Paper - A human embryo before the appearance of the myotomes (1918)
| Embryology - 27 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Ingalls NW. A human embryo before the appearance of the myotomes. (1918) Contrib. Embryol., Carnegie Inst. Wash. No.23 Publ. 227, 7:111-134.
| Online Editor Note |
|---|
|
Other papers by Ingalls
|
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
A Human Embryo Before the Appearance of the Myotomes
By N. William Ingalls.
(1918) With four plates and five text-figures.
- Contribution No.23: Figures | Plate 1 | Plate 2 | Plate 3 | Plate 4 | Plate 1 | Carnegie - Contributions to Embryology | Carnegie stage 8 | Category:Carnegie Stage 8 | Historic Embryology Papers
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
Reference
Ingalls NW. A human embryo before the appearance of the myotomes. (1918) Contrib. Embryol., Carnegie Inst. Wash. No.23 Publ. 227, 7:111-134.
Cite this page: Hill, M.A. (2024, April 27) Embryology Paper - A human embryo before the appearance of the myotomes (1918). Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Paper_-_A_human_embryo_before_the_appearance_of_the_myotomes_(1918)
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G
Introduction
The specimen which forms the subject of this paper came into my possession some time ago through the kindness of Dr. E. Peterka, of Cleveland. In the collections of embryology and teratology of the Department of Anatomy of Western Reserve University it is listed as embryo No. 1 (Western Reserve No. 1). On account of the very interesting and important stage of human development which it illustrates, a detailed investigation of its more essential features, especially as regards the embryo proper, has been undertaken. The extra-embryonic structures, chorion, body-stalk, and yolk-sac, and the evidence they offer on early blood and blood vessel formation, will not be dealt with in detail at this time.
The intact ovum, when it came into my hands, had been for about a month in alcohol of unknown strength, but was, on account of its small size, quite well preserved. The following brief history accompanied the specimen:
- April 2. Intercourse (also about two weeks before?).
- April 8. Period expected; regular 24 to 26 days.
- April 14. Bleeding commenced, gradually increasing.
- April 17. Ovum cast off.
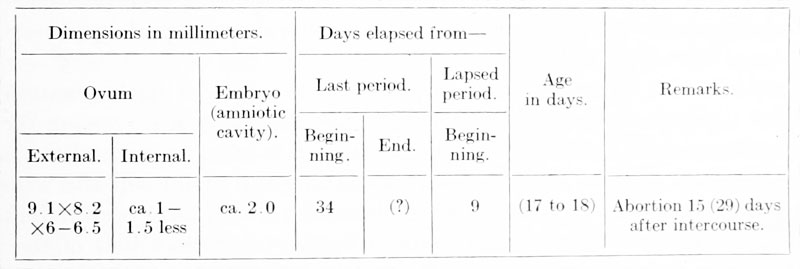
Before entering upon a discussion of the anatomical findings, something may be said as to the probable age of the specimen. Following the example of Bryce and Teacher (1908), which has been adopted so frequently, one can set up a similar table for the embryo in question :
The estimated age of 17 to 18 days was put in parenthesis in the above table because we could not bring ourselves to look upon it with any very great degree of confidence. The figures were obtained by comparison with embryos which were obviously in a stage of development either more or less advanced and by reference to the recent estimates of Triepel (1914) and Grosser (1914). Triepel's suggestion of subtracting about 18 days from 34 in this case would give an age of about 16 days. Embryo No. 1 is far in advance of both that described by Fetzer (1910) and the v. Herfi" embryo of Graf Spee (1896), the ages of which have been given as 15 and 17 to 18 days respectively. On the other hand, it is distinctly less advanced than Frassi's (1907) specimen, the age of which is estimated at (18) 19 days. The embryo Kl. 13 of Grosser (1913) is strikingly like our own, but we think a trifle more developed. Grosser gives the age as 19 days. A very similar stage of development is represented by the recent embryo of Strahl (1916), but concerning which there are no data as to age.
If one again adopt the method of Bryce and Teacher, it is possible to determine when, as regards the menstrual cycle, fertilization took place. The duration of the menstrual cycle in this case may be taken as 25 days - regular 24 to 26 - and if we let the age of the embryo be 18 days, then fertilization occurred on the seventeenth day of the previous menstrual month. Such a date w'ould harmonize very well with the findings of Frankel on ovulation, as interpreted by Grosser. The time thus assigned for fertilization could easily be pushed still farther toward the beginning of the menstrual month by either supposing that the embryo is more than 18 days old or that development was arrested by the hemorrhage some time before abortion occurred. As regards the last point, we see no reason to suppose that development stopped very long before the ovum was expelled. Fertilization on the seventeenth day of the menstrual cycle would mean that the intercourse of April 2 could not be considered in computing the age, since it falls on the twentieth of the cycle, 15 days prior to the abortion.
The history of intercourse two weeks before the one just noted is, however, subject to doubt; it would have occurred on the sixth day of the cycle. This would have called for a rather protracted sojourn of the spermatozoa within the tube namely, 11 days, a period over which they arc doubtless quite cai)al)lc of retaining their fertilizing power. The sixth day of the cycle would fall about the beginning of the so-called period of oestrus, and, in view of the reputed increase in libido at this time and of certain obstetrical experiences, one may suppose that not only is this a favorable time for insemination, but also favorable to a prolonged stay of the spermatozoa within the tubes.
The arrival of the ovum in the uterus and the date of its implantation are dependent upon the unknown factor of the time consumed in traversing the tube. Grosser points out that this may vary, depending upon tubal (menstrual) conditions, and he is inclined to raise the estimate of 10 days, given by himself and Triepel, to 14 days or even more. In either case, implantation would have occurred after the beginning of the lapsed period, and some influence other than that of the actual ovum upon the uterine mucosa would have to be invoked to inhiliit the intending menstruation. Such an influence, as is well known, has been .sought in the tiny ovum within the tube, acting alone or in conjunction with the newly formed corpus luteum. Assuming 10 days as the period of migration (7 days in the table of BryceTeacher), implantation would have occurred in our specimen on the second day of the cycle. It would therefore have found a mucous membrane especially adapted to its nutritional needs, possibly thus accounting for its large size as compared with the stage of development; but, on the other hand, the inhibitive action upon this same mucosa, from whatever source, may have come too late to save it, as seems also to have been the case with the Bryce-Teacher ovum.
It is not the purpose of this paper to enter into any discussion of the relations between ovulation and menstruation, the passage of the ovum along the tube, or other mooted questions which may have a bearing on the age and development of embryos, but a few words might be said regarding what seem to us to be certain aspects of this subject. Of the three cardinal embryonic features so often quoted, viz, age, size, and stage of development, only the last has any great practical importance, and even here there is more or less variation in different parts of the embryonic body. The fact that age, size, and development by no means rim parallel has been pointed out very clearly by Mall (1914; cf. also Rabl, 1915), and indeed there is no reason to expect that they would be exactly comparable. It seems to us that with ova of the same age, dating from the time of fertilization, discrepancies in their size and development may, and not without reason, be assigned to different environmental factors. Tubal conditions during migration, varying at different times in the menstrual cycle, might play a certain role, resulting in more or less rapid progress along the tube, accelerating or retarding development. Conditions in the uterus at the time of implantation, premenstrual, menstrual, or postmenstrual, etc., time of ovulation, or conceivably the actual size or potentiality of the ripe unfertilized ovum and other unknown or unappreciated factors, might bring about the variations so often observed. The relative independence of age, size, and degree of development is most strikingly evident in the case of pathological ova.
The variation in size at the same developmental stage is especially marked in the case of embryo No. 1 (see page 116). The chorionic vesicle is roughly of the same dimensions as that of the embryo Kl. 13 of Grosser or of the embryo of Eternod (1898), while the blastoderm of Xo. 1 is, as well as can be determined, about twice as long as in Grosser's case — but of about the same stage of development and a half longer than in Eternod's but far less advanced. In other words, the size of the vesicle is fairly commensurate with the assigned age, while the embryo is disproportionately large, both as regards the chorionic sac and the estimated age; it is, in addition, very large, considering the degree of development. The opposite disproportion between the sac and contained embryo is quite characteristic of pathological ova, and it may be that we are dealing here with the results of some subtle influence which has stimulated the growth of the embryo proper without, however, having disturbed unduly its orderly development or brought down the balance on the pathological side. A retardation in development but not in growth might account for observed conditions. In the face of the extensive literature on the early chapters of human development we can not claim to present a typical embryo of the middle of the third week, but simply a normal specimen of about that age. It is difficult enough to find one's way among the unnumbered variations of adult morphology, but as regards the embryo we have hardly scratched the surface. It would not be surprising if we had before us in this specimen one of those examples of embryonic variation which are so abundantly present later on (tail, pronephros, milk-ridge, fifth aortic arch, etc.j. For obvious reasons these individual variations become more plentiful as development proceeds, but at no time need they occasion any surprise and always may they be ranged under the same rubric.
