2009 Group Project 3
ZebraFish
Background Information
Model organisms, particularly vertebrates, have been researched extensively in science to reach a greater understanding of human embryo development without facing the ethical issues such studies would arouse if performed on human embryos. They are used, not only in the study of development of a healthy embryo but also for the study of diseases and in particular the role genetics plays in the presence and development of such diseases.
Why zebrafish?
Among these models, the zebrafish is one of the most useful due to unique characteristics, which allow it to be more easily studied than others. These advantages include the high reproductive rate of the zebrafish (something which is important for the study of genetics) and in addition to this, scientists are able to maintain them in breeding condition all year round. The fertilisation of the zebrafish is an external process; this means that the gametes can be fertilized individually by scientists, allowing for the manipulation of genetic material for the purpose of study of individual genes and the roles they play. Also, the unique transparency of the zebrafish embryo allows scientists to closely observe the development of the phenotypes without disturbing the process of development, enabling observation of the development of individual embryos from beginning to end.
"‘You can see different cell types, watch individual cells develop, do transplantation experiments’, Eisen enthuses, ‘and development is quick but not too quick’. Being able to watch individual neurons developing in real time opened up whole new avenues of research for Eisen and other neurobiologists."
(Bradbury, 2004)
In the study of most vertabrate models, development must be ceased in order to dissect the embryo for study. This hinders the study of the embryo greatly, forcing scientists to effectively ‘fill in the gaps’ of information they could not observe due to the disruption of embryo development. This however, is not necessary in the study of the zebrafish due to the transparency of the embryo.
History of Model Use
Timeline of the development of the zebrafish as a model organism
In the Beginning... George Streisinger (December 1927 - August 1984)
The use of the zebrafish as a model organism began in the late 1960s when a scientist at the University of Oregon,
George Streisinger, chose to study it based not only on the many advantages stated above but also on his love for tropical fish.
Streisinger wanted to analyse mutants among the zebrafish in order to study embryological development and stated that his aim was to “study features of the organism and embryological development of the vertebrate nervous system through the use of mutant strains” (Grunwald and Eisen, 2002). In order to do this, he decided that first the phenotypes showing rare recessive mutations must be used to reproduce heterozygous offspring. It wasn’t until 1976 that Streisinger and fellow scientist, Charline Walker managed to produce wholly or partially homozygous offspring using sperm to activate an egg but in such a way that the sperm (genetically impotent due to UV damage) did not contribute to the genetic make-up of the offspring. In order for the eggs to remain diploid without gaining an extra set of chromosomes, the second meiotic division was inhibited, giving the offspring the entire set of chromosomes needed. This allowed mapping out of genes and correlating them with phenotypes.
Charles Kimmel
Streisinger was not the only scientist working on the embryologic development of the zebrafish. Neurologist Charles Kimmel (also at Oregon) used the zebrafish to model the development of the nervous system and vertebrae. By the mid- 1970s, Kimmel uncovered the segmental structure of its brain.
Hesitations
Problems soon arose in the form of doubt when it came to using a zebrafish to understand human genetics. “There was little theoretical appreciation of the degree to which vastly diverged species would share the regulatory pathways that govern cell behaviour and embryonic development” (Grunwald and Eisen, 2002). At the time, there was no gene cloning and little understanding about genes, making the whole zebrafish project almost a gamble to follow through with. This put much strain on the funding given to Streisinger and the credibility of his work. This however did not affect Neurologists such as Kimmel as they were not much worried about the relevance of their work. According to them, as long as they were studying nerve cells, it was all relevant.
Taking the Plunge
The Institute of Molecular Biology at the University of Oregon seemed to nurture the gamble-like quality in Streisinger’s work and provided him with the right environment as well as facilities to develop his model. The attitude of the institute was one which encouraged personal endeavours and above all else, it was important to be “committed to answering a question” (Grunwald and Eisen, 2002).
Once a method to produce heterozygous diploids was established, non- mutant strains of zebrafish were created and then specific mutations induced to study the phenotype and genotype relations. A 1981 issue of Nature journal published the first article pertaining to the zebrafish research which outlined the methods used by Streisinger to produce the homozygous clones of the zebrafish.
Around this time, Kimmel had “described more identifiable neurons in the zebrafish than had been recognised in any other vertebrae” (Grunwald and Eisen, 2002). They also traced the growth of the axons of spinal cord motor neurons. The common ground on which Streisinger and Kimmel based their work lead to a collaborative effort to record “patterning and differentiation of the nervous system” (Grunwald and Eisen, 2002).
