2010 Group Project 5
Fetal Fibronectin
Introduction
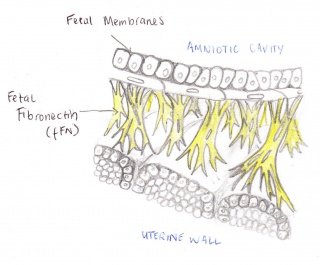
The Fetal Fibronectin (fFN) test measures the amount of secretions of fFN from a woman's vagina and cervix. This technique is used to determine the likelihood of a premature birth occurring. Fetal fibronectin is a protein based plasma that acts as a form of glue attaching the amniotic sac to the uterine wall. Fetal fibronectin is commonly present around the amniotic cavity between 22 to 35 weeks of pregnancy, but is released into the cervix and upper vagina toward the onset of labour. If the test finds fFN amongst the upper vagina, between this period, the woman will have a chance of going into early labour[1]. Hence, if there is no fFN found, it is most likely that they woman will not going into pre-term labour. Therefore, this test allows the woman to see if they are at risk or not.
About preterm births
Preterm births refer to labour that occurs early than expected. Preterm labour occurs prior to 37 weeks of gestation, Signs include:[2] - Contractions every 10 minutes or more
- Blood or clear, watery discharge from the vagina
- Low backache
- Heavy pelvic pressure
There are many groups of women who are at risk of preterm labour. They include:
- Women who have had a history of preterm births
- Women who are pregnant with twins or more
- Women who have cervical or uterine abnormalities
- Obese or overweight women[3]
- Women associated with an illness or disease (diabetes, high blood pressure)
- Smokers, alcoholics, drug users, stress and women who lack social support
Risk Factors of a pre-term birth
• A History of Pre-term births: women with preceding pre-term births prior to 37 weeks have a 55% rate of a subsequent pre-term birth[4]. Moreover, the incidence of a pre-term birth surmounts with every additional pre-term birth[5]. Gestational age is another factor that heightens the risk of a subsequent preterm birth, i.e. as the gestational age of the prior pre-term birth decreases, the risk of a subsequent pre-term birth increases[6].
• Short cervix: The Risk of a pre-term birth is manifested five-fold in the case of a woman with a cervical length of two centimetres as opposed to the expected four centimetres[7].
• Multifetal pregnancies: Approximately 60% of multifetal births occur prior to the 37th week[8].
Procedure of the Fetal Fibronectin Test
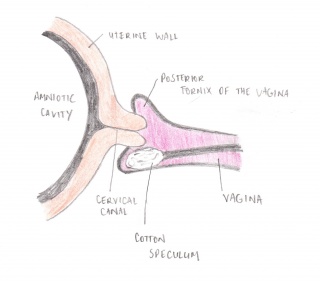
The fetal Fibronectin test involves a clinician placing a speculum into the vagina. A cotton swab is then used to collect a sample of the cervico-vaginal secretions in the cervical canal/posterior fornix. This sample is then sent to a Laboratory that analyses it, producing the results within 24 hours of the test. The necessity of the use of the speculum has been questioned in recent years. Studies have shown that the insertion of a finger to guide the cotton swab toward the posterior fornix of the vagina is just as effective as Adeza approved method. [9]
Laboratory procedure
The Laboratory test that is undertaken uses a specific monoclonal (FDC-6) antibody. The use of this antibody allows for rapid testing of the presence of fetal Fibronectin in the fFN assay. Prior to the use of this assay, ELIZA (Enzyme-Linked Immunosorbant Assay) was used but its inability to efficiently detect fetal Fibronectin saw it superseded by the current fFN assay. [10]
This assay is:
• Lateral flow; a type of Immunoassay that enables the test sample to exude along a solid substrate via capillary action. Another common example of this type of test is the take home pregnancy test[11].
