Genital - Male Development
| Embryology - 27 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Introduction
The male and female reproductive systems develop initially "indifferently", it is the product of the Y chromosome SRY gene that makes the "difference".
The paired mesonephric ducts (Wolffian ducts) contributes the majority of male internal genital tract.
Embryonic gonad development leads to the mesonephric/paramesonephric duct changes, while the external genitaila remain indeterminate in appearance through to the fetal period.
Importantly its sex chromosome dependence, late embryonic/fetal differential development, complex morphogenic changes, long time-course, hormonal sensitivity and hormonal influences make it a system prone to many different abnormalities.
There are also separate pages describing: Y Chromosome | spermatozoa | testis | epididymis | ductus deferens | prostate | penis | Category:Male
| Historic Embryology |
|
Caspar Friedrich Wolff (1734-1794) was a German embryologist and anatomist best known today for identifying the Wolffian duct (mesonephric duct; ductus deferens, epididymis), Wolffian body (mesonephros) and Wolffian cyst (mesonephric origin uterine broad ligament cyst) that bear his name. Thought also to be a founder of the germ layer theory. His doctorate dissertation Theoria generationis (1774) discarded the developmental theory of preformation. Later in his career, his teaching in Berlin was opposed by the professors of the Medical-Surgical College, who had guild privileges to teach medicine. |
Some Recent Findings
|
| More recent papers | |
|---|---|
|
This table allows an automated computer search of the external PubMed database using the listed "Search term" text link.
More? References | Discussion Page | Journal Searches | 2019 References | 2020 References Search term: Male Embryology | Male Development | Male Genital Development |
Testis Development |
| Older papers |
|---|
| These papers originally appeared in the Some Recent Findings table, but as that list grew in length have now been shuffled down to this collapsible table.
See also the Discussion Page for other references listed by year and References on this current page.
|
Textbooks
- Human Embryology (2nd ed.) Larson Chapter 10 p261-306
- The Developing Human: Clinically Oriented Embryology (6th ed.) Moore and Persaud Chapter 13 p303-346
- Before We Are Born (5th ed.) Moore and Persaud Chapter 14 p289-326
- Essentials of Human Embryology, Larson Chapter 10 p173-205
- Human Embryology, Fitzgerald and Fitzgerald Chapter 21-22 p134-152
- Developmental Biology (6th ed.) Gilbert Chapter 14 Intermediate Mesoderm
Movies
|
|
|
|
|
| Mouse Primordial Germ Cell Migration | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
| |||||||||
Development Overview
Three main stages during development, mesonephric/paramesonephric duct changes are one of the first male/female differences that occur in development, while external genitaila remain indeterminate in appearance for quite a while.
- Differentiation of gonad (Sex determination)
- Differentiation of internal genital organs
- Differentiation of external genital organs
The 2nd and 3rd stages dependent on endocrine gonad. Reproductive development has a long maturation timecourse, begining in the embryo and finishing in puberty. (More? Puberty Development)
Historic Images of Genital Changes
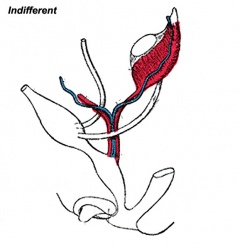
|
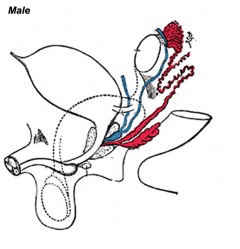
|
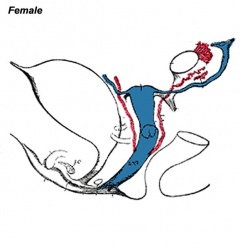
|
| Urogenital indifferent | Urogenital male | Urogenital female |
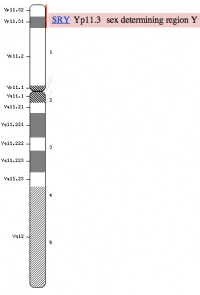
SRY
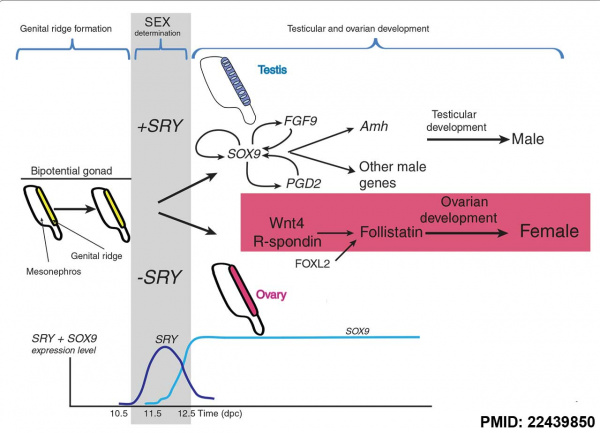
Sry (sex-determining region on the Y chromosome) gene was found in 1990 on the Y and the first SOX gene identified, the sry gene encodes a "testis-determining factor" a 204aa protein (Mr 23884 Da).