In 1982, Kimmel began a 10 year program to “illuminate the developmental steps that led to the origin and organisation of distinct tissue types in the zebrafish embryo” (Grunwald and Eisen, 2002). Although Streisinger died in 1984, the project went on to describe a crucial stage in embryonic development which occurred just before gastrulation. This study was groundbreaking as the step described was the one in which the entire body plan emerged for the embryo, giving future researchers a conceptual framework (or map) from which to explore and monitor the development of cells. It also “placed the zebrafish in the context of vertebral biology” (Grunwald and Eisen, 2002).
The Big Screen
Through combining the use of embryological, genetic and neurological studies, the 1980s saw Kimmel and other scientists exploring various gene mutations of the zebrafish. By combining these studies with other such studies of homologous genes and their phenotypes, mutations were found to exist across a range of animals.
For example, a zebrafish with no tail was found to have a mutation in the same gene as that of a mouse with no tail. Such findings interested Drosophila developmental geneticists and compelled them to begin a research program on the zebrafish by the late 1980s. The main aim of such a program, as led by Nusslein-Volhard (winner of a Nobel Prize for her work with the Drosophila) and Wolfgang Driever was to replicate “Drosophila screen for embryonic pattern mutants in a vertebrate” (Grunwald and Eisen, 2002). This program, later known as the ‘Big Screen’, began in 1993 and finished in 1996 and the resultant 37 papers (describing 4000 embryonic lethal mutants) were all published in volume 123 of the journal Development
The Big Screen had an enormous impact on the scientific community, pushing the zebrafish as the latest promising research model for embryology and the roles of genetics in disease and development. “The model for genetic analysis of development and physiology that had been established in Drosophila had been extended fruitfully to new vertebrate problems” (Grunwald and Eisen, 2002). The mutations described by the report were useful for the study of genetic disease processes across a range of vertebrates, including humans.
The Importance of Mutations
Around this time, a research team of undergraduate students, under the watchful eye of John Postlethwait, developed a method by which molecules across the zebrafish genome could be tagged and then used to map out genetic mutations. This development of a linkage map provided the tools needed for genetic analysis through positional cloning. Positional cloning is a process used to identify and locate a gene by first identifying its phenotype and then, using its already known approximate position on a chromosome (called the candidate region), narrowing down the position until the gene in question and its mutants are found.
With the development of this technique, the possibilities of genetic studies of zebrafish was brought to the attention of the director of the National Institute of Health(of the USA), Harold Varmus, who set up a project to study the genomic development of the zebrafish as advocated for by Len Zon, Marc Fishman (no, really) and Nancy Hopkins. Progress soon came about in the form of discoveries of genes and expressed sequence tags.
In the year 2000, the project to sequence the zebrafish genome was launched by the Sanger Centre. Also, Monte Westerfieldestablished both the ZFIN (Zebrafish Model Organism Database) project and ZIRC (Zebrafish International Resource Centre). Whilst ZIRC is a building at the University of Oregon in which many of the zebrafish being studied are kept, ZFIN is an internet based database which aims to be a central reference point for the studies into zebrafish embryology, linking it with other such embryological studies in other model organisms. Both ZIRC and ZFIN often work closely together.
Digital Zebrafish Embryo
In 2008 researchers at the EMBL (European Molecular Biology Laboratory) generated the first ever complete developmental imprint of a vertebrae using zebrafish as the model organism. This 'digital embryo' is a model which tracks every cell (its initial position, migration and divisions) in the embryo of a zebrafish for the first 24 hours after fertilisation. Click here to view the digital zebrafish embryo montaged with microscopy data. The varying colours on the left half of each picture (digital embryo) indicate the direction of movement of the individual cells whist the right half is the microscopy data shown at time points in development. To view the video of the reconstructed 3D model of the developing digital zebrafish embryo, click here.