One carries away from the perusal of the literature loearing on the age of young ova the relations between ovulation, menstruation, fertilization, implantation, etc. the impression that the actual age of a normal embryo has a value, for purposes of classification at least, more apparent than real if not in large measure fictitious, and the more so because this assigned age can be only a more or less defensible aj)i)ro.\imation. Complicating the more general factors touched upon above are the individual variations and pccuharities, pathological states it may be of the maternal organism if not also of the future ovum, temporary bodily or seasonal conditions and the like, not to mention possible paternal influences, a variety of factors which it is difficult or impossible to evaluate, and we are imperceptibly carried into problems of fecundity, absolute and relative sterility, and other clinical, racial, and sociological questions. In the end one can appreciate the perplexity of Hyrtl when he wrote long ago in his characteristic vein: "So weit ware nun Alles recht. Xur begreifft man dabei nicht, warum die Frauen nicht fortwahrend schwanger sind, imd aus dem Schwangersein ihr Lebelang nicht herauskommen."
The entire specimen was stained in bulk with hematoxylin, and after sectioning at 10 microns was counterstained with eosin-safranin. The plane of section, which it was supposed would be transverse to the embryo, the interior of the vesicle having been examined somewhat before embedding, came out quite obliquely, as can be seen in the various text-figures. While the staining reactions are not always what could be desired, still there is no doubt that the essential features have been preserved. Occasional mitoses are in evidence, as will be noted later.
1. The Chorionic Vesicle. Gross
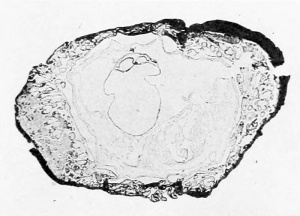
The following account is taken from our notes made soon after receiving the specimen. The intact vesicle (plate 1, figs. 1 and 2) is quite regularly formed and distinctly flattened; the surface showing the circular area of free villi is slightly more convex than the opposite. The form tends to be roughly quadrangular with the corners rounded off. To the touch the vesicle feels quite firm and resistant. Measured under a magnification of 5 diameters, the ovum shows the following dimensions: length 9.1 mm., breadth 8.2 mm., thickness 6 to 6.5 mm. The internal measurements are from 1 to l.o mm. less.
One surface of the ovum presents a large, sharply defined area of free chorion and its villi, .situated at one end and extending about to the middle. The villi vary greatly in size and shape. They may assume the form of long, slender processes or of thick, broad, irregular masses, often in clumps together and leaving a few small areas free. There arc a few straw-colored areas as from blood-stains. The remainder of this surface of the ovum is smooth and varies in color from a straw through a red (fresh meat) to almost a purple.
The opposite side of the ovum is much smoother, covered partly by a much thinner layer of maternal tissue through which project more or less freely the villi of the chorion. These villi appear to be rather more pointed and slender than those previously noted, resembling ])apilliP filiformes. There seems to be no part of the sac which does not possess villi.
Upon making an incision along one side of the sac to facilitate embedding, a large cavity is found into which projects the embryonic anlage attached to the side showing the free villi. Regarding the embryo proper nothing more than a small, whitish, globular mass (yolk-sac) can be made out for fear of injuring the embryonic structures. At this time there were seen a few minute but distinct strands traversing the cavity (exoccelom) and connecting the inner surface of the vesicle with the yolk-sac. Traction upon the margins of the opening in the vesicle could be seen to have a very distmct effect upon the yolk-sac on account of the attachment of the above-mentioned filaments.
2. The Embryo and Adnexa
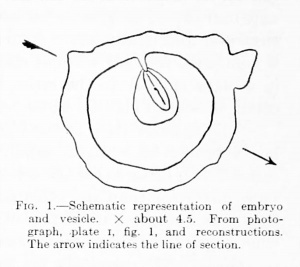
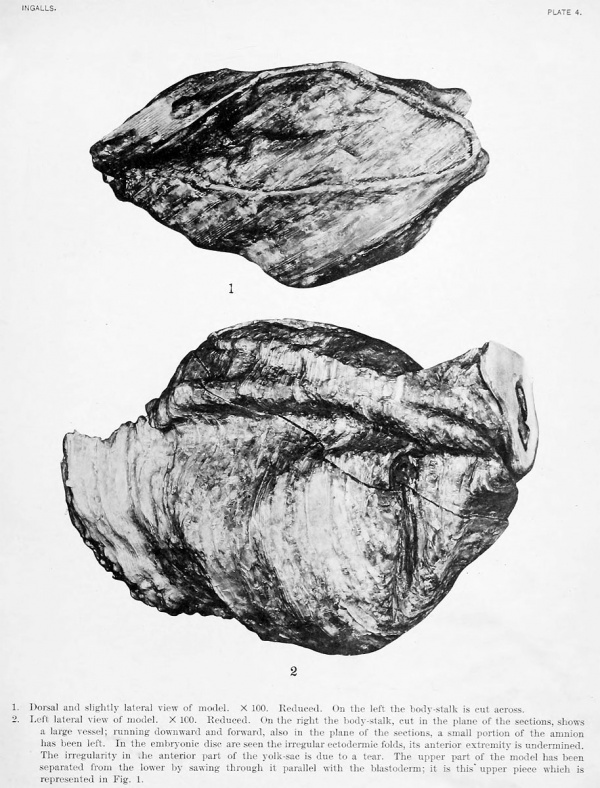
The main features of the embryonic anlage are shown in the text-figures 1, 2, and 3 and in the photographs of the model (plate 4, figs. 1 and 2). The general shape of the blastoderm is not unlike that of the Frassi embryo, but narrower and as a whole very much larger. Its dimensions, determined on the reconstructions (X 100), are 2 mm. in extreme length by about 75 mm. in breadth at the widest point. The ventral surface of the embryonic disk presents a very slight ventral concavity in the sagittal plane, while at right angles to this the same surface is for the most part convex, i. e., projecting slightly into the yolk-sac. The dorsal surface is in general more strongly convex, owing to the presence of the prominent ectodermic folds. The amnion above completes roughly the curvature of the yolk-sac below. The anterior extremity of the blastoderm is quite regularly rounded and, especially on the left side, is undermined by shallow extensions of the exoccelom; the posterior half tapers evenly to a point.
As may be seen in the dorsal view of the model, the appearance of the posterior third of the embryonic disk is quite different from that of the middle and anterior thirds. This posterior part contains the cloacal membrane and about the caudal half of the primitive streak and is the most regularly formed part of the entire blastoderm. The dorsal surface presents here, on either side of the median fine, two rather steep, even slopes (plate 3, fig. 1), the left slightly more extensive, which extend from the region of the cloacal membrane and primitive streak to the attachments of the amnion laterally. The primitive groove appears in the model simply as the central, deepest portion of this valley-like area. The ventral surface of the region in question is strongly convex from side to side. Distinct primitive folds can not be made out.
The central third of the embryo includes the anterior half of primitive streak and the head-process region in front of it. Most conspicuous here are the two large folds of ectoderm which extend from near the middle fine to the attached border of the amnion; the fold on the left side is broader and more regular than that on the right. Separating these prominent folds (plate 3, figs. 2 and 3) lies, in their caudal halves, the anterior end of what we may call the primitive groove, here very deep and narrow. The groove between these folds in their cephalic portion is much shallower and finally lost. Near the posterior end of this shallower groove, which is continued forward without distinct interruption from the primitive groove, but in a plane slightly to the right, lies the minute dorsal opening of the archenteric canal (so-called chordal canal) slightly to the left in the bottom of the groove.
The anterior third of the blastoderm is in general slightly convex, but its surface is broken up by many small, irregular folds to which one can attach no significance. It is certain that the ectoderm in the anterior half of the embryo has suffered more distortion than any other part. The result has been an obliteration, as far as they may have been indicated, of the early medullary folds anteriorly, coupled with what seems to be their accentuation and prolongation posteriorly. That these last mentioned folds, occupying the center of the blastoderm, have anything to do with the medullary folds is, considering the stage of development, very doubtful. The posterior ends of these folds, especially on the right, have a remote resemblance to the caudal lobes of a later date.
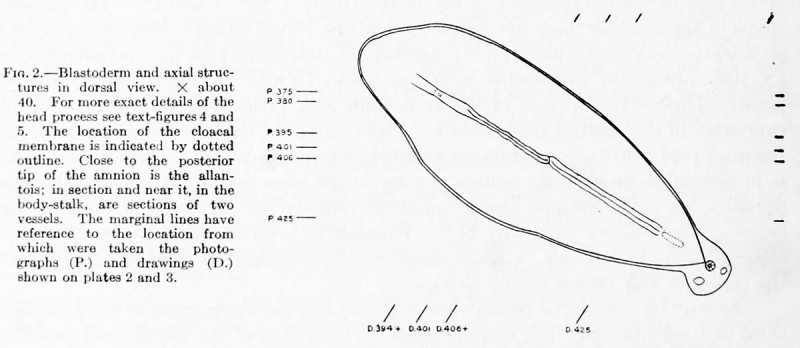
Fig. 2. Blastoderm and axial structures in dorsal view. X about 40. For more exact details of the bead process see text figures 4 and 5. The location of the cloacal membrane is indicated by dotted outline. Close to the posterior tip of the amnion is the allantois; in section and near it, in the body-stalk, are sections of two vessels. The marginal lines have reference to the location from which were taken the photograph (P.) and drawings (D.) shown on plates 2 and 3.