Sry acts as a transcriptional activator (HMG type-high mobility group) binding to DNA and initiating male sex determination then regulating male development. The protein sequence is shown on this current page and the full genebank entry can also be seen. The sry protein has a HMG box that binds DNA by intercalating in the minor groove. Read about the mapping of the testis determining factor which is SRY.
The actual gene targets of SRY are still being determined but at least one downstream gene Sox9 has been identified. Another gene Dax1 (nuclear hormone-receptor superfamily member) when expressed as a transgene will antagonize Sry and also force dosage-sensitive sex reversal.
Mouse sex determination genes[8]
- Links: SRY | Y chromosome | OMIM - Sry | 3d Structure Of The Human Sry-Dna Complex
| Human SOX Family | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
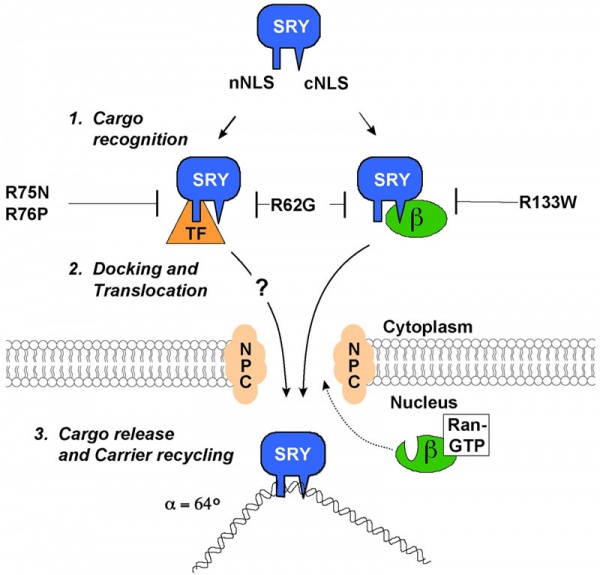
SRY Nuclear Import
A model for nuclear import of SRY from normal males and XY females.[9]
The distinct nuclear localization signals (NLSs) of SRY use different import pathways.
- cNLS - recognized by IMPβ, docks the transport complex at the nuclear pore complex (NPC) and is then translocation through. After nuclear entry of the complex, RanGTP binds to IMPβ to trigger release of SRY; DNA binding by SRY may also facilitate the release process.
- nNLS - mediates nuclear import through a novel pathway not utilizing conventional nuclear import factors such as IMPs but an unidentified "transport factor" (TF) suggested to be calcium-calmodulin.
Sry Target Genes
Cerebellin 4 precursor (Cbln4) - encodes a secreted protein expressed in Sertoli cells in the developing gonad.[10]
Androgen receptor - SRY interacts with and negatively regulates this receptor transcriptional activity.[11]
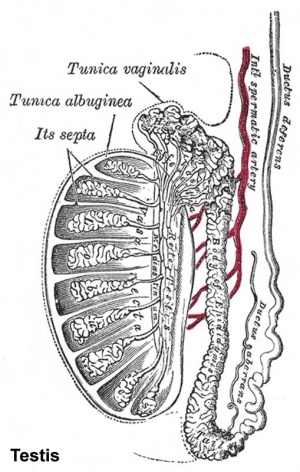
Gonad - Testis
See the detailed notes on testis development.
