue takes the form of a brush of fibres (fig. 10) curling around the medial side of the ventral process of the paraseptal cartilage. At a later stage, 58 and 65 mm., independent ossification is seen in this brush (fig. 11), forming a bone which, deep in front, narrows rapidly behind to pass ultimately under the vomer (fig. 16).
From its ultimate relation to the medial ventral process of the paraseptal cartilage, this bone may be looked upon as a covering bone to that process of cartilage.
FIG. 12. Coronal section, 80 mm. embryo.
N .S., nasal septum; P.S.C., paraseptal cartilage; P.P.P., separate centre of palatine process of premaxilla; S.D., upper end of Stenson’s duct.
FIG. 13. Horizonta1 section, 42 mm. embryo.
C., canine tooth germ ; I2, I1, incisor tooth buds; H.P., hard palate.
FIG. 14. Horizontal section, 42 mm. embryo, a little higher than in fig. 6. Note that the incisor tooth germs have behind each of them a small mass of bone which elevates the periosteum behind them.
H.P., hard palate; S.D., Stenson’s duct or canal ; B., bony masses behind incisors which will be confluent with the main mass higher up; I1. , incisor tooth germ ; C., canine tooth germ.
FIG 15. Horizontal section through 42 mm. head, a little higher than fig. 7. Note the two masses B in fig. 7 have been incorporated in Pr.m.
P.M., palatine process of maxilla; C., canine tooth germ; Pr.m., premaxilla; S.D., Stenson’s duct. 392 Professor Fawcett
At older stages this bone assumes a large size, but then it has fused with the facial part of the premaxilla and forms the inner permanent wall of the anterior palatine canals.
Now a word as to the fissures which appear on the oral surface of the facial part of the premaxilla.
These are due to the fact that the backs of the two incisor teeth are early covered, each of them, by downgrowths of bone from the premaxillary mass; and these coverings, not only growing downwards, but at the same time backwards, produce elevations which, imperfectly meeting, result in intervening fissures. Such elevations are quite visible in horizontal sections at the 42 mm. stage (figs. 13, 14«, 15). ‘I propose at a later date to go into the question of the development of the premaxilla more fully, so no more may be said on the question at present.
The Vomer
The Vomer has been variously described as arising in membrane by one or two centres. Of late years it has been the custom to say that the Vomer arises by two centres in membrane at the lower border of the septal cartilage of the nose, and that these centres fuse together at their lower borders to form a V— or Y-shaped bone which encloses the septum between its two limbs.
Bland Sutton, however, describes the Vomer as arising from one centre deposited in the lower border of the perichondrium of the ethmo-vomerine plate as early as the eighth week of foetal life; and quite recently H. Fuchs, as the result of examination of two human embryos of 5-,‘; cm. in length. in which the Vomer in one was completely paired and in the other for the most part unpaired, thinks that there must be individual variations, sometimes paired, sometimes unpaired. On this point, however, he says: “ Hier miissen spezielle Untersuchungen erst klarheit schafi'en.”»
Phylogenetically, it seems to be admitted that the vomer is paired, but that, ontogenetically, in most mammals it is unpaired.
My own researches, conducted over a very large number of human specimens, satisfy me that the. Vomer in every case in man arises by two points of ossification placed at the lower part of the septum nasi and at a little distance behind the paraseptal cartilages, but at a plane medial to them. In no case have I met with a single anlage younger than the 45 mm. stage.
Each centre, cut coronally, is in appearance pear-shaped, the large end of the pear being downwards and inwards, the smaller end upwards and outwards.
At the 30 mm. stage the height of these centres is 40 ,u., and they rapidly increase both in height and length, so much so that they soon spread forward into the area between the two paraseptal cartilages, and the latter overlap them. Doubtless there is here intercurrent growth.
FIG. 16. Corona.l section, 35 mm. embryo.
N .S., nasal septum ; V., vomer ; P., fused soft tissues of hard palate.
FIG. 17.Coronal section, 65 mm. embryo.
