Paper - Phases of Maturation and Fertilization in Human Ova
| Embryology - 6 Mar 2026 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Hamilton WJ. Phases of maturation and fertilization in human ova. (1944) J Anat. 78: 1-4.
| Online Editor Notes |
|---|
| William James Hamilton (1903-1975) was originally at the St Thomas's Hospital Medical School and in 1936 became Professor of Anatomy in the University of London at St Bartholomew's Hospital Medical College. He was Regius Professor of Anatomy at the University of Glasgow from 1945 to 1948. Finally, he was Professor of Anatomy at Charing Cross Medical School and then Dean of the School from 1956 to 1962.
|
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
Phases Of Maturation And Fertilization In Human Ova
By W. J. Hamilton
Department of Anatomy, St Bartholomew’s Hospital Medical College, London
During the past year it has been possible, with the co-operation of Miss G. H. Dodds, M.D., F.R.C.S., and Miss Josephine Barnes, M.D., F.R.C.S., of University College Hospital, London, to secure two living human ova from cases under their care.
The first specimen, ovum no. 1, at the stage of the second maturation spindle, was secured from a woman (Mrs G.) aged 29 years who had previously had two children. As she was suffering from severe mitral stenosis it was considered desirable that she should be sterilized. She stated that her menstrual cycle was of the 28-day type and that menstruation lasted 2 days. The cycle, however, previous to the one in which the ovum was recovered was of 30 days duration; the first day of that cycle was 18 May 1942 and the next menstrual cycle began on 17 June. The operation was performed on 3 July 1942 at 2 p.m., i.e. on the 17th day of the cycle or 14 days before the estimated onset of the next cycle assuming that the cycle from which the ovum was secured was of the 30-day type. On the evening before operation the right ovary, on vaginal examination, was found to be enlarged and slightly: tender. At operation, when the ovaries were examined, the left ovary was normal; the right ovary showed that a follicle had recently ruptured and a small amount of blood was escaping from the opening of the collapsed follicle. A bilateral salpingectomy was performed.
The second specimen, ovum no. 2, at an early stage of fertilization, was obtained from a woman (Mrs S.) aged 40 who had had five previous pregnancies, the last two of which were associated with severe toxaemia and terminated as miscarriages, the last of which occurred in February 1942. On account of her clinical condition it was considered advisable that she should be sterilized. Previous to her last miscarriage her menstrual cycles were of the 28-day type, menstruation lasting 6-7 days. Her last menstrual period before the operation commenced on 16 April 1942. Coitus took place on 27 and 29 April and the operation was performed at 9.30 a.m. on 1 May, i.e. on the 16th day of the cycle. On opening the abdomen a small amount of free fluid was found in the peritoneal cavity. The left ovary was normal. The right ovary showed a recently ruptured follicle from the opening of which a small amount of blood was escaping. There was also a cyst in this ovary. A bilateral salpingectomy and right oophorectomy were performed.
Technique
After the uterine tubes were removed at operation they were tied at both ends and placed in Locke’s solution until they were taken to the laboratory. On arrival there, the mesosalpinx and peritoneum were removed from the tubes so as to prevent kink.ing and to allow a free flow of fluid to pass during the subsequent flushing with Locke’s solution which was done from the uterine to the fimbriated end into watch-glasses. In each case the ovum was recovered in the first few c.c. of the washings from the tube corresponding with the ovary from which ovulation had occurred. The ova were photographed, while presumably still living, with a Vickers Projection Microscope at a magnification of 480 diameters. They were fixed by the gradual addition of Bouin’s fluid (Allen’s modification, P.F.A. 3) to the Locke’s solution. Dehydration was carried out in the usual way. They were double embedded in celloidin and paraffin. Unfortunately, during these manipulations the second specimen was lost. Complete serial sections at 8);. were cut of the first ovum by Mr E. Park.