- Links: Testis Development
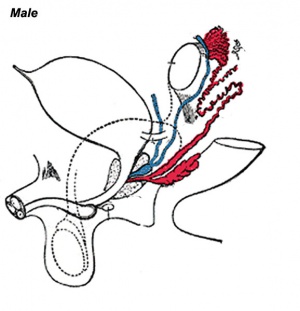
Internal Genital
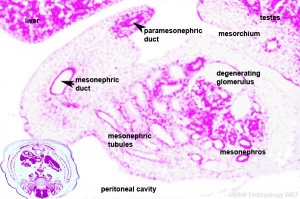
Mesonephric duct or Wolffian duct differentiates to form the male internal genital tract the vas deferens (ductus deferens, vas deferens or simply vas). Associated with the duct are the male prostate and accessory glands.
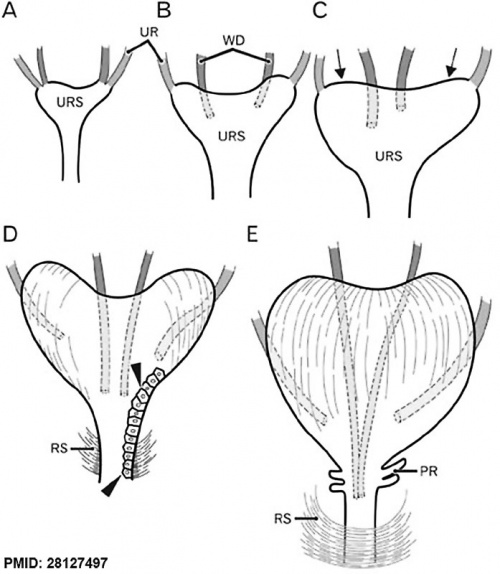
| Human Mesonephric Duct position (week 6 to 11) | |
|---|---|
|
Schematic representations of the descent of the mesonephric duct (Wolffian duct, WD) or vas deferens. Anterior view.[12]
|
| Adult Ductus deferens | ||
|---|---|---|
| ||
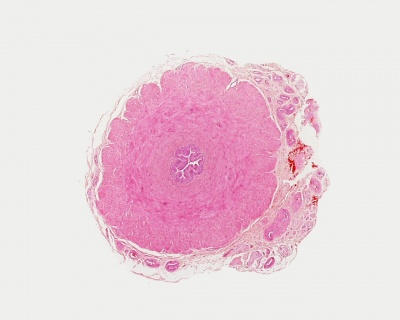
| Adult Prostate | ||

|
|
|
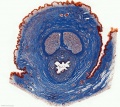
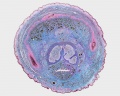
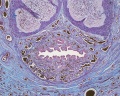
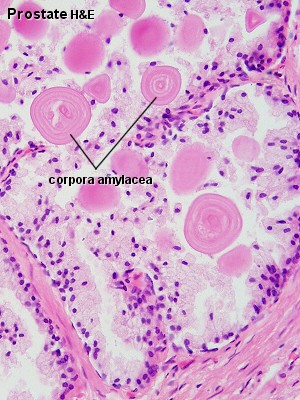
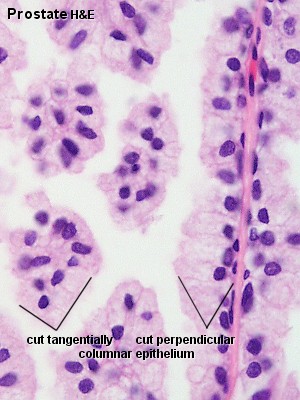
| Human prostate histology | Corpora Amylacea | Submucosal gland |
| (adult, low power overview) | (adult, detail) | (adult, high power detail) |
External Genital
- external genitalia are initially identical and undergo male and female differentiation under the influence or absence of steroidal sex hormones.
- Indifferent stage ‐ cloaca divided by proliferating mesenchyme forming the urorectal septum which separates the ventral urogenital sinus from the dorsal rectum.
- Difference stage ‐ locally in this region the presence or absence of dihydrotestosterone (DHT), generated from testosterone, determines male/female development.
Hormones
Anti-Müllerian Hormone
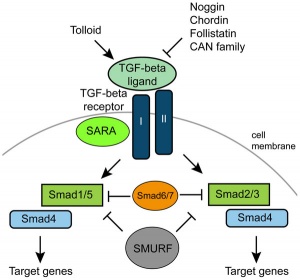
Anti-Müllerian Hormone (AMH, Müllerian Inhibiting Substance, MIS, Müllerian Inhibiting Factor, MIF) is a secreted glycoprotein factor of the transforming growth factor-beta, TGF-beta superfamily, that regulates gonadal and genital tract development. (Gene locus 19p13.3)
In the male embryo, the Sertoli cell secrete AMH and inhibit paramesonephric (Mullerian) duct development. This secreted hormone also acts to differentiate the Leydig cells (interstitial cells).