• Solid-phase Immunosorbent assay device; also known as reverse ELISA. This process involves an immunosorbent polystyrene rod which has distended ogives that have been sensitised to the fetal fibronectin specific reagent. The sensitised rod is then placed into the fetal fibronectin sample that is extracted from the patient. The ogives on the rod, which have now been exposed to the potential fetal fibronectin, undergo a series of washing, incubation in conjugate and incubation in chromogen (to develop the colour that will determine the partiality or impartiality of fetal fibronectin to the test and thus the premature presence of it in the cervix)[12]
• Qualitative detection of fFN in cervico-vaginal specimens; the test concludes in either a positive or negative result.[13]
Test Results
Maintaining the quality of the sample
The integrity of the test requires that the vagina and the cervix remain undisturbed. Such disruptions include:
• Physical examinations/digital vagina examinations such as a transvaginal ultrasound
• Sexual intercourse within 24 hours of the test as Semen contains a degree of fibronectin which may lead to a false positive result. [14].
• Preeclampsia [15]
Also, a False negative may occur if the Mother is suffering from Candida Albicans (yeast infection) as it binds with the Fetal Fibronectin. [16]
What do the test results mean?
The results of the fetal Fibronectin test appear as a numeric figure that is either above or below a specified ng (of fetal fibronectin)/mL (of sample collected). If the Figure is below 50ng/mL the test result is negative; if it is greater than 50ng/mL the test result is positive [17]
What does this mean?
With 95% accuracy a negative result predicts that labour is not going to occur within the following two weeks. A positive result, however, is an inconclusive result. Its main value is providing healthcare workers and the mother with the opportunity to negate for as long as possible the pre-term labour.
What`s following the positive results?
From the perspective of infant health,preventions or treatments for fetal fibronection test positive woman can be divided into 3 types:
a.Primary prevention - turning preterm birth to at term birth.
b.Secondary Prevention - intervening before preterm birth.
c.Tertiary Prevention - treatments for premature infants.
However,the progress has mainly been made in the latter two preventions.
The secondary prevention involves the regionalisation of perinatal and neonatal care which ,including matenal transport of impending preterm births and the use of antepartum steroid to speed up the maturation of postnatal lungs.
Tertiary prevention involves developments in neonatal intensive care such as ventilator management and surfactant treatment. It has had a substantial impact on infant mortality yet has less notably affected rates of long-term sequelae.
The secondary and tertiary prevention measures together have lowered gestational age-specific neonatal mortality rates.[18]
The predicted results attained by the Fetal fibronectin test determine the actions of the mother and the health care workers within the next two week period. If the results are positive, some or all of the following actions will follow thereafter:
• Tocolytic agents; these inhibit uterine contractions and delay subsequent birth by approximately 4-5 days. They allow for substantial time to administer betamethasone, which is a glucocortocoid drug that sharply accelerates the rate of fetal lung maturity (usually reaching optimum load in 1-2days)[19]
• Progesterone; this is administered as it decreases the incidence of preterm births in mothers who have had previous pre-term births. [20]
• Corticosteroids; These also aid in fetal lung maturity.[21]
• Hospital transfer and bed rest; the mother may need to be transferred to a medical institution that has the facilities to deal with premature babies.
Targeted individuals
The American College of Obstetricians and Gynecologists doesn’t recommend pregnant women who have no symptoms and are not at high risk to be put to test, as the test hasn’t shown the accuracy predicting asymptomic woman.[22]
Only 2 groups of pregnant woman would be recommended to put to test, which are the groups that have contractions or other symptoms of preterm labor in between week 24th and 34th.The other group would be the group that are at high risk for preterm labor, such as women with prior preterm delivery, multifetal gestations, uterine abnormalities and cervical factors , but have no symptoms.[23]
The test should only be used on those pregnant woman who are at high risk and show symptoms of preterm labor to help make a more accurate diagnosis. For example, women who have intact amniotic membranes, a cervix that has not dilated more than 3 cm, only slight vaginal bleeding, no cervical cerclage (a cervix that has been sewn shut during pregnancy to keep the baby in the uterus).[24]
Current Research
How does a preterm birth happen?
The fFN(fetal fibronectin) remains relatively very low levels throughout the first 22 weeks of gestation. The following dramatically increase of the concentration that reaches 50 ng/mL or over from 22nd week onwards associates with the increase of the incidence of preterm labor before week 37th of gastation.[25]
What is the indicator of a preterm birth?