N .S., nasal septum; V., vomer; P.S., connective tissue representative of paraseptal cartilage;
At the 50-60 mm. stage the two anlagen fuse at their lower borders to form a transversely disposed plate, the appearance of the vomer then being U- or Y-shaped in section (fig. 17). At a later stage than this, 100 'mm., the bone becomes Y-shaped in section,.more especially in the region between the hinder ends of the anterior paraseptal cartilages (fig. 18). At the 65 mm. stage the single vomer is seen to divide anteriorly and posteriorly into two spines, of which the anterior project over the hinder ends of the centres for the palatine processes of the premaxillae.
Up to the 100 mm. stage, the vomer is entirely derived from membrane ; but at the above-mentioned stage it becomes added to by ossification of the hinder end of the anterior paraseptal cartilage, thus coming into harmony with the condition observed by Zuckerkandl and Fuchs in the cat.
It is only the hinder end of the anterior paraseptal cartilage which is ossified in this way, but it will be shown that the vomer in all probability is very largely formed at the expense of the deeper layers of the connective tissue remnant of the common paraseptal cartilage (see section dealing with paraseptal cartilage).
From the hinder end of the ossified anterior paraseptal cartilage a plate of membrane bone descends by the side of a limb of the Y-shaped membrane bone vomer, and at a later stage this plate fuses with the outer edge of the plate connecting the two limbs of the Y (150 mm. stage). In this region the vomer is composed of four lamellae, as in fig. 19. This is quite clearly the condition seen in the cat, and represented both by Fuchs and Zuckerkandl.
Zuckerkandl, however, states that the vomer is also ossified partly from periosteal cartilage which is placed mesial to the posterior paraseptal cartilage. Fuchs has not seen this, nor have I been able to observe it in man—at any rate, as late as the 150 mm. stage. It is, however, possible that at a later stage one might come across it, as Zuckerkandl observes it in a comparatively old cat embryo, viz. 85 mm. long. Zuckerkandl would also derive the vomer in part from the posterior paraseptal cartilage; that, I feel certain, is not the case in man.
The whole question is one of great interest because, seeing the more intimate relation of the vomer to the paraseptal cartilages than to the septum nasi, and from the fact that the vomer actually invades that cartilage, it looks very much as if the vomer were at once a covering bone to the paraseptal cartilage and at the same time a mixed bone because it, during the process of its growth, invades and makes use of the paraseptal cartilage in its formation. If, then, the vomer is to be regarded as originally a covering bone to the paraseptal cartilage, it is clear that it must phylogenetically arise by two centres, and that the single condition is one acquired later; but to prove such a theory a careful examination of the conditions prevailing in those animals in which a complete cartilago paraseptalis communis is present must be made. Moreover, the vomer leaves its close relationship to the paraseptal cartilage at its posterior end.
FIG. 18. Coronal section, 100 mm. head. Notice the Y-shaped vomer.
V., vomer; P.S., paraseptal cartilage; N .S , nasal septum. The paraseptal cartilage is ossified on one side, and from it descends membrane bone.
FIG. 19.. Coronal section of 150 mm. embryo, showing quadrilaminar vomer. A., part derived from paraseptal cartilage ; B., footplate ofgvomer ; P.P., palatine process of maxilla.
Summary
- The vomer arises, in membrane at about the 28-30 mm. stage, at the hinder end of the anterior paraseptal cartilage on each side of the middle line in two separate anlagen, and at a plane. within the paraseptal cartilages.
- The two halves unite ventrally at about the 50 mm. stage.
- That growth proceeds mainly backward at the expense of the deeper layers of the connective tissue representative of the middle segment of the common paraseptal cartilage.
- That the vomer invades the hinder end of the anterior paraseptal cartilage at the 100 mm. stage, and becomes through this at the 150 mm. stage quadrilaminar.
- That it is possibly to be regarded as a covering bone‘ to the paraseptal cartilages rather than to the septum.
- That it is both covering and replacing bone (mixed) in origin.
The Paraseptal Cartilages
Our conception of the paraseptal cartilages in man is practically limited, so far as one can judge from descriptions, to two small cartilages found by the side of the anterior part of the septum nasi in man and at a lower level than the organ of Jacobson; in fact, these cartilages are designated somewhat unhappily the J acobsonian cartilages, and from most of the text-book descriptions it seems quite uncertain if the form, position, and relation of these cartilages is really understood, for in not a few of them they are described as partially enveloping the organ of Jacobson.