Description
First specimen
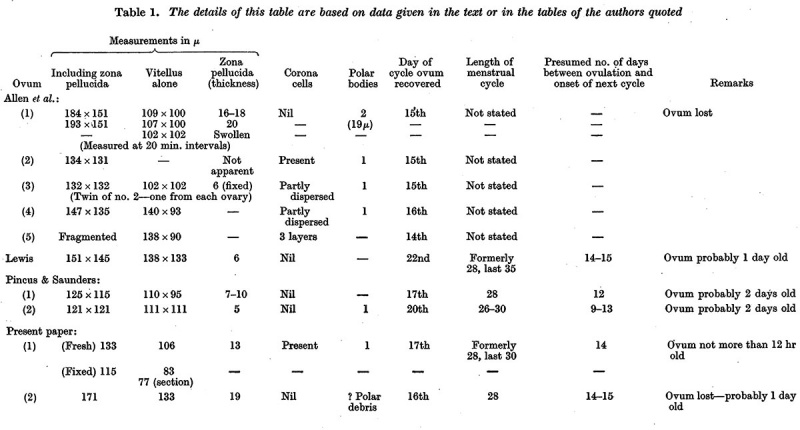
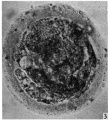
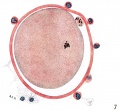
When examined in the fresh state several layers of loosely arranged cumulus cells were found attached all around the zona pellucida which had a homogeneous appearance and was closely applied to the vitellus (P1. 1, fig. 1). Owing to the presence of the cumulus cells the ovum did not roll freely in the watch-glass and at no time was a polar body visible nor could any details of nuclear material within the ovum be recognized. The light yellowish, uniformly granular vitellus completely filled the zonal cavity. There were no such subdivisions of the yolk material as described by Lewis (1931). The dark areas shown in the photograph are due to the presence of cumulus cells on the upper or lower surface of the zona.
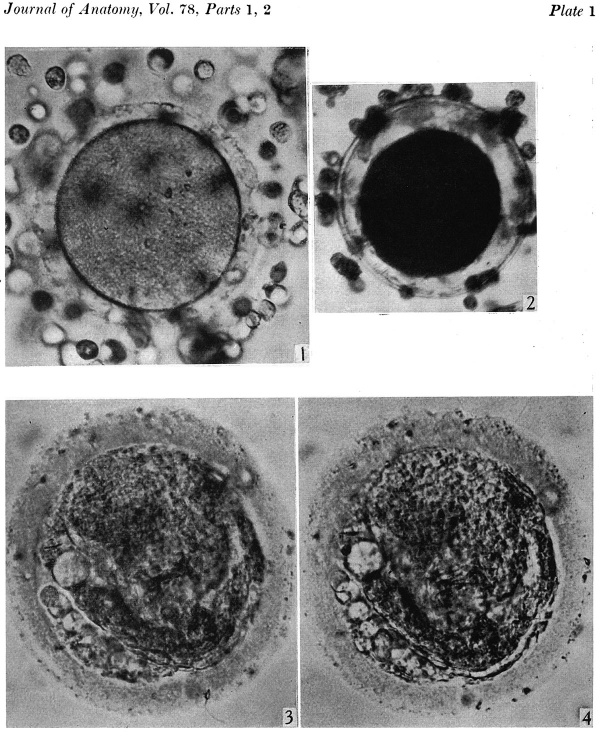
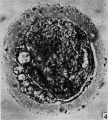
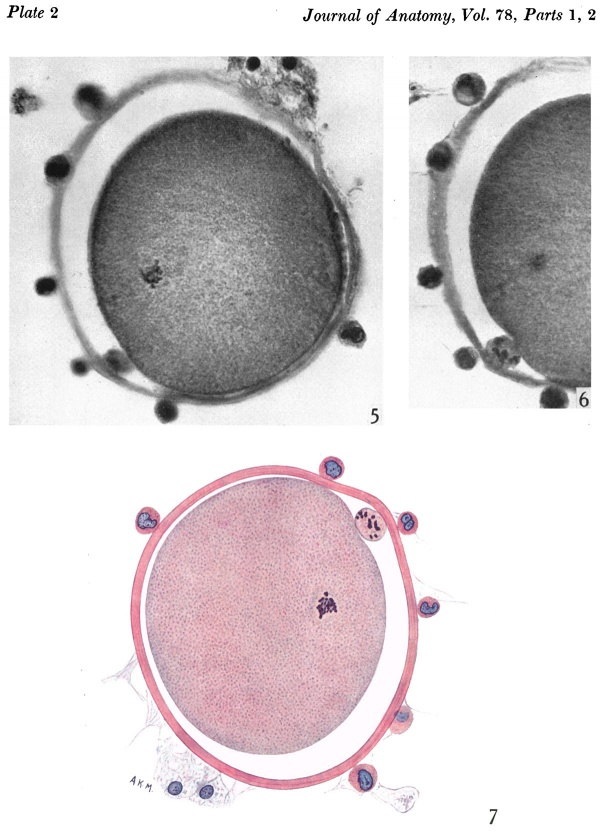
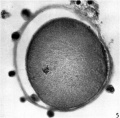
After fixation and dehydration many of the follicle cells were dispersed so that in the sectioned ovum relatively few are attached to the zona. With fixation the vitellus underwent a shrinkage greater than that of the zona pellucida so that a perivitelline space appeared (Pl. 1, fig. 2). After staining with modified Harris’s haematoxylin and eosin the cytoplasm appears uniformly granular and shows no evidence of polarity (Pl. 2, figs. 5, 7). The 2nd maturation spindle, cut slightly obliquely, is present at the stage of metaphase. The first polar body shows scattered chromosomes in its cytoplasm (Pl. 2, figs. 6, 7).
Second specimen
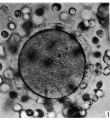
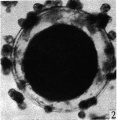
When examined in the fresh state this was found to be free of cumulus cells. The zona pellucida appeared as a homogeneous membrane in which many complete sperms could be seen. Some were lying obliquely, others transversely (Pl. 1, figs. 3, 4). The vitellus did not completely fill the zonal cavity; it was coarsely granular, and at one depth of focus showed, in a clearer zone towards the centre, what may have been the spindle of the , lst cleavage. The general appearance of the vitellus, however, is that of early degeneration. Between the vitellus and the zona at one side a number of globules are seen which are probably the remains of the disintegrating polar bodies. It was unfortunate that it was not possible to verify the structures seen in this ovum by subsequent sections.