The amnion lies close to the embryonic ectoderm anteriorly, while farther back it is lifted high above it by being incorporated in the body-stalk. Any indications of the presence or recent disappearance of an amniotic duct as noted by Grosser (1913) and Strahl and Bcneke (1910) are wanting. This amniotic duct may very well be one of those instances of embryonic variation referred to above, not only variable but (juitc possibly very transient, and in this same category may be placed a peculiar feature of our embryo to which we would here draw' attention. As can be seen in the accompanying illustrations, the caudal tip of the amnion lies in very close proximity to the allantois, a short distance above the connection of the latter with the yolk-sac. In at least two sections there is a very distinct though tiny, narrow outpocketing of the amniotic cavity toward the allantois (not .shown in the figures). Here the epithelium of the amnion is of a low cuboidal type in contrast with its squamous character in the immediate vicinity. There is no connection between the cavities of the amnion and allantois, but their epithelial cells fuse into a single mass over a small area of contact. The lumen of this amniotic diverticulum, which is also very short, is only a few microns in diameter, tapering slightly toward the allantois. This structure may well be compared with the secondary connection set up between the same cavities in certain reptiles, the canalis amnioallantoideus of 8trahl (Schauinsland, 1902).
Histologically the amnion is composed of two layers of cells which are generally frankly squamous. The transition of the ectodermic cells to the flattened type is as a rule quite abrupt. In certain places, however, the cells, near the attached border of the amnion, are cubical and become squamous only at some distance from the line of reflection. The cells of the mesodermic layer of the amnion have slightly smaller, more densely staining nuclei and seem to present a clean, even surface to the exoccelom. Throughout most of the membrane its two layers are in close contact, often almost indistinguishable, but along the borders there is frequently a considerable space between the two, across which run numerous irregular cell processes connecting the ectoderm and mesoderm but apparently belonging rather to the latter. Scattered mesodermic cells in the interval between the layers are quite common. Posteriori}^, where the membrane is cut tangential!}-, the ectodermic elements appear polygonal in outline, with large, pale nuclei which almost fill the cell.
In the consideration of the embryo proper we shall begin with the most posterior structures and gradually work forward. In like manner we shall endeavor to separate the following observations from the speculations which they indicate.
Mention has already been made of the indications of a canalis amnio-allantoideus in the caudal extremity of the amnion. A short distance in front of this, in the axis of the blastoderm and at the posterior end of the primitive streak, lies the cloacal membrane. As shown in figures 2 and 3, it measures about 0.12 mm. in length. This measurement and likewise the figures are at best approximations, maximum Limits in any case, since it is difficult to determine the exact line, if there be one, between the membrane and the primitive streak. There is in the cloacal region a well-defined groove in the ectoderm, less conspicuous, however, than the primitive groove with which it is directly continuous. In certain sections it is quite evident that the ectoderm and entoderm are in immediate contact, the mesoderm being at some httle distance. In other sections, largely on account of the irregular lower surface of the ectoderm, the picture is very much like that of the primitive streak. The conditions found here in the cloacal membrane are such as would be expected from the gradual and not entirely regular transformation of the streak into the membrane. All that is requu-ed is an arrest of mesoderm formation and the subsequent separation of the upper and middle germ-layers. The entoderm below is a perfectly distinct layer the cells of which have nuclei larger and paler than those of the other layers. The condition of the ectoderm is such that the real character of its cells can not be made out. Its free surface is of course distinct, but the lower surface is often markedly regular and frayed out. In the region under discussion at present it is undoubtedly of the columnar type, in most places, if not everywhere, pseudostratified with one, two, or occasionally three layers of nuclei. Farther back and laterally the ectoderm is frankly one-layered, with low columnar or even cuboidal cells. Throughout most of the embryo, however, the arrangement of its nuclei in several layers, the character of its lower surface, and it.s often irregularly varying thickness make impossible any definite statements as to its real structure. Cell boundaries are not commonly visible.
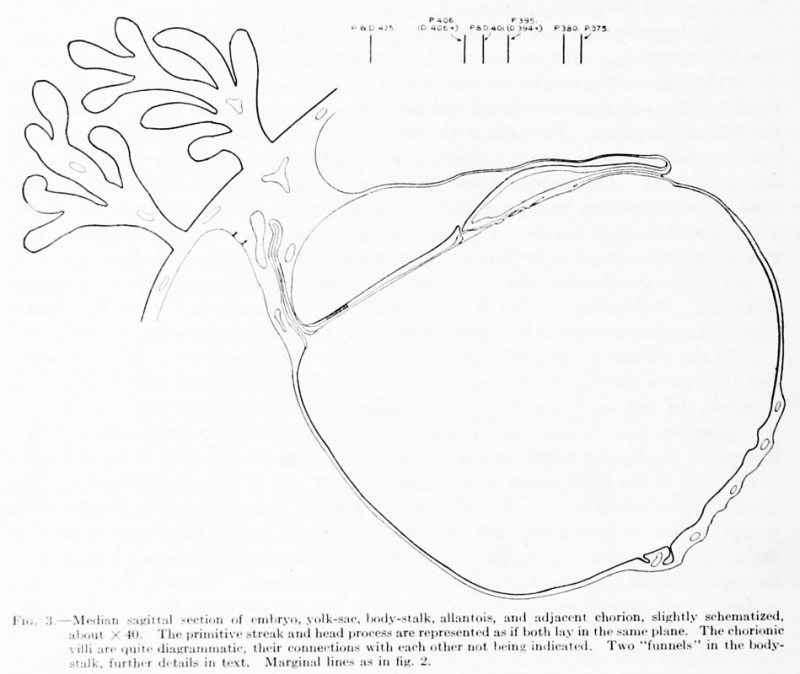
Fig. 3. Median sagittal section of embryo, yolk-sac, body-stalk, allantois, and adjacent chorion, slightly schematized, about X 40. The primitive streak and head process are represented as if both lay in the same plane. The chorionic villi are quite diagrammatic, their connections with each other not being indicated. Two "funnels" in the bodystalk, further details in text. Marginal lines as in fig. 2.
The primitive streak (figs. 1, 2, and 3; plate 2, fig. 1 ; plate 3, fig. 1) is very long in this embryo, making up about one-third of the axis of the blastoderm, the center of the latter being just in front of the anterior end of the streak. Its length, measured from the anterior limit of the cloacal membrane to the dorsal opening of the archenteric canal, is about 0.G5 mm. In position it is not exactly central, but is displaced a trifle to the right. The primitive groove is well defined throughout, the continuation, as noted above, of its anterior end passes slightly to the right of the opening of the archenteric canal and the beginning of the head process. This condition, in which the primitive streak and its head process do not lie in the same sagittal plane, is not uncommon in a variety of forms, and apparently the head process is usually on the left, as in this case. The anterior limit of the streak is of course easily determined, and our reason for assigning the posterior limit is that at this latter point there is not only a conspicuous connection of mesoderm and ectoderm, but also a rather sudden separation of the entoderm from the cell-mass just above. This cleft between the entoderm and the primitive streak is present throughout its caudal half, while anterior to this the entoderm lies very close to the newly formed mesodermic elements.
The primitive groove is best defined at its posterior end, where it appears as a sharply outlined groove between the more gentle slopes of the ectoderm on either side. Farther forward, where the high ectodermic folds are found, this fine median furrow can not be distinguished, being simply the bottom of a deep, narrow trough. In its most caudal part the primitive groove possesses a narrow, flat floor bounded by perpendicular walls of distinctly greater extent. The breadth of the floor may be estimated at about 0.01 to 0.015 mm. This dimension is accentuated in the photographs on account of the obliquity of the sections. Followed forward, the groove varies in shape and width, its floor soon disappears, and it is finally lost in the general slope of the embryonic ectoderm. In the primitive groove the outlines of the ectodermic elements, free surface, and cell boundaries are more distinct and the arrangement of the nuclei is rather more regular than elsewhere.
In those places where there is a well-defined floor one can sometimes .see, in one side of the floor, a deep, distinct secondary groove, usually on the right. A few sections show a definite lipping of the primitive groove, i. e., the lateral wall; this being observed only on the left side, bulges into the groove, forming a small recess between the floor and the wall. These peculiar conditions occur only near the posterior end of the primitive groove. The ectoderm of the groove presents 2 to 3 or 4 layers of nuclei and is thickest in front. The floor is often composed of but a single layer of low cells, remarkably thin in places but always intact.