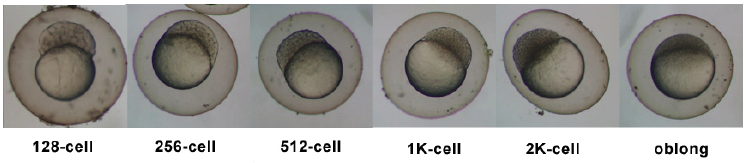
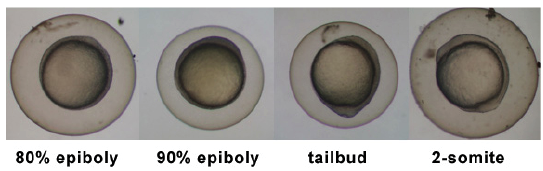
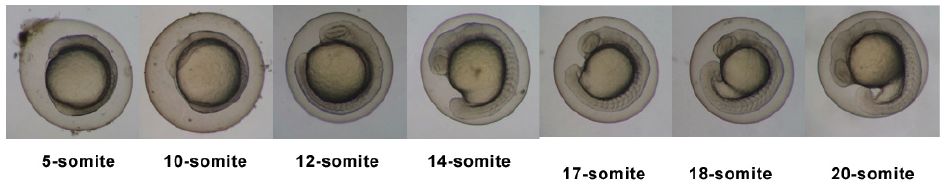
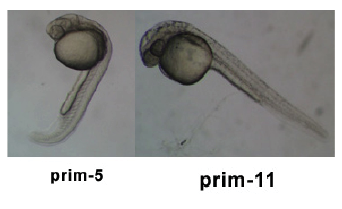
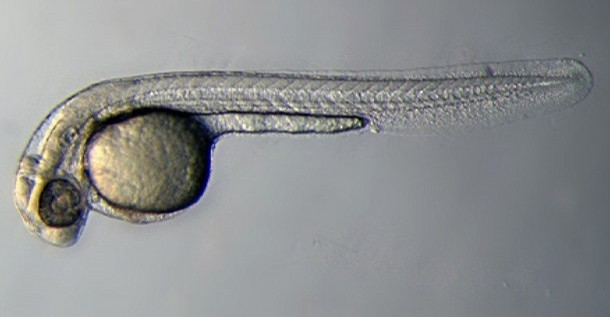
Timeline and Stages of Embryonic Development
Links: Cleavage Period / Blastula Period / Gastrula Period / Segmentation Period / Pharyngula Period / Hatching Period / Larval Period / 2009 Project 3 - Zebrafish
- Student animal model - zebrafish project contributed page 2009.
Genetics of the ZebraFish and Embryology
The Danio rerio (zebrafish) genome has not yet been fully sequenced. It is made up of 25 pairs of chromosomes and is approximately half the length of the human genome. As of September 24, 2009, 1,543,637,863 base pairs have been sequenced of an estimated total of 2,011,175,423 bps.
Danio rerio Genome Sequencing Project
Zv8, the 8th integrated Whole Genome Shotgun assembly of the zebrafish was released in June, 2008 by the Wellcome Trust Sanger Institute. This project gives us a genome length of 1,481,241,295 bps and the accompanying gene sets is comprised of 24,147 genes which code proteins.
For more information on the Zv8, follow the link to The Sanger Trust institute Website
Danio rerio Mitochondrial DNA Sequence
The zebrafish mitochondrial genome has been sequenced and was found to be 16,596 base pairs in length and contain 13 protein-coding genes. The order and content of these genes are that of the common vertebrate genome. Mitochondria are the major source of ATP in eukaryotes through oxidative phosphorylation. Mitochondria are therefore crucial for cell life but are also important in the regulation of apoptosis. The similarity between zebrafish mitochondria and that of humans makes it possible for us to use zebrafish as an accurate mitochondrial model which will allow us to study normal and pathological mitochondria.
Genetics and Embryology
The zebrafish provides an excellent model for the study of vertebrate development. Embryonic development of the zebrafish takes place externally and the embryo is completely transparent. Development is simple and rapid, developing to a free swimming larva 120 hours after fertilisation, with a generation time of 10-12 weeks. This means that we can learn the effects of genetic mutation in a relatively short period of time and discover how they affect development. Identification of genes can be achieved through analysis of mutant phenotype, that is, the best way to determine the role of a specific gene is to mutate that gene so that it cannot function normally and observe what affect this has on the organism. Not only can genetic studies involving gene mutations of the zebrafish allow us to identify the role of genes but they provide us with a model to study vertebrate development and various human diseases.
How are mutations achieved?
Mutagens, such as N-ethyl-N-nitrosourea (ENU), gamma rays and X-rays are used to induce a mutation in the zebrafish. Usually the zebrafish will receive multiple treatments with a mutagen during mutagenesis. A breeding scheme is then implemented to achieve a stable mutation (this usually requires at least three generations) before analysis of mutant phenotype. Genetic screening is used to help determine where the mutation occurs and if it is present. Screens commonly used in studying the zebrafish are premeiotic and postmeiotic mutagenesis and screens of haploid and diploid progeny form a given fish. Many mutagenised fish die due to environmental stress. It is important for researchers to bear this in mind when conducting these experiments and take extra care to ensure that temperature and oxygen saturation of the water is kept constant and even that noise levels are kept to a minimum so as not to jeopardise the experiment.