Ligand of TGF-beta (transforming growth factor-beta) superfamily and receptor binds to the anti-Mullerian hormone receptor type 2. Signalling pathway activate SMAD family transcription factors that regulate gene expression.
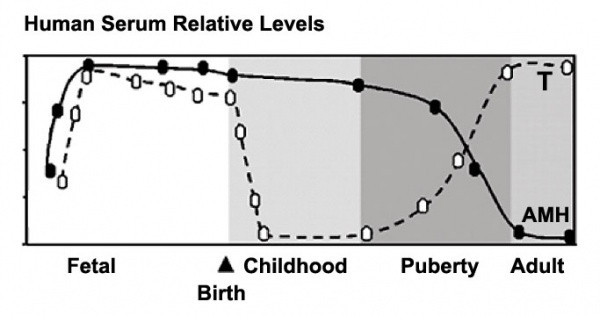
In postnatal males, AMH increases during the first month, reaching peak level at 6 months of age, and then slowly declines during childhood falling to low levels in puberty.
In reproductive age women, AMH is produced in the ovary by the granulosa cells surrounding preantral and small antral follicles and serum levels may reflect the remaining follicle cohort and decrease with age.
Sertoli cells release mainly a prohormone (proAMH), that is cleaved by subtilisin/kexin-type proprotein convertases or serine proteinases. The cleaved protein forms a stable complex (AMHN,C). Therefore the circulating AMH is a mixture of proAMH and AMHN,C. It has been suggested that proAMH may be activated within the gonads and also by its endocrine target-cells.
Preproprotein proteolytically processed to generate N- and C-terminal cleavage products, that homodimerize and associate to form a biologically active noncovalent complex. (see Protein Atlas)
Male testosterone and AMH levels
Ovary AMH
During ovary follicle development, the granulosa cells secrete AMH and it may have a role in follicular recruitment and development.[14] and may also function in postnatal elevation of FSH secretion in females.[15]
Other AMH Tissues
- The placenta has also been shown to both synthesise AMH and express its receptors.[16]
- AMH receptors have been identified in both the pituitary and brain.[15]
Links: TGF-beta | OMIM - AMH
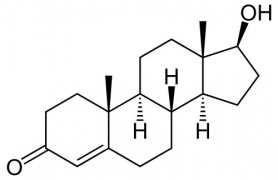
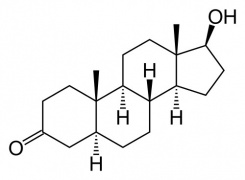
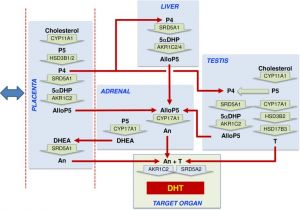
Dihydrotestosterone (DHT)
Male presence of Dihydrotestosterone (DHT, 5α-dihydrotestosterone, androstanolone, 5α-androstan-17β-ol-3-one).
|
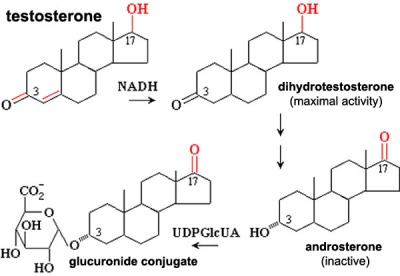
|
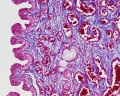
Histology
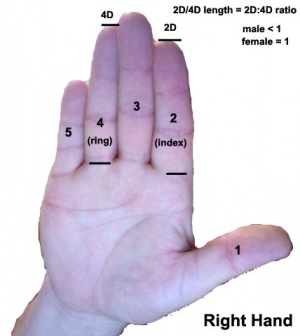
Androgen and Digit ratio (2D:4D)
The ratio of 2nd and 4th finger (D, digit) length. This ratio has been suggested to relate to high fetal testosterone concentration (males have lower 2D:4D than females) and has been shown for several species.[17] Although a study in mice has not shown the same correlation.[18] There have been some suggestions that the ratio may also be an indicator of various neurological abnormalities.