From the w'alls of the primitive groove, much less conspicuously from its floor, where this is well marked, the ectoderm is continued directly into the mesoderm lying laterally, while the floor of the groove, often much thinner than the side-walls, is widely separated from the entoderm, in which space occasional free cells may be seen. There are in a few places interruptions in the transition from ectoderm to mesoderm, probably artefacts due to the loose character of the latter layer. The width of the primitive streak, i. e., of the zone of proliferation of mesoderm, is about 0.05 mm. Towards its anterior end this zone gradually becomes more massive, the connection between ectoderm and mesoderm more extensive, and the entire median fine between ectoderm and entoderm becomes filled with closely packed mesodermic cells. At the very anterior end of the streak, just behind the primitive node, the lower germ-layer is again widely separated from the cells dorsal to it. Everywhere, however, in the primitive streak can the entoderm be seen as a distinct, definite cell-layer.
The mesoderm on either side of the middle fine forms a well-defined, loosely cellular stratum of slightly varying thickness. It is continuous laterally and. posteriorly with the outer layers of the yolk-sac and amnion, anteriorly with the mesoderm on either side of the head process, and mesially with the ectoderm of the primitive streak. This layer of cells is thinnest behind and lies uniformly close to the entoderm, to which its cells are attached by numerous fine processes. The constituent cells vary considerably in size and shape; most of them possess a number of larger or smaller, partly anastomosing processes, while some seem to have a smooth rounded cell-body. One finds quite generally a fine, sharp line on that surface of the mesoderm toward the ectoderm, very much like a basement membrane of connective-tissue origin, the membrana prima of von Spec. There is no indication anywhere of an arrangement of the mesodermic cells in two layers, as has been repeatedly described in the primitive-streak region.
The majority of the mitotic figures observed in this specimen are found near, or at a short distance from, the primitive streak; by far the greater number of the.se occur in the ectoderm and mesoderm, especially in the former; only rarely are they seen in the entoderm. In tho,se cases in which the axis of the spindle can be determined it is found in nearly all instances parallel to the surface of the ectoderm or mesoderm and at right angles to the median line.
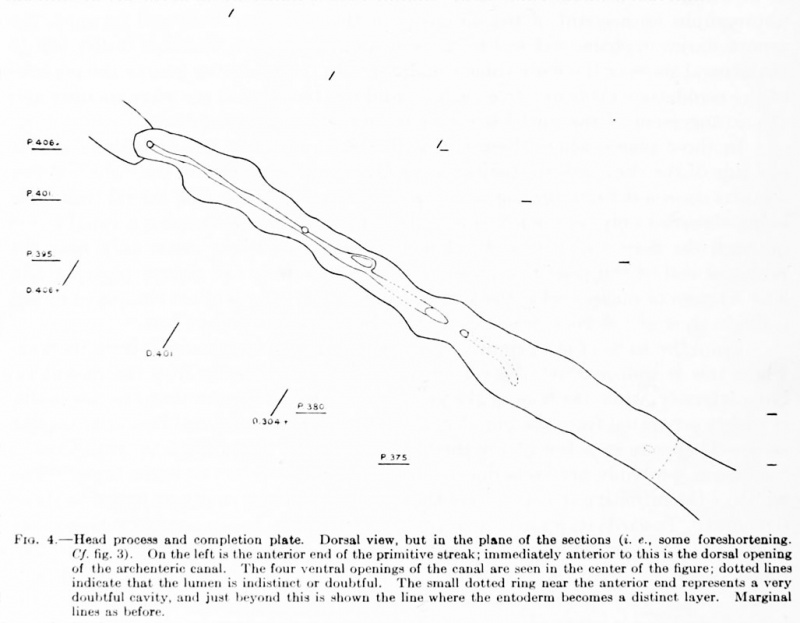
Fig. 4. Head process and completion plate. Dorsal view, but in the plane of the sections (i.e., some foreshortening. Cf. fig. 3). On the left is the anterior end of the primitive streak; immediately anterior to this is the dorsal opening of the archenteric canal. The four ventral openings of the canal arc seen in the center of the figure: dotted lines indicate that the lumen is indistinct or doubtful. The small dotted ring near the anterior end represents a very doubtful cavity, and just beyond this is shown the line where the entoderm becomes a distinct layer. Marginal lines as before.
As mentioned above, the anterior part of the primitive groove is but the bottom of a deep, median furrow in the blastoderm. This furrow becomes rapidly wider and is continued some distance farther forward, but distinctly to the right of the head process, where it gradually fades out. Here, at the anterior end of the primitive streak, is the primitive or Hensen's node. There is, strictly speaking, no real node, knot, or distinguishable enlargement at this point, and nothing to indicate any separation between the groove and the archenteric canal. Immediately caudal to the beginning of the canal the ectoderm becomes thinner and there appears a wide interval between the entoderm and the la&t of the primitive streak dorsal to it.
Lateral to the node, or better in it, i. e., in the walls of the first part of the canal, the ectoderm and mesoderm are in broad connection. Just anterior to the node the head process has freed itself from the overlying ectoderm, is continuous with the mesoderm on either side, and fused with the entoderm below. The posterior ectodermic opening of the archenteric canal is very minute, being only about 0.005 mm. in diameter (plate 2, fig. 2; plate 3, fig. 2). The actual opening on the surface can hardly be made out, since it is bounded only by the slightly staining cytoplasm of the surrounding cells, the nuclei of which, in contrast to other regions, are here at a greater distance from the free surface. From this point the canal passes directly ventral through the substance of the primitive node, turns forward and to the left, and again forward in the fine of the head process (figs. 2, 3, and 4) . It is at the node, and here only, that the three germ-layers are fused with each other (plate 3, fig. 5).
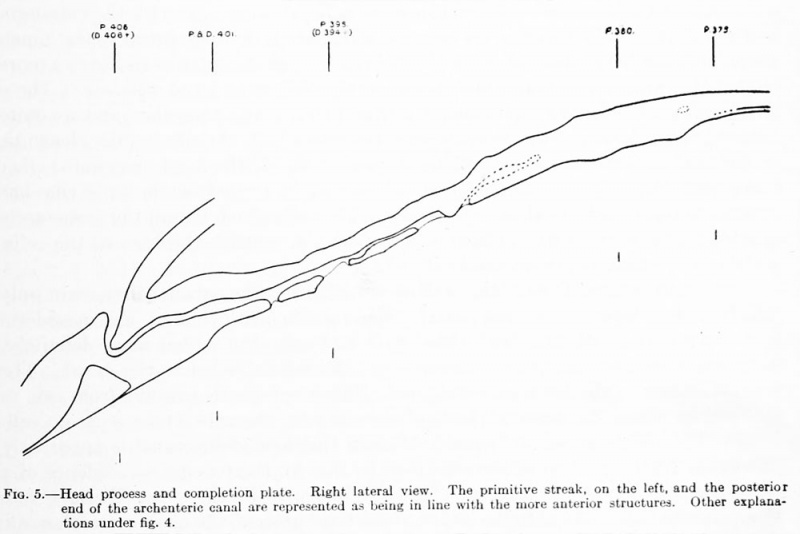
Fig. 5. Head process and completion plate. Right lateral view. The primitive streak, on the left, and the posterior end of the archenteric canal are represented as being in line with the more anterior structures. Other explanations under fig. 4.
Extending cpphalad from tlio primitive node in the axis of the blastoderm is the head process of the primitive streak (figs. 1 to 5). This structure, including the completion plate in front, is slightly longer than the primitive streak, measuring about 0.75 mm. in length; its diameter is variable, but in general gradually increases from behind forward. The posterior half, or head process proper, varies in width from 0.03 to 0.05 mm., its lumen from O.OOG to 0.01 mm., while the length of its lumen, the archenteric or canal of Lieberkiihn, is 0.34 mm. The average breadth of the completion plate is about 0.00 mm.
The head process is an axially placed, hollow, cylindrical mass which, at its origin in the primitive node, is directly continuous with the superficial ectoderm and the substance of the primitive streak, as well as with the mesoderm on either side. It very soon becomes free from the ectoderm above and fuses with entoderm below; its lumen, which is at first nearer the dorsal surface of the process, takes up a central position, while at the same time the dorso-ventral diameter diminishes somewhat. In considering this structure we shall begin at its posterior end, at the point where it has just disengaged itself from the surface ectoderm. It appears here in section as a roughly pyramidal or wedge-shaped mass projecting well into the space below the ectoderm. Shari)ly limited above, this mass is fused at its base with the entoderm and mesoderm. The lumen is yet eccentrically placed; the cells dorsal to the lumen are much fewer in number, more deeply staining in their cytoplasm, and have a more epithehal arrangement than those between the lumen and the yolk-sac. These latter cells are much more numerous, more irregularly massed together, and are quite indistinguishable from the neighboring mesodermic and entodermic elements. A few sections in advance (plate 2, fig. 3; plate 3, fig. 3) the head process is rather lower and distinctly broader, its free outlines more curved, while its cavity has increased in size and lies about the center. The cells which bound the archenteric canal dorsally are frankly epithelial; their nuclei are nearer the base of the cells, while the cytoplasm is deeply stained.