They have been the subject of much study and inquiry, more especially in the lower animals, and there is quite a formidable mass of literature concerning them. . T
The most exhaustive, perhaps, of recent times is that of Zuckerkandl, but unfortunately it does not much concern man. Moreover, that part which does concern man is limited to a description of microscopical sections, and ignores the aid of models, by which alone can a true conception of the conditions be ascertained. The same may be said for Mihalkovics’s elaborate descriptions.
These later descriptions of the cartilages which have appeared have not taken into account the fibrous tissue of the nose, so that the J acobsonian cartilages seem to be left stranded as independent entities by the side of the septum nasi. That was my own conception before studying the conditions, as presented in section, of the head of a 65 mm. human embryo stained by Mallory’s triple connective tissue method. This, with the aid of a model made from the sections, entirely altered my conception of the morphology of these cartilages; and a perusal of the work especially of Zuckerkandl on the cat, kindly lent by Professor Thane, and the work of Max Voit, kindly sent by the author, together with that of the work by Gaupp, enables me to bring the conditions seen in man into entire harmony with those seen, by the authorities quoted, in other animals. The 65 mm. stage is a very convenient one to begin one’s description with, firstly, because there is the model to go by, and secondly, because the anterior paraseptal or J acobsonian cartilage has reached a stage in development which can only be augmented by increase in size, not in complexity.
Position of the Jacobsonian Cartilage.— ( I use the term Jacobsonian cartilage at present only for convenience; later it will be abandoned.) The cartilage at this stage lies at the side of the swollen lower edge of the nasal septal cartilage, immediately behind the ventro-lateral process of that septum, a process described by Spurgat as the ventro-lateral process, and further described by Zuckerkandl, who adopts the same name—processus lateralis ventralis.
The cartilage lies at some distance below the J acobsonian organ, and never at any time has any close relationship to it in man. In fact, so remote is the relation of the latter to the cartilage that considerable dispute has arisen as to the question of its homology with that organ in those types in which there is a close relation between the two (Mihalkovics).
The appearances presented by the cartilage in coronal section are such that unless a model is made it is almost impossible to get anything like a true conception of its real form. There are so many bends and twists in it; moreover, so many processes arise from it, that in one and the same section one gets the idea of a number of separate cartilages lying near the septum —as, indeed, Mihalkovics described.
A model shows us that the cartilage is essentially an antero-posteriorly directed plate, which in front divides into two short processes which come into contact with two like processes of the processus lateralis ventralis (fig. 20). Posteriorly the plate comes to a point; from the side of the lower border of the plate, and near its front end, a flattened process starts outwards and upwards, running under the narial passage towards the processus alaris of the lateral nasal capsule, but not quite reaching it, the View of the cartilage from the front being like the letter V; from the apex of this (fig. 21) there runs backwards a small rounded process which Zuckerkandl. has described and figured (fig. 20‘, p. 33 F. of Zuckerkandl’s paper). This process is of some interest, because it is very closely related to the palatine process of the premaxilla; in fact, this bone may almost be regarded as a covering bone to this process of the J acobsonian cartilage (fig. 20, M.V.P.). As we trace the main plate of cartilage back we find that it comes to a point, so that, seenfrom the side, it appears like a right-angled triangle, the apex backwards (fig. 20), but, as the cartilage disappears behind, its place is taken by a thick lamina of connective tissue which corresponds very much in thickness, and position, with regard to the septum nasi, to the plate of cartilage which it replaces (fig. 20, C.T.); and if this mass be traced backwards, it will in course of time give place to a piece of cartilage (fig. 20, PRO.) which, commencing in a pointed extremity, rapidly increases in height, and, turning outwards, becomes continuous at the cupola posterior (fig. 20, P.Cu.) with the lateral nasal capsule.
FIG. 20. Side view of nasal septum with associated structures. Lateral nasal capsule for ’ the most part removed. 65 mm. (Fawcett).