According to a study carried out in a teaching hospital, the presence of fetal fibronectin in the cervicovaginal secretions of preterm birth symptomatic women indicates a significant risk for subsequent preterm birth while the absence of fetal fibronectin in this group of woman is a very strong indication that subsequent preterm birth is unlikely to occur.[26]
How accurate is fetal fibrinectin in preterm birth prediction?
The findings are, the preterm birth (before 37 weeks of gestation) rate in the population studied was 19.1%. Fetal fibronectin predicted preterm birth with sensitivity of 63%, specificity of 95.6%, positive predictive value of 77.3%, and negative predictive value of 91.6%. A negative test accurately excluded (97.9%) the chance of subsequent birth during the three weeks interval following sampling.[26]A study with objective to determine the accuracy with which cervico-vaginal fetal fibronectin predicts preterm delivery using systematic quantitative overview of the available literature, concludes that fetal fibronectin test is the most accurate in predicting spontaneous preterm birth within 7 to 10 days after the test of symptomatic woman before advanced cervical dilatation.
The variation of fFN accuracy in asymtomatic and symtomatic women
A total of 26 876 women involved in the study and among asymptomatic women the best summary likelihood ratio for positive results was 4.01, 95% confidence interval 2.93 to 5.49 for predicting birth before 34 weeks gestation, with corresponding summary likelihood ratio for negative results of 0.78. Among symptomatic women the best summary likelihood ratio for positive results was 5.42 for predicting birth within 7-10 days of testing, with corresponding ratio for negative results of 0.25.
What does the current research say about this test?
Although the test has been commonly used in labour and delivery units to help in the management of preterm labour asymptomatic woman, yet it is not an official screening method to be used of pregnant woman in general, as there is not sufficient evidence to recommend its use as a screening method. Fetal fibronectin test, if combined with clinical findings, has a potentially important role in clinical management of women with symptoms suggestive of preterm labour.[26] Since this review found an association between knowledge of fFN results and a lower incidence of preterm birth before 37 weeks, further research should be encouraged.[27]
Significance
Advantages
Infants that are born preterm are mainly associated with high chances of mortality and getting health and development problems as they grow up. Complications associated include acute respiratory, gastrointestinal, immunologic, central nervous system problems, hearing, and vision defects, longer-term motor, cognitive, visual, hearing, behavioral, social-emotional, health, and growth problems. The birth of a preterm infant can also bring considerable emotional and economic costs to families and have implications for public-sector services, such as health insurance, educational, and other social support systems.[22] Therefore, being able to predict preterm birth would eliminate those problems listed above. The fetal fibronectin test is also a non-invasive test that can be completed quickly.
Disadvantages
There is no side effects in this test, rather the use of fFN test in routine clinical practice allows management and resources to be targeted more appropriately and may limit unnecessary interventions.[28]
External Links
Glossary
Amniotic cavity - innermost sac containing amniotic fluid that encloses the embryo.
Antenatal - prenatal, before birth.
Asymptomatic - producing or showing no symptoms of a condition.
Cervical cerclage - stitching a malfunctioning (open) cervix closed during pregnancy to prevent miscarriage.
Corticosteroids - group of steroid hormones with metabolic functions and inflammatory treatment.
Fornix - On both sides of the cervix the vagina bulges out into a pocket, which is called a fornix
Gestation - process of the developing embryo, where the fetus is carried in the womb between conception and birth.
Immunosorbent - An antibody that is used to remove a specific antigen from a mixture; An antigen that is used to remove a specific antibody from a mixture
Monoclonal Antibody - an antibody produced by means of recombinant DNA technology to recognise one specific substance
Multifetal - involves two or more fetuses in the womb during pregnancy.
Preeclampsia - is a medical condition where hypertension arises in pregnancy (pregnancy-induced hypertension) in association with significant amounts of protein in the urine
Speculum - a medical instrument for dilating a bodily passage or cavity in order to examine the interior
Symptomatic - involves producing symptoms of a condition.
Tocolysis - medications used to supress preterm labour.
Uterine wall - the wall of the uterus in the female reproductive system, superior to the cervix.