To measure (2D:4D) - using your right hand palm up, measure the index finger (2) and ring finger (4) length from palm to tip. Dividing the index finger by the ring finger gives the 2D:4D ratio, average women ratio is 1, average men is 0.98.
Additional Images
References
- ↑ 1.0 1.1 O'Shaughnessy PJ, Antignac JP, Le Bizec B, Morvan ML, Svechnikov K, Söder O, Savchuk I, Monteiro A, Soffientini U, Johnston ZC, Bellingham M, Hough D, Walker N, Filis P & Fowler PA. (2019). Alternative (backdoor) androgen production and masculinization in the human fetus. PLoS Biol. , 17, e3000002. PMID: 30763313 DOI.
- ↑ Ortega EA, Salvador Q, Fernandez M & Ward MA. (2018). Alterations of sex determination pathway in the genital ridges of males with limited Y chromosome genes. Biol. Reprod. , , . PMID: 30285093 DOI.
- ↑ Gredler ML, Seifert AW & Cohn MJ. (2015). Tissue-specific roles of Fgfr2 in development of the external genitalia. Development , 142, 2203-12. PMID: 26081573 DOI.
- ↑ Ishiguro T, Tamagawa S & Ogawa H. (1992). [Changes of pupil size in brain death patients]. Seishin Shinkeigaku Zasshi , 94, 864-73. PMID: 1484906
- ↑ Funke S, Flach E, Kiss I, Sándor J, Vida G, Bódis J & Ertl T. (2010). Male reproductive tract abnormalities: more common after assisted reproduction?. Early Hum. Dev. , 86, 547-50. PMID: 20674196 DOI.
- ↑ Lin C, Yin Y, Veith GM, Fisher AV, Long F & Ma L. (2009). Temporal and spatial dissection of Shh signaling in genital tubercle development. Development , 136, 3959-67. PMID: 19906863 DOI.
- ↑ Wu X, Ferrara C, Shapiro E & Grishina I. (2009). Bmp7 expression and null phenotype in the urogenital system suggest a role in re-organization of the urethral epithelium. Gene Expr. Patterns , 9, 224-30. PMID: 19159697 DOI.
- ↑ de Lau WB, Snel B & Clevers HC. (2012). The R-spondin protein family. Genome Biol. , 13, 242. PMID: 22439850 DOI.
- ↑ Harley VR, Layfield S, Mitchell CL, Forwood JK, John AP, Briggs LJ, McDowall SG & Jans DA. (2003). Defective importin beta recognition and nuclear import of the sex-determining factor SRY are associated with XY sex-reversing mutations. Proc. Natl. Acad. Sci. U.S.A. , 100, 7045-50. PMID: 12764225 DOI.
- ↑ Bradford ST, Hiramatsu R, Maddugoda MP, Bernard P, Chaboissier MC, Sinclair A, Schedl A, Harley V, Kanai Y, Koopman P & Wilhelm D. (2009). The cerebellin 4 precursor gene is a direct target of SRY and SOX9 in mice. Biol. Reprod. , 80, 1178-88. PMID: 19211811 DOI.
- ↑ Yuan X, Lu ML, Li T & Balk SP. (2001). SRY interacts with and negatively regulates androgen receptor transcriptional activity. J. Biol. Chem. , 276, 46647-54. PMID: 11585838 DOI.
- ↑ Jin ZW, Abe H, Hinata N, Li XW, Murakami G & Rodríguez-Vázquez JF. (2016). Descent of mesonephric duct to the final position of the vas deferens in human embryo and fetus. Anat Cell Biol , 49, 231-240. PMID: 28127497 DOI.
- ↑ Pang K, Ryan JF, Baxevanis AD & Martindale MQ. (2011). Evolution of the TGF-β signaling pathway and its potential role in the ctenophore, Mnemiopsis leidyi. PLoS ONE , 6, e24152. PMID: 21931657 DOI.
- ↑ McLennan IS & Pankhurst MW. (2015). Anti-Müllerian hormone is a gonadal cytokine with two circulating forms and cryptic actions. J. Endocrinol. , 226, R45-57. PMID: 26163524 DOI.