The measurements of both ova, together with other human ova, are given in Table 1.
Discussion
Allen, Pratt, Newell & Bland (1930) give an account of the attempts that have been made to secure human tubal ova. They succeeded in obtaining five ova by reverse irrigation of the uterine tube from the uterus. The photographs of the living eggs found by them are unsatisfactory and the histological details leave much to be desired. Undoubtedly some of the ova are undergoing degenerative changes. Lewis (1931) obtained an unfertilized ovum by flushing the uterine tube which had been previously removed with a myomatous uterus. The vitellus was dark and everywhere in contact with the zona pellucida and showed three types of yolk bodies which were irregularly distributed in it. Two further ova have been recovered by Pincus & Saunders (1937). One of these ova contained a dark coarse cytoplasm with-several large dark yolk particles, similar to those of the Lewis ovum, and the vitellus was in close contact with the zona pellucida. This egg was cultured and the movements that occurred in the cytoplasm were apparently of a degenerative nature. The second ovum contained no dark yolk masses. It was entangled, however, in a mass of cells which were thought to represent the remains of the cumulus oophorus and which obscured the details of the cytoplasm in the photograph. It was thought that this ovum had only recently been shed.
In ovum no. 1 of the present account, at the second maturation spindle stage, the cytoplasm is uniformly granular and thus more closely resembles the second ovum of Pincus & Saunders than any so far described. This uniformity of cytoplasm is in contrast to that described by Allen et al. (1930) who found that the central cytoplasm of the vitellus was coarsely granular and the peripheral ‘exoplasm’ was finely granular. It seems probable that ovulation had occurred recently. Histologically this ovum shows no degenerative changes. Hertig (1942) report the isolation of a ‘perfect unfertilized tubal ovum’ but give no details concerning it.