The cells ventral to the lumen show no definite arrangement; they stain only faintly and no layer of entoderm can be made out beneath them. The mesoderm is directly continuous with both these groups of cells, but rather more definitely, on account of their staining reactions, with the cell-mass below the canal. The ventral surface of the head process is, near its posterior end, concave from side to side and at about its margins the entoderm can be recognized as a sejiarate celllayer. The thinning out and eventual loss of the floor of the canal is apparently due to the rearrangement of the cells lu>re (cf. fig. 5), there being no evidence of a corresponding loss or destruction of cells. We have in these two distinct cell groups, dorsal and ventral to the lumen of the head process, the plaque notochordale and placiue lecithoenti^rique respectively of van Beneden (1899). To these we shall take occasion to recur later. Near its i)osterior end, where the lumen is more dorsally placed, the cells of the i)la(|ue notochorilale are only about half as numerous as those of the i)la(|ue lecithoeiifericiue; at the point shown in the illustrations they are approximately equal in iiuinhcr.
A little anterior to the sections just mentioned is found the first ventral opening of the archenteric canal. It is very small, located entirely in one section, and appears as a narrow passage connecting the canal with the cavity of the yolk-sac. Just beyond this is the second ventral opening (plate 2, fig. 4; plate 3, fig. 4), very large, mainly on the left side, and limited dorsally by the beautifully epithelial notochordal plate. The epithelial cells of the plate are here columnar, their nuclei are slightly nearer the base than the free surface, and their cytoplasm stains rather intensely. In spite of their character these cells are not to be separated laterally from the adjoining mesoderm and entoderm. In the sections which follow, the notochordal plate varies considerably both in breadth and distinctness. The canal likewise is not always well defined; its floor varies in thickness or may even appear deficient. It can be traced forward, however, to the third and last definite ventral opening, where again the notochordal plate is very conspicuous, while in the floor appear a few cells, some of which may be free. This last portion of the head- process hardh^ projects above the level of the neighboring mesoderm.
Anterior to the head process and continuous with it lies the so-called completion plate, the Erganzungsplatte (des Urdarmstranges) of Bonnet. Its posterior limit may be placed just in front of the third and most anterior opening of archenteric canal, while at its opposite extremity it is gradually lost as the two lower layers of the blastoderm becomes distinct. Its length, with the limits just noted, may be taken as about 0.4 mm.; its width, averaging about 0.06 mm., is greater than that of the head process proper. In structure it differs very markedly from the typical head process just described. The transition between the two appears to be gradual, at least so far as can be determined on a transver.se series. On following the sections forward it is seen that the conspicuous dorsal cells (notochordal plate) rapidly lose their epithelial character, and the lumen (which was such a prominent feature before) becomes very doubtful if not actually wanting (indicated by the dotted outlines in figs. 3, 4, and 5, solid line in fig. 2). At the same time there is a gradual but not uniform increase in the breadth and thickness of the plate until it reaches nearly twice the dimensions of the head process, bulging below into the yolk-.sac and above into the space between the ectoderm and mesoderm. Along its lateral borders, which are never sharply marked, it is directly continuous with the mesoderm, as this layer is with the head process farther back. At its anterior end it is gradually lost in an ill-defined, axial condensation of mesoderm, and very soon this also disappears. Structurally the completion plate is made up of a rather closely packed mass of cells in which no details can be made out. The entoderm beneath does not lose its identity to quite the extent which it does in the head-process region, but still can hardly be recognized as a distinct layer. Toward the anterior limits of the plate the entoderm appears as a definite layer of large, thick, almost cuboidal cells. At certain points there are indications of a sort of doubling in the plate, due to the presence of a shallow furrow on its dorsal surface. Here, and also where this feature is not apparent, the faintly defined cavity lies distinctly on the right side. Far forward, near the extremity of the plate, there is again a faint indication of a small cavity. One peculiar feature of the plate is the presence in or between the cells (one can not in this case say which) of numerous rather large, rounded, intensely staining granules, very similar to those described by Bonnet (1901) in the completion plate of the dog. Although a few of these granules can be seen in other locations, as also figured by Bonnet, they are by far most numerous and conspicuous in the completion plate.
The variations in size of the head process and completion plate, especially as regard their breadth and the roughly corresponding variations in the lumen, are shown in figure 4. Although such variations are recorded {cj. Rabl, /. c, Taf. iv), they are unusually distinct and regular in this case. What significance may attach to them we can not say. They seem too small to correspond with the future segmentation of the mesoderm lateral to them, and we have been unable to discover any special features in this mesoderm, such as possibly more active proliferation of cells in relation to the enlargements, either opposite or between them.
The mesoderm in the anterior half of the blastoderm is essentially the same as that which we have already described; far anterior it becomes very thin.
Any indications of a folding of? of the embryo, of a proamnion, or buccopharyngeal membrane are wanting.
The structures thus far considered comprise the essential features of the embryonic anlage. Nowhere, as far as we can make out, is there any sign of future segmentation, and nowhere in the embryo are there either blood-vessels or blood-cells; but at the very anterior end of the embryonic disk there occur a number of prolongations of the exocoelom under the embryonic ectoderm. These exoccelomic diverticula have a very small, distinct, Init quite irregular lumen lined by cells similar to those on the neighboring yolk-sac and amnion. They appear as rather long, irregular, tubular ingrowths which take their origin from the exoccelom at the point where the mesoderm of the amnion and yolk-sac meets the embryonic mesoderm. The two anterior diverticula arise in the shallow groove under the anterior edge of the blastoderm. Of these ingrowths there can be made out about four, two on either side. The anterior pair, longer and more distinct, reach nearly to llie middle line. Of the posterior pair the right is very .short, while the left runs parallel to and just within the margin of the blastoderm. Judging from their location, they might stand in some relation to the future pericardial coelom.
The Yolk Sac
Only approximate dimensions can be given here on account of the folding, partial collapse, and a somewhat extensive tear near the anterior end of the sac. We may estimate its antero-posterior measurement at about 2.5 mm., in a dorsoventral line at about 2 mm., and a little less than this latter figure from side to side. As seen in the illustrations (fig. 3; plate 1, fig. 3; plate 4, figs. 1 and 2) it is still very large as compared with the embryo projecting well beyond it on all sides, particularly in front and on the right. Originally it was doubtless quite regular in shape. The surface of the sac is for the most part quite smooth and regular, but over a certain area on the fundus anteriorly it has the characteristic uneven, nodular appearance arising from the early blood formation in this region.
The epithelium lining of the umbilical vesicle varies considerably in different parts. In the axis of the embryo the entoderm consists of flattened cells which form a distinct layer except in the region of the primitive node, head process, and completion plate. Elsewhere in the embryo the entoderm is a definite layer of flattened elements whose nuclei stain less deeply and are possibly a trifle larger than those of the mesoderm just above. In the immediate vicinity of the embryo the walls of the yolk-sac consist of two thin layers of cells, usually closely applied to each other, particularly toward the anterior end, but between the layers occur scattered mesoderm cells which are much more numerous in the posterior part of the sac. Farther from the embryo the entoderm cells gradually become thicker, their cell-bodies become more definite, and they take the stain more readily. Over the fundus the lining cells are in general cubical, with well-marked boundaries. Here there is extensive formation of blood-cells and blood-vessels which we shall not discuss at present, except to say that there is no connection between these vessels and those of the body-stalk. In the fundus of the sac there occur two small outpocketings of the entoderm into the covering mesoderm (fig. 3). In these diverticula the epithelium is higher and its cells larger than elsewhere.
The Allantois
The allantois is given off from the yolk-sac a short distance behind the cloacal membrane. It immediately enters the body-stalk, running at about right angles to the plane of the embryo and, as noted on page 118, is at one point in intimate contact with the amnion. Its length, without reference to its slightly curved course, is about . 65 mm. The lumen is largest just above its origin in a small funnel-shaped depression in the yolk-sac. Its free, slightly coiled extremity has a cavity almost as large as at its origin, while between these the duct and its lumen are narrowest. The average outside diameter is about . 04 mm. Its walls are composed of low columnar cells containing large, densely-staining nuclei. As it appears in the sections the duct lies in a large space due to the shrinking away of the surrounding tissue.
The Body Stalk
The body-stalk is short and distinctly flattened from side to side. Embedded in the loose mesenchyme of which it is composed are the allantois, the posterior part of the amnion, and numerous vessels filled with nucleated blood-cells. No attempt has been made to reconstruct or learn the exact disposition of these channels. In places their walls seem to be deficient and they take on the character of the unlined spaces. The outer covering of the body-stalk is a rather prominent mesothelium, which is best marked near the embryo and also on what we may call the posterior aspect of the stalk. Toward the chorion this covering stops abruptly, at a varying but short distance from the attachment of the stalk, and there also appear to be deficiencies in this covering, especially on the anterior surface of the stalk. From this mesothelial layer there are a number of more or less definite ingrowths, a few of them forming quite distinct "funnels." Other findings in the body-stalk are the unlined spaces, angiocysts, and angioblast cords described by Bremer (1914).