P.L.V., processus lateralis ventralis of septum: A.P.S. J acobsonian or anterior paraseptal cartilage; . M.V.P., median ventral process; C.T., connective tissue bridge between A.P.S. and P.P.G.;
s.M., suspensory membrane; P. EL, palatine process of premaxilla.
FIG. 21. Coronal section of 37 mm. Minot embryo.
P.8., paraseptal cartilage. Notice median and lateral plate united in a V-shape.
FIG. 22. Left side of model from 100 mm. embryo of nasal septum, with anterior paraseptal cartilage, with its processes showing the part which becomes ossified (a), and the lamella of bone derived from the paraseptal cartilage and its connective tissue backward continuation (b).
This mass of connective tissue seems to be suspended from the septum, as indeed the J acobsonian cartilage does, by a sheet of connective tissue less dense than itself, a sheet which gradually increases in height or depth from before backwards, a sheet which blends above with the perichondrium of the cartilage of the nasal septum, but which at the hinder end of the nasal cavity sweeps outwards around the interior of the cupola posterior on to the cartilage of the lateral nasal capsule, and may be called the suspensory membrane (fig. 20, S.M.).
It is clear, then, that posteriorly the J acobsonian cartilage is connected, by a dense connective-tissue bridge much like it in thickness and height, with another cartilage also lying by the side of the septum.
Now, this is a condition -which is almost exactly comparable with that in the .cat and many other animals examined by Zuckerkandl, who says:
“ Das die cartilago paraseptalis communis bei keinem der untersuchten Tiere ihrer ganzen Lange nach erhalten bleibt. Die Leiste zerfallt zunachst in eine vordere und hintere Halfte. ‘Die erstere Wandelt sich‘ in den J acobson’schen knorpel" um, die letztere, die cartilago paraseptalis posterior, geht als knorpel zu grunde . . . aber, zum Aufbau des Pfiugscharbeins verwendet.” ;
FIG. 23. Part of foetal skull of rabbit (Voit), ventral view. Bones on left half removed.
C.P.C., cartllago paraseptalis communis; F.B., fenestra basalis; Fn.', fenestra; L.T.A., lamina. transversalis ’ anterior; L.T.P., lamina transversalis posterior; M., maxilla; P.p., palatme process of premaxilla; S.C., septa! cartilage; V.,,vomer. .
If we compare the conditions here, in man with those in the foetal rabbit, we find that in the rabbit a paraseptal cartilage stretches along the whole length of the nasal septum, turning outwards anteriorly to join the lateral nasal capsule as the lamina transversalis anterior, turning outwards posteriorly to form a lamina transversalis posterior, as is very well seen in Voit’s model of the chondrocranium of the rabbit embryo, fig. 23.
I give three figures in which the conditions may be compared in the mole, fig. 25 (Gaupp), the rabbit, fig. 23 (Voit), man (Fawcett), fig. 24. On comparing for a moment these figures, it will be evident to all that the conditions present in man are almost identical with those in the mole, and
FIG. 24. Ventral View of nasal capsule of 65 mm. human embryo (Fawcett). Bones chiefly removed on left half, left on right half.
A. P.C., anterior paraseptal cartilage; I.f1‘., inferior turbinal cartilage ; C.T., connective-tissue bridge connecting ant. and post. paraseptal cartilages; L.T.A., rudimentary lamina transversalis anterior; M. maxilla ; M.P., medial posterior process of ant. paraseptal cartilage; M.T., middle turbinal cartilage; F.R.\;., flssura rostroventralis; P.L.V., processus lateralis ventralis; P.P., palatine centre of premaxilla; P.P.C., post. paraseptal
cartilage; P.p., papilla palatina; S.C., septal cartilage; S.D., Stenson’s duct; S.T., superior turbinal cartilage; V. , vomer.
the animals—especiall.y the cat—investigated by Zuckerkandl, and that both are degenerate conditions of those existing in the rabbit. In the rabbit, fig. 23, there is a complete paraseptal cartilage, the cartilage paraseptalis communis, stretching along the Whole length of the nasal septum, Whereas in man—like the cat, mole, etc.—this common paraseptal cartilage has degenerated in its median greater part so as to leave at its anterior end the anterior paraseptal cartilage (the so-called J acobsonian cartilage), and at its posterior end the posterior paraseptal cartilage. The intermediate part has become the dense connective-tissue bridge connecting the two (fig. 24, C.T.).
