Developmental Mechanism - Cell Migration
| Embryology - 1 Mar 2026 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Introduction
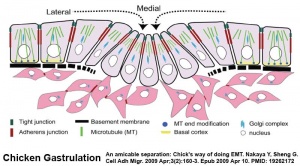
The process of cell migration occurs at different stages throughout embryonic development and involves other developmental mechanisms. The first key migration occurs during gastrulation. Later key migratory events also occur during somitogenesis and neural crest migration.
This mechanism involves several cellular processes including: cytoskeletal reorganisation, adhesion, extracellular matrix, and chemotactic signaling.
Some Recent Findings
|
| More recent papers |
|---|
|
This table allows an automated computer search of the external PubMed database using the listed "Search term" text link.
More? References | Discussion Page | Journal Searches | 2019 References | 2020 References Search term: Developmental Cell Migration | Neural Crest Cell Migration |
| Older papers |
|---|
| These papers originally appeared in the Some Recent Findings table, but as that list grew in length have now been shuffled down to this collapsible table.
See also the Discussion Page for other references listed by year and References on this current page. |
Neural Crest Development
- Links: Neural Crest Development
References
- ↑ Bazzi H, Soroka E, Alcorn HL & Anderson KV. (2017). STRIP1, a core component of STRIPAK complexes, is essential for normal mesoderm migration in the mouse embryo. Proc. Natl. Acad. Sci. U.S.A. , 114, E10928-E10936. PMID: 29203676 DOI.
- ↑ Creuzet SE, Viallet JP, Ghawitian M, Torch S, Thélu J, Alrajeh M, Radu AG, Bouvard D, Costagliola F, Borgne ML, Buchet-Poyau K, Aznar N, Buschlen S, Hosoya H, Thibert C & Billaud M. (2016). LKB1 signaling in cephalic neural crest cells is essential for vertebrate head development. Dev. Biol. , 418, 283-96. PMID: 27527806 DOI.
- ↑ Scarpa E & Mayor R. (2016). Collective cell migration in development. J. Cell Biol. , 212, 143-55. PMID: 26783298 DOI.
Textbooks
Reviews
Scarpa E & Mayor R. (2016). Collective cell migration in development. J. Cell Biol. , 212, 143-55. PMID: 26783298 DOI.
Barriga EH & Mayor R. (2015). Embryonic cell-cell adhesion: a key player in collective neural crest migration. Curr. Top. Dev. Biol. , 112, 301-23. PMID: 25733144 DOI.
Aman A & Piotrowski T. (2010). Cell migration during morphogenesis. Dev. Biol. , 341, 20-33. PMID: 19914236 DOI.
Articles
Breau MA & Schneider-Maunoury S. (2015). Cranial placodes: models for exploring the multi-facets of cell adhesion in epithelial rearrangement, collective migration and neuronal movements. Dev. Biol. , 401, 25-36. PMID: 25541234 DOI.
Wada N. (2011). Spatiotemporal changes in cell adhesiveness during vertebrate limb morphogenesis. Dev. Dyn. , 240, 969-78. PMID: 21290476 DOI.
Search PubMed
Search Pubmed: Epithelial Mesenchymal Interaction
External Links
External Links Notice - The dynamic nature of the internet may mean that some of these listed links may no longer function. If the link no longer works search the web with the link text or name. Links to any external commercial sites are provided for information purposes only and should never be considered an endorsement. UNSW Embryology is provided as an educational resource with no clinical information or commercial affiliation.
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2026, March 1) Embryology Developmental Mechanism - Cell Migration. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Developmental_Mechanism_-_Cell_Migration
- © Dr Mark Hill 2026, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G