Connections between these last-named structures and the mesothelial ingrowths are not especially in evidence, but we have not gone into a detailed study of them in this respect. The occurrence of similar conditions in the wall of the yolk-sac we would not like to exclude, because the histological pictures arc here less satisfactory than anywhere else in the whole specimen.
3. The Chorion (microscopic) and Exocoelom
Examination of the sections (plate 1, figs. 3, 4, and 5) shows that the villi in the equatorial zone are much more developed, longer, larger, more numerous, and more richly branched than on the two flattened poles of the vesicle. The mesodermic portion of the chorionic wall is a thin, fairly uniform stratum, the ragged exoccelomic surface of which is in marked contrast with the same surfaces of the embryonic structures. Within the larger villi, even far from the embryo, are found numbers of open spaces, some of the smaller having a fairly distinct endothelial lining. Some of the unlined spaces in the wall of the sac are very large, and they may be brought about in part or at least accentuated by the pulling away of the mesodermic from epithelial constituents of the chorion which is in evidence almost everywhere. Occasionally short strands resembling the angioblast cords are seen, even in the bases of the villi, but these and undoubted vessels in the villi are by far most frequent near the attachment of the body-stalk.
The villi possess a loose mesenchymal core which in the shorter ones extends quite to their free ends, while in the longer and larger equatorial villi this core is not so extensive. The inner layer of the epithelial covering of the villi and also of the chorionic wall is made up of distinct cellular elements, polygonal in outline and varj'ing from thick squamous to low cuboidal, constituting the cytotrophoblast on the layer of Langhans. Cell-boundaries are here uniformly distinct, and both the cytoplasm and the nuclei stain more lightly than the same parts of the overlying syncytium. Often the line between the Langhans layer and syncytium is very sharp, again decidedly vague, while in numerous places either layer may be so reduced as to seem the only covering of the mesodermic core. Most frequently it is the syncytial layer which is so markedly thinned or apparently absent. The fact that the line between these two layers can not always be seen, and the occurrence in the deeper portions of the syncytium of what seem to be indistinct cell-boundaries, would point to the close genetic relationship of the two layers. Distally the cellular layer of the villi passes over into the cell-columns by means of which the villi are extensively united. This is especially conspicuous in the case of the equatorial villi, among which are alsf) found extensive irregular masses, the trophoblastic cell-islands, which on the surface toward the ovum gradually merge into the cell-columns of the villi. These cell-islands are composed of large, very pale cells with distinct boundaries and large, pale nuclei. The constituent elements are for the most part irregularly polygonal, but they may take on an elongated, spindle-like form, as if actively drawn out. A faint vacuolization is not infnMiuent. In many places, but most marked in the neighbourhood of the embryonic attachment, these cellular masses form practically an inclosing shell over the intervillous spaces beneath.
The syncytium, or plasmoditrophoblast, over the vesicle wall and the bases of the villi consists of a thin layer of slightly varying thickness, but as a rule thinner than the cellular layer beneath it. Both its cytoplasm and nuclei stain very densely. Traced outward upon the villi, the syncytium rapidly thins out on the cell-columns and soon disappears. The largest syncytial masses are found in the equatorial zone just outside the cell-islands. Here it forms large, often extensively vacuolated or spongy masses which can not always be definitely separated from the cell-islands. Scattered through the intervillous spaces, some of them close to the wall of the vesicle, are free syncytial masses of every possible size and shape. The nuclei vary widely in number; they may be small and stain quite densely, or large and pale, and this in the same bit of syncytium. "Prickle processes" are seen quite distinctly on some of these masses and their protoplasm is often very finely vacuolated. Smaller fragments of syncytium often lie in shallow pits or excavations in the cell islands or trophoblastic columns. These masses are often very small, with one or more nuclei, and are only very lightly stained. Here again there seems to be a direct transformation of cytotrophoblast into plasmoditrophoblast. If there are evidences of cell-division in the chorion they have so far eluded us.
The amount of maternal blood in the intervillous spaces varies considerably in different localities. In a few places it is very abundant, in others almost wanting. It is most plentiful on the flattened poles of the ovum, where the villi are fewer and shorter and where the cell columns and islands and syncytial masses are least in evidence. It would appear as if the anastomosing cell-columns around the equator of the ovum had prevented the entrance of maternal blood, except very indirectly through the more distant intervillous spaces. That the blood should have drained out more readily from these deeper spaces, many of which are closed externally by the remains of the trophoblastic shell, seems quite improbable. Over much of the ovum externally is a layer of clotted blood in which leucocytes are more numerous than in the blood in the intervillous spaces.
In concluding this account of the chorion mention may be made of a small cyst-like structure faintly seen on plate 1, figure 3. It is composed of tissue to all appearances like the mesoderm of the chorion and lies close to, but seemingly not in connection with, the vesicle wall. No indications of a chorionic duct have been encountered.
Concerning the magma in the exoccelom, it will be recalled that upon gross examination of the ovum a few fine strands were observed connecting the yolk-sac and chorion. At that time it could be seen that traction upon these strands was not without effect upon yolk-sac. In the sections there can be found only some ragged wisps of a finely fibrillar nature, which at various points grade insensibly into irregular clumps of a finely granular or fibrous character extensively present in the cavity of the vesicle. In a few places where the larger strands have an attachment to the chorion there occur very intensely staining nuclei. Where best developed the fibrils are very conspicuous; they form loose bundles and stain very dark with hematoxylin. Over the amnion and the yolk-sac near it is a very thick, condensed layer of a finely granular texture (plate 2, figs. 5 and 6).
4. General Discussion
The embryo which we have just described represents an extremely interesting and instructive stage in the ontogenesis of man. In it are found as many important features of early development as could well be expected in one and the same specimen. Besides presenting so may typical and classical features, it has the added advantage of showing them on an unusually large scale. This size, as already mentioned, may be considered simply as a variation; accentuated it may be by unknown influences. It is w-ell known that certain developmental stages are quite ephemeral ; that there is further a greatly varying susceptibility in different tissues and in these at different times, and herein may lie some explanation of the conditions described above, perhaps an unusual development or late persistence from unknown causes. We may recall here that Rabl makes repeated mention of considerable variations in size, age, and development in the areffi embryonales of rabbits, often insisting that they can not be looked upon as either abnormal or distorted, although offering no explanations. We may quote in this connection his own words {I. c, p. 378; cf. also Taf. iv) regarding embryos with one somite: "Da habe ich denn von einer sehr merkwiirdigen Erscheinung zu berichten. Ich habe namlich zwei Arten von Embryonen dieser Entwicklunsstufe beobachtet: die eine war kurz, breit und gedrungen, die andere lang, schmal und schlank." The gist of the above is that we consider our embryo normal, though not typical.
Any discussion of the findings in this embryo naturally revolves around the question of gastrulation and the formation of the germ-layers. We shall not at this time attempt an extended treatment of the subject, but give simply our own interpretation of w'hat we have observed in this particular case. Naturally one should not conclude too much from a single stage, either as to antecedent or later conditions; but every stage must be in harmony with those which precede or follow, and the truth is not always commensurate with the extensiveness of the evidence. On many problems of development this embryo of course throws no light whatsoever, being far too advanced.
As regards the formation of the amniotic cavity and the yolk-sac, we may accept them as currently given. The question of the mesoderm is not so easily disposed of. In spite of its precocious development, we can not yet see the necessity of denying that it may still be, in principle, peristomal mesoderm. Considering the recent attempts of Rabl in this respect and the similar difficulty regarding the entoderm, it would .seem to us that the inherent questions of gastrulation and homology should be more definitely disproven before an entirely new and foreign mode of development is invoked.
In the primitive streak we have a closed blastopore, howbeit radically altered. At its anterior end is an opening and what is theoretically at least an invagination, the head process with its archenteric canal. The posterior end of the streak is, in this stage, marked by the cloacal membrane which is later also open, at present in process of formation. Between the two joints there is extensive mesoderm formation, as witnessed by, the mitoses in this region, peristomal mesoderm. If one were inclined to carry the comparison still farther, the peculiar features of the primitive groove mentioned on page 121 might be interpreted as an attempt at the formation of lateral blastoporic lips. How much of the mesoderm of the embryo appears first as strictly peristomal we of course can not say.
The consideration of the head process involves also the tangled question of the entoderm. The head process of the primitive streak (Kolliker), I'ebauche de I'archenteron of Van Beneden, Bonnet's Urdarmstrang, or the MesodermsJickchen of 0. Hertwig, is one of the most important features of the area embryonahs. In its formation, and that of the primitive streak, we have the essentials of gastrulation in man; in the cavity of the head process, the archenteric canal (Urdarmkanal), is retained all that is left of the canity of the primitive gastrula, the archenteron. From this head process are derived, to what extent it is impossible to say, gastral mesoderm, further chorda, for the most part, and (for aught we know) more or less of the entoderm of the gut-tract. From the foregoing it will be clear that we do not agree with Keibel (1910, 1913) and Hubrecht (1905, 1909) in considering the formation of the two-layered stage, ectoderm and entoderm, as constituting the process of gastrulation. That entoderm formed by delamination is essentially secondary or yolk entoderm, the paraderm of von Kupffer, Wenckebach's caenogenetic entoderm, the lecithophor of van Beneden.