But we have seen that the common paraseptal cartilage in the rabbit is connected anteriorly by a lamina transversalis anterior with the lateral nasal capsule, and posteriorly with this same lateral nasal capsule by a lamina transversalis posterior, the two laminae bounding in front and behind an anterio-posterior fissure known as the fenestra basalis, the lamina transversalis anterior separating that fissure from the anterior narial aperture—the fenestra narina.
In man, a true lamina transversalis posterior fails-—unless, indeed, one regards the under margin of the posterior cupola as its representative whilst the lamina transversalis anterior is incompletely developed. It, in fact, is represented by the flattened outwardly and upwardly directed process described as proceeding from the outer side of the lower margin‘ of the anterior end of the main plate of the J acobsonian (anterior paraseptal) cartilage. Seeing that a complete lamina transversalis anterior fails in man, the fenestra basalis and the fenestra narina are confluent with one another, forming a fissura rostroventralis, as described by Gaupp.
FIG. 25. Foetal skull of mole, ventral view (Gaupp). A.P.C., anterior paraseptal cartilage; F.B., fenestra basilis; F.N., fenestra narina; L.T.A.,
lamina transversalis anterior; L.T.P., lamina transversalis posterior ; M., maxilla; P.P.C., posterior paraseptal cartilage. .
When one examines the anterior paraseptal cartilage in an older embryo, one finds that whilst its general external form is maintained, it has undergone a striking internal change, for that pointed posterior extremity described in the 65 mm. stage has undergone ossification, having been invaded by bony growth from the vomer (fig. 18); and not only is the cartilage seen to be undergoing entochondral ossification, but the perichondrium surrounding it is also converted into bone, and the bone spreads down in the connective tissue below the pointed posterior extremity of the anterior paraseptal cartilage until ultimately, at the 150 mm. stage, it has reached the footplate of the vomer.
‘The posterior paraseptal cartilage does not, so far as I can ascertain, ossify in man; it becomes converted into fibrous tissue, and can be seen in that condition’ in the 150 mm. stage.
The connective-tissue bridge connecting the anterior and posterior paraseptal cartilages in man, becomes converted into bone, the bone being the vomer, which is formed at first, at any rate, in the deeper layers of this bridge.
FIG. 26.—Coronal section 65 mm. embryo through posterior paraseptal cartilages. ‘Notice the suspensory membrane.
L.N.C., lateral nasal capsule; S.M., suspensory membrane of posterior paraseptal cartilage; P.P.C., posterior paraseptal cartilage ; V., vomer; N .P., naso-pharyngeal passage.
If one now examines the paraseptal cartilages in stages younger than the 65 mm. stage, one will see very marked differences in form, more especially in the case of the anterior one.
In the 24: mm. stage it is merely a flattened, obliquely inclined plate attached to the septum by a connective-tissue bridge. iMihalkovics states that it detaches itself, whatever that may mean, from the septum at the third month. My sections show that it is chondrified independently of the septum at the 24 mm. stage. Even at this early stage a cellular anlage of the lamina transversalis unconnected, it is true, with the lateral nasal wall—is to be seen passing outwards from the under margin of the anterior paraseptal cartilage underneath the nasal cavity, but not reaching, as before said, the lateral capsule of the nose, and it is not until the 37 mm. stage that this process becomes chondrified. It is the anlage of the imperfect lamina transversalis anterior of man.
Having now ascertained the form, position, and relation of the so-called J acobsonian cartilage, having seen that it is but the persistent cartilaginous anterior remnant of what in lower animals like the rabbit is the cartilago paraseptalis communis, having seen that in man a posterior cartilaginous remnant of the common paraseptal cartilage exists and that the median greater part is only in a connective-tissue condition, the term J acobsonian cartilage may well be dropped, and the better one, anterior paraseptal, used in its place.
The question next arises as to the morphological position of this common paraseptal cartilage.
Is it an offshoot of the septum, or is it an offshoot of the lateral nasal capsule? If the latter, it may be derived either from the floor of the capsule, as the majority of the later authors——e.g. Gaupp, Zuckerkandl, Voit, etc.think, or it may be a part of the median wall, or even roof, of that capsule.