- ↑ 15.0 15.1 Garrel G, Racine C, L'Hôte D, Denoyelle C, Guigon CJ, di Clemente N & Cohen-Tannoudji J. (2016). Anti-Müllerian hormone: a new actor of sexual dimorphism in pituitary gonadotrope activity before puberty. Sci Rep , 6, 23790. PMID: 27030385 DOI.
- ↑ Novembri R, Funghi L, Voltolini C, Belmonte G, Vannuccini S, Torricelli M & Petraglia F. (2015). Placenta expresses anti-Müllerian hormone and its receptor: Sex-related difference in fetal membranes. Placenta , 36, 731-7. PMID: 25972076 DOI.
- ↑ McIntyre MH. (2006). The use of digit ratios as markers for perinatal androgen action. Reprod. Biol. Endocrinol. , 4, 10. PMID: 16504142 DOI.
- ↑ Yan RH, Bunning M, Wahlsten D & Hurd PL. (2009). Digit ratio (2Dratio4D) differences between 20 strains of inbred mice. PLoS ONE , 4, e5801. PMID: 19495421 DOI.
Reviews
Cohn MJ. (2011). Development of the external genitalia: conserved and divergent mechanisms of appendage patterning. Dev. Dyn. , 240, 1108-15. PMID: 21465625 DOI.
Larney C, Bailey TL & Koopman P. (2014). Switching on sex: transcriptional regulation of the testis-determining gene Sry. Development , 141, 2195-205. PMID: 24866114 DOI.
Rey RA & Grinspon RP. (2011). Normal male sexual differentiation and aetiology of disorders of sex development. Best Pract. Res. Clin. Endocrinol. Metab. , 25, 221-38. PMID: 21397195 DOI.
Biason-Lauber A. (2010). Control of sex development. Best Pract. Res. Clin. Endocrinol. Metab. , 24, 163-86. PMID: 20541146 DOI.
Koopman P. (2010). The delicate balance between male and female sex determining pathways: potential for disruption of early steps in sexual development. Int. J. Androl. , 33, 252-8. PMID: 19845801 DOI.
Wilhelm D, Palmer S & Koopman P. (2007). Sex determination and gonadal development in mammals. Physiol. Rev. , 87, 1-28. PMID: 17237341 DOI.
Sharpe RM. (2006). Pathways of endocrine disruption during male sexual differentiation and masculinization. Best Pract. Res. Clin. Endocrinol. Metab. , 20, 91-110. PMID: 16522522 DOI.
Warne GL & Kanumakala S. (2002). Molecular endocrinology of sex differentiation. Semin. Reprod. Med. , 20, 169-80. PMID: 12428197 DOI.
Adham IM, Emmen JM & Engel W. (2000). The role of the testicular factor INSL3 in establishing the gonadal position. Mol. Cell. Endocrinol. , 160, 11-6. PMID: 10715534
Hiort O & Holterhus PM. (2000). The molecular basis of male sexual differentiation. Eur. J. Endocrinol. , 142, 101-10. PMID: 10664515
Articles
Chawengsaksophak K, Svingen T, Ng ET, Epp T, Spiller CM, Clark C, Cooper H & Koopman P. (2012). Loss of Wnt5a disrupts primordial germ cell migration and male sexual development in mice. Biol. Reprod. , 86, 1-12. PMID: 21900680 DOI.
Cools M, Wolffenbuttel KP, Drop SL, Oosterhuis JW & Looijenga LH. (2011). Gonadal development and tumor formation at the crossroads of male and female sex determination. Sex Dev , 5, 167-80. PMID: 21791949 DOI.
Search PubMed
Search Pubmed: Male Genital System Development | mesonephric duct
Terms
- mesonephric duct - (Wollfian duct) An early developing urogenital paired duct system that initially runs the length of the embryo, that will differentiate and form the male reproductive duct system (ductus deferens). In females, this duct degenerates occasionally some remnants may remain associated in broad ligament.
- Wolffian duct - (mesonephric duct, preferred terminology), A developmental duct that runs from the mesonephros to cloaca. The duct in male differentiates to form the ductus deferens and in female the same structure regresses. Historically named after Caspar Friedrich Wolff (1733-1794), a German scientist and early embryology researcher and is said to have established the doctrine of germ layers. (More? Caspar Friedrich Wolff)
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2024, April 27) Embryology Genital - Male Development. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Genital_-_Male_Development
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G