To what extent this first-formed layer is concerned in the formation of the digestive tract we do not know; certainly in some forms its role is by no means an exclusive one. The fact that this yolk entoderm fuses with the head process but not with the primitive streak is but evidence as to its caenogenetic character. The only support of the views of Keibel and Hubrecht is the supposition that this secondary entoderm is the sole and only source of the gut entoderm. The theory and the entoderm stand or fall together. In the walls of the head process, i. e., bounding the archenteric canal, we would expect to find primary or protentoderm, Bonnet's Urentoderm, the palingenetic entoderm of Wenckebach. If the lumen of the head process is in reality an archenteric canal, then we would expect it to give rise to mesoderm (segmented), chorda, and gut entoderm and such, with the reservations given above, seems to be the case. If the head process is simply the anlage of the chorda plus some mesoderm (whence the misnomers chordal or notochordal canal, chordulation, etc.), why should it contain a definite although inconstant canal communicating with the exterior; why so much more material than is required for the chorda, and why its fusion and communication with the yolk-sac? The answer is that in the formation of the head process and not in the delamination of the secondary entoderm we have a process which can be designated as gastrulation.
As concerns the derivatives of the head process, the case of the chorda is perfectly clear. At this stage its anlage is contained in the dorsal, epithelial wall of the canal, the notochordal plate. The fate of the ventral wall or floor of the canal, the plaque enterique of van Beneden, is uncertain. It fuses early with the yolk entoderm or lecithophor immediately beneath to form the plaque lecithoenterique. The.loss of the floor, from the rearrangement of its cells, results in the confluence of the archenteric canal and the cavity of the yolk-sac. This process is naturally cienogenetic, since the yolk entoderm und its inclosed cavity are ca;nogenetic features. There are thus restored the original conditions in which the anlage of the chorda and mesoderm (enterocoele) are situated in the dorsal wall of the gut. To what extent there is any formation of gastral mesoderm from the head process is a question. In any case, even if the mesoderm had a jieristomal origin, its continuity with the walls of the canal is sufficient to indicate the interpretation of the latter as potential sources of gastral mesoderm. With the disappearance of the floor of the canal there is ushered in the stage of the so-called intercalation of the chorda in the entoderm. This obviously takes place quite irregularly and the picture is exactly that seen on such a large scale in Reptilia, but clearly marked in many other forms. This stage is shown on plate 2, figure 4, and plate 3, figure 4. To be exact, this is not an intercalation of the chorda in the entoderm. The notochordal plate is in connection laterally not only with the entoderm, but much more extensively with the mesoderm. If one suppose that there may still be mesoderm formed from the borders of the plate (and there is here no evidence to the contrary) it would be possible to raise objection to the use of the term "notochordal plate," since it would contain chorda and gastral mesoderm. Not, however, until there is a definite separation of the plate from the mesoderm and its continuity with the entoderm alone can one speak of an intercalation of the chorda.
The extent, if any, to which the plaque l^cithoenterique (Dotterdarmplatte) contributes to the formation of the wall of the future digestive tract is difficult to determine and certainly not to be decided by any one stage. There are a number of facts, however, which seem to point to such a participation. The marked disproportion between the notochordal and enteric plates in the posterior, least differentiated part of the head process and the retention of the former, practically intact throughout its whole extent, indicate unmistakably that there is formed from the primitive node and head process a considerable mass of material which is not extended in the formation of the chorda. If this material be not actually lost, then it must find its way into the mesoderm or entoderm or into both. In view of the large mass of material produced, much greater than that destined for the chorda, and considering also its peculiar mode of development, virtually an invagination, the simplest solution is to suppose that both mesoderm and entoderm are formed from the side-wall and floor of the head process. If the development of gastral mesoderm is small or wanting, so nuich more material for the entoderm. It may l)e recalled here that the digestive tract in the embryo is very small below the pharynx and no very great amount of material would be required to form its walls. The fact that in certain animals the primary entoderm is concerned in the formation of the epithelial wall of the gut seems to us very significant. It would seem that the absence of definite evidence that the entoderm of the future embryo is not, in part at least, primary entoderm, is outweighed by the above considerations and by the fundamental homologies which they tend to preserve.
With the liead process and i)rimitive streak we have not yet exhausted the possibilities for the discussion of fundamental problems; there remains the question of the completion plate, Bonnet's Ergilnzungsplatte, the protochordal plate of Hubrecht. Our knowledge of this structure is less extensive than that of the region we have just been considering, and our remarks will be correspondingly brief. Bonnet's term is a very fitting one, since the derivatives of the plate are the same as those of the head process and directly continuous with them. For Rabl (I. c, p. 239) the completion plate is simply "das vorderste Ende des in Lecithophor vorgeschobenen Kopflfortsatzes oder Urdarmsackchens." For Bonnet and Hubrecht it is developed from the yolk entoderm independently of the head process. The evidence in this particular case would seem rather to support this latter view. The second view, however, is not so easily reconciled with our ideas of gastrulation as the first, and we shall not carry the discussion farther at this time. Concerning the future of this plate, which has been recognized in a variety of forms and given a variety of names, there is much more unanimity of opinion. There arise from the completion plate in the dog, according to Bonnet (/. c, p. 286) : "1. Mesoderm des ^^orderkopfes, 2. die Chorda des Vorderkopfes, und 3. ein pramandibulares Darmrudiment. Es bildet dieses Gebiet also thatsachlich ein Erganzungsstiick des Urdarmes, indem es dieselben Derivate wie dieser aus sich hervorgehen lasst." The anterior part of the chorda, which, as compared with that derived from the head process is very short, remains long in connection with the entoderm. The formation of mesoderm is also continued here for some time.
The significance of the apparent cavity formation in the completion plate is a matter of uncertainty. It might be compared with the secondary canals which sometimes appear in the chorda as it separates from the entoderm. One could perhaps look upon them as attempts at the development of an archenteric cavity, or they might conceivably stand in some relation to the rarely appearing head cavities. As far as we can make out, the buccopharyngeal membrane would have appeared close to the anterior limit of the completion plate, with the possibility of the plate contributing in its formation.
From the observations here presented and from the consideration of other human embryos, one may conclude that the essential features of gastrulation in man are directly comparable with the classical features of that ancient and important process. Significant parallels may be drawn between early human ontogenesis and that of many other representative vertebrates. The conditions in man are manifestly simpler and more primitive than in many cases which have been extensively studied, these being often very specialized or aberrant forms. Hand in hand with specialisation and advancement there is the appearance in ontogeny of caenogenetic features which always tend to obscure the original picture. If man has retained much that is primitive and generalized, then we should expect to find some expression of this in his earliest development.
Cleveland, Ohio, September 27, 1917.
Literature Cited
VAN Beneden, feo. Sur la presence chez I'homme d'un canal archenterique. Anat. Anz., 1899, xv, 349-350.
Bonnet, R. BeitriVge zur Embryologie des Hundes. Erste Fortsetzuiig. Anat. Hefte, 1901, xvi, 231-332.
Bremer, J. L. The earliest blood vessels in man. Amer. Jour. .\nat., 1914, xvi, 447-405.
Bryce, T. II.. and J. H. Teacher. Contributions to the study of the early development and imbedding of the human ovum. Glasgow, 1908.
Eternod, a. C F. Premiers stades de la circulation sanguine dans I'oeuf et Tembryon humains. Anat. Anz., 1898, xv, 181-189.
Fetter, — . Ueber ein durch Operation gewonnenes menschliches Ei, das in seiner Eutwickelung etwa dem Peters'schen Ei eutspricht. Verhandl. d. anat. Gesellsch., 1910, xxiv, 110-126.
Frassi, L. Ueber ein junges menschliches Ei in situ. Archiv f. mikr. .\nat., 1907, l.xx, 492-505. Grosser, O. Ein menschlicher Embrj-o mit C'hordakanal. Anat. Hefte, 1913, xlvii, 049-680. , Altersbestimmung junger menschlicher Embryonen; Ovulations- und Menstruationstennin. Anat. Anz., 1914, xlvii, 264-283. Hubrecht, A. A. W. Die Gastrulation der Wirbeltiere. Anat. Anz., 1905, xxvi, 353-366.
Hubrecht, A. A. VV. Die Saugetierontogenese in ihrer Bedeutung fijr die Phylogenie der Wirbeltiere. Jena, 1909.
Keibel, F. Manual of human embryology. Keibel and Mall, vol. I, 1910. . Die Entwicklungsgeschichte der Wirbeltiere. Die Kultur der Gegenwart, iii, iv, 2, 1913.