There is very little to say in favour of the view that it is derived from the septum, unless that septum be regarded as a compound structure. It certainly cannot be derived from a septum limited in its origin to the united trabeculae, if for the moment we admit, for purposes of argument, trabeculae in the human chondrocranium.
An origin from the floor of the lateral nasal capsule, such as is supported by the above-mentioned authors, seems certainly better grounded; for have we not evidence that the cartilago paraseptalis communis is connected with the lateral nasal capsule both in front and behind by the laminae transversales anterior and posterior respectively? And it is quite feasible to look upon the lateral nasal capsule as being folded in under the nasal passages, to be at its inner end applied to the lower part of the septum nasi, continuation of this unfolding only being interrupted in the case of the rabbit by the fenestra basalis, and in man by the fissura rostroventralis. But if this be the manner of formation of the cartilago paraseptalis communis, why does it appear to be suspended from the nasal septum by the thin membranous sheet before described, and represented in the model of this region in the 65 mm. human embryo? And why should this membranous sheet be.quite distinct in greater part from the perichondrium of the true nasal septal cartilage? Why, moreover, should this sheet turn outwards at the posterior cupola nasi to become continuous with the internal perichondral lining of the lateral nasal wall ? We haveseen that this—1et us call it—suspensory membrane is quite narrow in height where connecting the anterior paraseptal cartilage with the septal Wall, but that, as it is traced backwards, it gradually increases in height, rising almost to the top of the septum, that part of the septum which will have attached to it the cribriform plate of the future ethmoid, before blending with the perichondrium of the septum proper.
The question cannot be satisfactorily answered by mere speculation, but requires an exhaustive inquiry into the conditions prevailing in a large range of the lower animal series. But there seems to be very strong evidence that the paraseptal cartilage belongs rather to the roof than the floor of the nasal cavity. At all events, this suspensory ligament descends to it from the lateral nasal capsule through the roof and alongside the septum towards it; that is quite evident Where the true septum is not so deep—-as posteriorly, but not so evident anteriorly, Where the great depth of the septum has stretched out this ligament to such an extent that it has for the most part blended with the perichondrium of the nasal septum.
References
CALLENDER, Phil. Trans., London, 1869.
RAMBAUD and RENAULT, Origine et développement des os, 1864.
BLAND SUTTON, J ., Proceedings of the Zoological Society of London, 1884.
THOMSON, Cunningham’s Text—Boolc of Anatomy, 1906.
HOWDEN, Gray’s Anatomy, 1909.
QUAIN, 1890.
GAUPP, HertWig’s Handbuch, 2. Teil., Band iii., 1906.
TOLDT, C., Handb. d. gerichtl. Med., Tiibingen, 1882.
MIHALKOVICS, Anat. Hefte, 1898.
Mall FP. On several anatomical characters of the human brain, said to vary according to race and sex, with especial eeference to the weight of the frontal lobe. (1909) Amer. J Anat. 9: 1-32. MALL, F., American Journal of Anatomy, 1909.
SAPPEY, Traite’ d’anatomie descriptive, 1888-9.
PORTAL, Anatomic médicale, vol. i., 1803.
HUMPHRY, The Human Skeleton, 1868.
FUCHS, Anatomischer Anzeiger, “ Verhandlungen der anatomischen Gesellschaft a. d. dreiundzwanzigsten Versammlung in Giessen,” April 1909.
ZUCKERKANDL, “ Uber den J acobsonschen Knorpel und die Ossification des Pflugscharbeines,” Sitzungsberichten der Kaiserl. A/cad. der Wissenschaften in Wien, Bd. cxvii., Ab. iii., November 1908.
Vorr, “ Das Primordialcranium des Kaninchens . . . .” Anat. Hefte, Heft 116 (38. Band), 1909.
Cite this page: Hill, M.A. (2024, May 27) Embryology Paper - The development of the human maxilla, vomer, and paraseptal cartilages (1911). Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Paper_-_The_development_of_the_human_maxilla,_vomer,_and_paraseptal_cartilages_(1911)
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G