References
- ↑ 2010 March of Dimes Foundation http://www.marchofdimes.com/professionals/14332_1149.asp
- ↑ 2010 March of Dimes Foundation http://www.marchofdimes.com/professionals/14332_1149.asp
- ↑ <pubmed>20647282</pubmed>
- ↑ Meis PJ, Klebanoff M, Thom E, et al. Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate. N Engl J Med. 2003;348:2379-85.
- ↑ Adams MM, Elam-Evans LD, Wilson HG, Gilbertz DA. Rates of and factors associated with recurrence of preterm delivery. JAMA. 2000;283:1591-96.
- ↑ Mercer BM, Goldenberg RL, Moawad AH, et al. The preterm prediction study: effect of gestational age and cause of preterm birth on subsequent obstetric outcome. Am J Obstet Gynecol. 1999;181:1216-21.
- ↑ Iams JD, Goldenberg RL, Meis PJ, et al. The length of the cervix and the risk of spontaneous premature delivery. N Engl J Med. 1996;334(9):567-72.
- ↑ . Singer E, Pilpel S, Bsat F, et al. Accuracy of fetal fibronectin to predict preterm birth in twin gestations with symptoms of labor. Obstet Gynecol. 2007 May;109(5):1083-87
- ↑ <pubmed>18355785</pubmed>
- ↑ Review of Rapid Fetal Fibronectin Assay in the Management of Suspected Preterm Labour. http://www.health.alberta.ca/documents/AHTDP-fFN-synthesis.pdf
- ↑ Review of Rapid Fetal Fibronectin Assay in the Management of Suspected Preterm Labour. http://www.health.alberta.ca/documents/AHTDP-fFN-synthesis.pdf
- ↑ Review of Rapid Fetal Fibronectin Assay in the Management of Suspected Preterm Labour. http://www.health.alberta.ca/documents/AHTDP-fFN-synthesis.pdf
- ↑ Review of Rapid Fetal Fibronectin Assay in the Management of Suspected Preterm Labour. http://www.health.alberta.ca/documents/AHTDP-fFN-synthesis.pdf
- ↑ American College of Obstetricians and Gynecologists (ACOG). Management of Preterm Labor. ACOG Practice Bulletin, number 43, May 2003 (reaffirmed 2008)
- ↑ <pubmed>1536222</pubmed>
- ↑ Fetal Fibronectin; Lab Test online. http://www.labtestsonline.org/understanding/analytes/ffn/test.html
- ↑ <pubmed>10739521</pubmed>
- ↑ <pubmed>PMC1508187</pubmed>
- ↑ Tocolytic Agents - Protocols for Administration for Threatened Preterm Labour. http://www.health.nsw.gov.au/policies/PD/2005/pdf/PD2005_249.pdf
- ↑ Effectiveness Of Progesterone In Reducing Preterm Births May Be Altered By Genetic Predisposition. http://www.sciencedaily.com/releases/2009/01/090130084153.htm
- ↑ Corticosteroids For Fetal Lung Maturity. http://medicalcenter.osu.edu/PatientEd/Materials/PDFDocs/medicatn/gendrug/corticos-fetal.pdf
- ↑ 22.0 22.1 Institute of Medicine (US) Committee on Understanding Premature Birth and Assuring Healthy Outcomes; Behrman RE, Butler AS, editors.Washington (DC): National Academies Press (US);2007
- ↑ <pubmed>7738935</pubmed>
- ↑ American College of Obstetricians and Gynecologists (ACOG). Assessment of Risk Factors for Preterm Birth. ACOG Practice Bulletin, number 31, October 2001 (reaffirmed 2008).
- ↑ Akush Ginekol (Sofiia). 2010;49(2):39-42. Bulgarian.
- ↑ 26.0 26.1 26.2 <pubmed>8688390</pubmed>
- ↑ <pubmed>18843732</pubmed>
- ↑ <pubmed>16953860</pubmed>
2010 ANAT2341 Group Projects
Project 1 - Ultrasound | Project 2 - Chorionic villus sampling | Project 3 - Amniocentesis | Group Project 4 - Percutaneous Umbilical Cord Blood Sampling | Project 5 - Fetal Fibronectin | Project 6 - Maternal serum alpha-fetoprotein | Group Assessment Criteria
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2024, April 27) Embryology 2010 Group Project 5. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/2010_Group_Project_5
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G