As can be seen from Table 1 there is considerable variation in the size of the different ova described, the smallest being that described by Pincus & Saunders (1937) and the largest ovum no. 2 of the present series.
Maturation
There is a comprehensive literature on the maturation of the oocyte in different mammals, but up to the present only a few cases have been described in the human subject.
In all mammals so far examined, with the possible exception of the dog (Van der Stricht, 1923), the second maturation division begins in the ovary but is not completed until after fertilization. The first description of the formation of the first and second polar bodies in man was given by Thomson (1919) and was based on examination of follicles which undoubtedly show either atretic or degenerative changes and, therefore, need not be considered further. He believed that the second polar body was formed when the ovum was still in the ovary.
The first maturation stage of the human oocyte was described by Stieve (1926). He found a primary ovarian oocyte with two distinct masses of nuclear material close to the periphery. A further stage of the first maturation was described by Hoadley & Simons (1928); the nucleus was at the ‘mesophase’. Recently, Rock & Hertig (1942) report having recovered two oocytes at the first maturation division from ovarian follicles. Dixon (1927) described an oocyte in the ovary at the second maturation spindle stage and concluded, without sufficient evidence, in my view, that the second polar body is formed before the oocyte leaves the ovary and before fertilization has taken place. The appearances seen in ovum no. 1, which is at the metaphase and is already shed, and the description given by Allen et al. (1930) for two of their shed ova, and also by Pincus & Saunders(l937) do not support the opinions advocated by Dixon. There is no doubt that the second maturation spindle persists until after ovulation, but the period of time that the oocyte remains at the second maturation division stage, or whether it is completed in the absence of fertilization, cannot be stated until more material is available.
Time of ovulation
The recovery of ovum no. 1, on the 17th day of the menstrual cycle in a woman who stated that normally her cycle was of 28 days, although the previous cycle, which was accurately timed, was of 30 ‘days’ duration, and of ovum no. 2, on the 16th day of the cycle in a woman who had a cycle of 28 days, raises the question of the time of ovulation.
It is probable that ovum no. 1 had only recently been shed less than 12 hr. previously (see clinical history and findings on vaginal examination). The cumulus cells are still present, as they are in some other ova described (see Table 1), and the specimen is well fixed and stained and shows no degenerative changes. From the comparative aspect it is worthy of note that Pincus (1930) found, in the rabbit, that the cumulus cells persisted for 10 hr. after ovulation in unfertilized eggs and only for about 5 hr. in fertilized ones. In the mouse (Lewis & Wright, 1935) in both fertilized and unfertilized eggs the cumulus cells disappear at about 12 hr. after ovulation.
Ovum no. 2 was probably shed at least one day previously, all the cumulus cells having disappeared and many sperms being found in the zona pellucida. There is just a possibility that this egg had been fertilized; in a clear area towards the centre there are dark rod-like bodies suggestive of chromosomes on the spindle of first cleavage (of. Pincus, 1930), in the rabbit). The recovery of these eggs shows that ovulation occurs, in women with a ‘regular’ cycle, at approximately the middle of the menstrual cycle.
On the subject of the time of ovulation in the menstrual cycle there is an extensive literature which has been fully reviewed by a’ number of investigators (see Knaus, 1934; and others). Evidence, however, is accumulating which seems to show that ovulation is more closely related to the succeeding menstrual period than to the beginning of the preceding one (see Knaus, 1934; Ogino, 1934; Pryde, 1941; and Smith, 1942). Rock & Hertig (1942), by comparing the ages of young embryos with the associated endometrial histology in seven cases, and especially in two which were well controlled, believe that ovulation occurs 14 (15 to 13) days before the anticipated date of the next menstrual period. Further, if the menstrual histories of the cases from which human ova have been recovered are analysed the data. tend to show that ovulation is more closely related to the beginning of the succeeding period.