Mall, F. P. On stages in the development of human embryos from 2 to 25 mm. long. Anat. Anz., 1914, XLVi, 78-84.
Rabl, C. Edouard van Beneden und der gegenwiirtige Stand der wichtigsten von ihm behandelten Probleme. Archiv f. mikr. Anat., 1915, Lxxxviii, 1-470. ScHABiNSLAND, — . Article in Hertwig's Handbuch, Bd. I, 1, 1902.
Spee, F. Graf. Neue Beobachtungen ijber sehr friihe Entwickelungsstufen des menschlichen Eiea. Archiv f. Anat. u. Entwickelungsgesch., 1890, 1-30. Strahl, H. Ueber einen jungen menschlichen Embryo, nebst Bemerkungen zu C. Rabl's Gastrulations theorie. Anat. Hefte, 1916, liv. , and R. Beneke. Ein junger menschlicher Embryo.
Wiesbaden, 1910. Triepel, H. Altersbestimmung bei menschlichen Embryoneii. Anat. Anz., 1914, xlvi, 385-398.
Figures
Plate 1
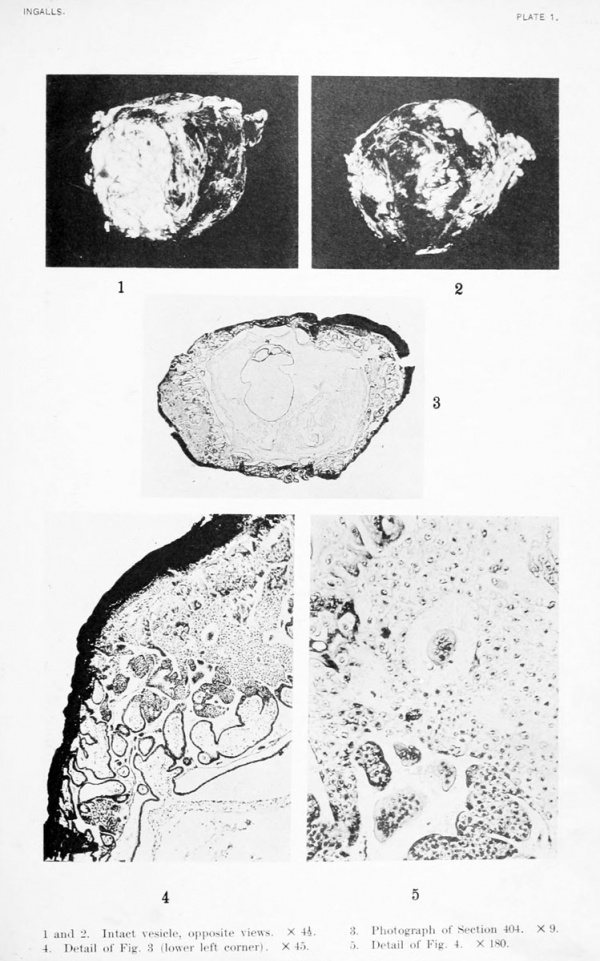
1 and 2. Intact vesicle, opposite views. X 4.5
3. Photograph of Section 404. X 9
4. Detail of Fig. 3 (lower left corner). X 45).
5. Detail of Fig. 4. X I80.
Plate 2
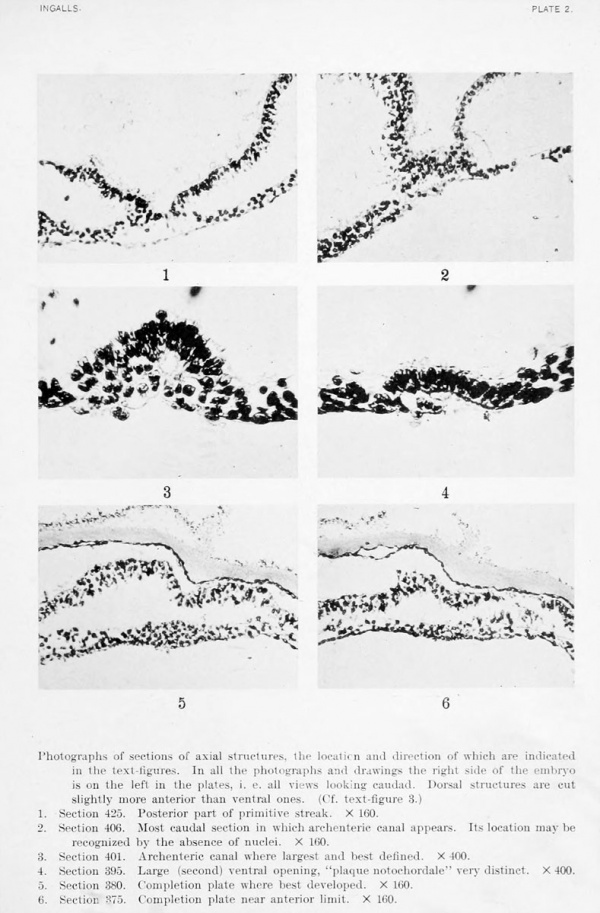
Photographs of sections of axial structures, the location and direction of which are indicated in the text-figures. In all the photographs and drawings the right side of the embryo is on the left in the plates, i. e. all views looking caudad. Dorsal structures are cut slightly more anterior than ventral ones. (Cf. text-figure 3.)
1. Section 425. Posterior part of primitive streak. X 160.
2. Section 406. Most caudal section in which archenteric canal appears. Its location may be recognized by the absence of nuclei. X 160.
3. Section 401. Archenteric canal where largest and best defined. X 400.
4. Section 395. Large (second) ventral opening, "plaque notochordale" very distinct. X 400.
5. Section 380. Completion plate where best developed. X 160.
6. Section S75. Completion plate near anterior limit. X 160.
Plate 3
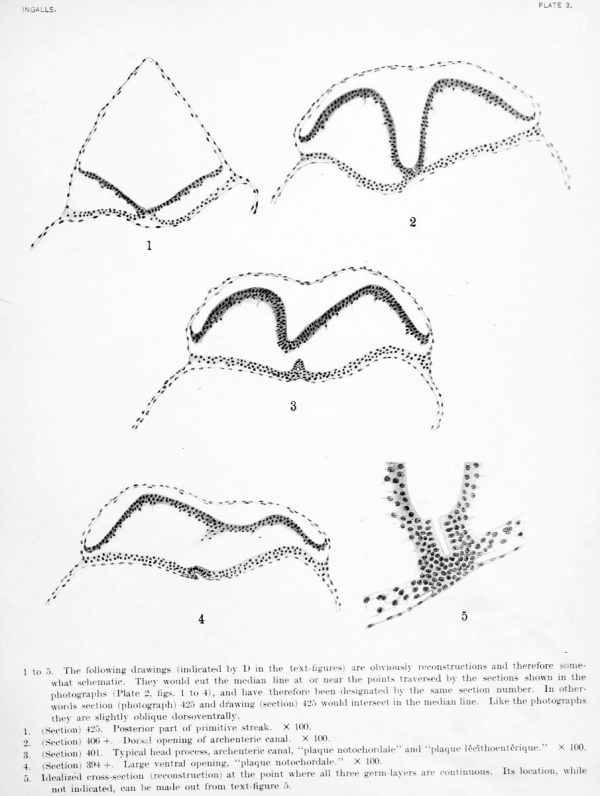
1 to 5. The following drawings (indicated by D in the text-figures) are obviously reconstructions and therefore somewhat schematic. They would cut the median line at or near the points traversed by the sections shown in the photographs (Plate 2. figs. 1 to 4), and have therefore been designated by the same section number. In otherwords section (photograph) 425 and drawing (section) 425 would intereect in tlie median line. Like the photographs ihey are slightly oblique dorsoventrally.
- (Section) 425. Posterior part of primitive streak. X 100.
- (Section) 406 +. Dorsal opening of archenteric canal. X 100.
- (Section) 401. Typical head process, archenteric canal, "plaque notochordale' and "plaque lec'ithoenterique." X 100.
- (Section) 394 +. Large ventral opening, "plaque notochordale." X 100.
- Idealized cross-section (reconstruction) at the point where all three germ-layers; are continuous. Its location, while not indicated, can be made out from text-figure 5.
Plate 4
Dorsal and slightly lateral view of model. X 100. Reduced. On the left the body-stalk is cut across.
Left lateral view of model. X 100. Reduced. On the right the body-stalk, cut in the plane of the sections, shows a large vessel; running downward and forward, also in the plane of the sections, a small portion of the amnion has been left. In the embryonic disc are seen the irregular ectodermic folds, its anterior extremity is undermined. The irregularity in the anterior part of the yolk-sac is due to a tear. The upper part of the model has been separated from the lower by sawing through it parallel with the blastoderm; it is this upper piece which is represented in Fig. 1.
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2024, April 27) Embryology Paper - A human embryo before the appearance of the myotomes (1918). Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Paper_-_A_human_embryo_before_the_appearance_of_the_myotomes_(1918)
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G