The Lewis ovum (1931) was recovered on the 22nd day of the cycle. As ovulation had probably occurred 24 hr. earlier and assuming that this cycle would have been of 35 days’ duration, as was the preceding one, then ovulation occurred 15 days previous to the beginning of the succeeding cycle. In ovum no. 1 of Pincus & Saunders (1937), ovulation is estimated to have occurred 12-14 days before the beginning of the next cycle, and in their ovum no. 2, 9~13 days before the 1st day of the next menstrual period, depending upon whether the present cycle was of 26 or 30 days’ duration. The menstrual histories of the two cases from my series described in this paper also indicate that both ovum ,no. 1 and ovum no. 2 were ovulated 14-15 days be fore the presumed beginning of the next menstruation.
Summary
- A description is given of two human tubal ova.
- Ovum no. 1 in the living state was surrounded by cumulus cells. The vitellus completely filled the zonal cavity.
- On section this ovum was found to be at the metaphase of the second maturation division.
- Ovum no. 2 showed spermatozoa in the zona pellucida.
- The time of ovulation in women is discussed.
I wish to acknowledge my indebtedness to the Publications Fund of the University of London for a grant towards the cost of the coloured illustration of this communication. I also wish 0 thank Mr A. K. Maxwell for the natur-treu figue; and also Mr A. E. Westwood for the photographs.
References
Allen, E., Pratt, J. P., Newell, Q. U. & Bland, L. J. (1930). Contr. Embryol. Carneg. Instn, 22, 45.
Dixon, A. F. (1927). Irish J. Med. Sci. p. 149.
Hoadley, L. & Simons, D. (1928). Amer. J. Anat. 41, 497.
Kivus, H. (1934). Periodic Fertility and Sterility in Woman. Vienna.
Lewis, W. H. (1931). Johns Hoplc. Hosp. Bull. 48, 368.
Lewis, W. H. & Wright, E. S. (1935). Ooritr. Embryol. Carney. Irtatn, 25, 113.
OGINO, K. (1934). Conception Period in Women (trans. Y. Mujagawa). Harrisburg: Medical Arts Co.
Pmcus, G. (1930). Proc. Roy. Soc. B, 107, 132.
Pmcus, G. & Ssusmsas, B. (1937). Amt. Rev. 69, 163.
Pryde, J. (1941). Brit. Med. J. 1, 12.
Roan, J. & Hertig, A. T. (1942). Amer. J. Obstet. Gynaec. 44, 9173.
Smith, G. P. (1942). Brit. Med. J. 2, 38.
Srmvm, H. (1926). Z. milcr.-amt. Forsch. 6,.231.
Thomson, A. (1919). The Maturation of the Human Ovum J. Anat., Land, 53, 172.
Van Der Stricht, 0. (1923). Arch. Biol., Paris, 83, 229.
Explanation of Plates
The photomicrographs have been reproduced without retouching.
Plate 1
Fig. 1. Photomicrograph of ovum no. 1 in the living state. The dark areas on the vitellus are cumulus cells on the upper or lower surface of the zona. x 480.
Fig. 2. The same ovum immediately after fixation. x 480.
Fig. 3. Photomicrograph of ovum no. 2. A complete sperm is seen in the zona pellucida; other sperm heads are visible. At the lower left hand corner of the photograph globular detritus is seen. x 480.
Fig. 4. Photomicrograph of the same egg at a diflerent focus. In a clearer area towards the centre of the vitellus rod-like bodies (? chromosomes) are seen. x 480.
Plate 2
Fig. 5. A section of ovum no. 1 showing the metaphase of the second maturation division. The flrst polar body (out of focus in the lower left hand comer) is seen. The vitellus has shrunk ffom the zona pellucida and most of the cumulus cells have been cast off (of. P1. 1, fig. 1). x 840.
Fig. 6. Photomicrograph of part of same section as in fig. 5 with the polar body in focus. x 840.
Fig. 7. A drawing of same section as that in figs. 5 and 6 showing the details of the second maturation spindle and the state of the 1st polar body. The cytoplasm is seen to be uniformly granular. x 840.
Cite this page: Hill, M.A. (2026, March 6) Embryology Paper - Phases of Maturation and Fertilization in Human Ova. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Paper_-_Phases_of_Maturation_and_Fertilization_in_Human_Ova
- © Dr Mark Hill 2026, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G