Vertebrate Heart Development
The zebrafish has a major advantage over previous models for the study of vertebrate heart development in that because of their small size, embryos are not wholly reliant on the cardiovascular system. They receive enough oxygen through passive diffusion to be able to survive with fairly normal function for several days without any blood circulation. This allows researchers to thoroughly analyse severe defects of the cardiovascular system. Many mutations have been identified and analysed which cause a change the development of the heart in the zebrafish. Below is a table which identifies some known mutations and resultant phenotype changes related to heart development.
| Mutation | Gene | Phenotype | When is Mutation First Observed |
|---|---|---|---|
| bonnie and clyde | bon | Cardia bifida | Blastula |
| casanova | Unknown | Cardia bifida | Blastula |
| two-of-hearts | Unknown | Cardia bifida | Mid-somitogenesis |
| heart-and-soul | Unknown | Defects in heart tube formation | Mid-somitogenesis |
| open-eyed pinhead | oep | Absence or reduction of nkx2.5 expression, cardia bifida, reduction of cardiac ventricular tissue | Blastula |
| tell-tale-heart | isl | Contractility defect | Mid-somitogenesis |
| silent heart | Unknown | Prevents contraction |
Adapted from: Stainier, D. (2001). Zebrafish Genetics and Vertebrate Heart Formation. Nature Reviews , 2:39-48. and Glickman, N., & Yelon, D. (2002). Cardirac development in zebrafish:coordination of form and function. Cell and Developmental Biology , 13:507-513.
Current Embryology Research
The most commonly used organism for studies of development and function is the zebrafish embryo. The zebrafish embryo has been observed for many years from 1980s to 2009. Although the embryo was consistently studied over the years, it was most commonly utilized in 2004-2006 from the use of the zebrafish in drug testing to fight cancer, to being the first blueprint of a vertebrae embryo. [1] Recent developments have allowed for further understanding about genetics; expression and function, eye disorders, organ and vertebrae development as well as reproductive studies. These studies utilize the zebrafish embryo due to its rapid proliferation rate and the transparency of the embryo.
Cancer Research
In 2005, under the guidance of Mary J.C. Hendrix from the Northwestern University Feinburg School of Medicine, Illinois, a laboratory implanted the embryo of a zebrafish with human melanoma cells; these cells divided but did not express themselves as tumors within the embryo. They were however maintained as plastic phenotypes, expressing specific cell types including endothelial, neural and stem cells. Hendrix stated that cancer cells are unspecific plastic phenotypes that are similar to embryonic stem cells. Due to the ability of the zebrafish embryo to maintain the plastic phenotype and to suppress the tumor from forming in the microenvironment, Hendrix hypothesized that there may be a causal relationship between the formation of cancer cells and the role of the environment. Hendrix concluded that further study into the factors that suppressed the formation of tumor cells in the zebrafish could potentially reverse the phenotype of tumor cells.
The Massachusetts Institute of Technology (MIT), Boston also depicted a mutant zebrafish embryo, where the embryo was injected with a mutagenesis and subsequently resulted in the failure of the synthesis of melanin. Hence MIT determined that the failure of the synthesis of melanin resulted in the mutant zebrafish lacking adequate black pigments in melanocytes, as depicted in the file: A Zebrafish Pigment Mutant.
Genetic Mutations Associated with Sight
Nephronophthisis (NPHP) is a cystic kidney disease, caused by mutations of nine genes and is also associated to cerebellar defects, situs inversus and retinitis pigmentosa where the mutation of NPHP5 and NPHP6 is associated to progressive blindness. The Renal Division of the University Hospital in Freiburg, Germany determined that gene products, such as nephrocystin5 and nephrocystin6, contributed to maintaining photoreceptor homeostasis. Zebrafish embryos were utilized to analyze the genetic interaction of the gene products once NPHP5 and NPHP6 was depleted. The following link provides further information about the usefulness of the zebrafish as a model for eye disorder research
Significance of Notochord
One of the most significant characteristics of the embryo is the notochord; a midline structure required for skeletal development of the vertebrae and is the main central structure through which the embryo develops. The Laboratory of Molecular Genetics and Developmental Biology, China isolated a contig of expressed sequence tags from the zebrafish ovary. This cDNA sequence was cloned and expressed within the oocyte, which further progressed through the cleavage stage to the blastula stage. At gastrulation, the specific gene sequence was evident in the dorsal region, restricted to the notochord and pectoral fin, during embryonic development at 48 and 72 hours. Hence the zebrafish notochord related gene (znrg) was established due to its abundant availability in the notochord, which was determined to play a vital role in notochord development within the zebrafish.
Brain and Developmental Deficiencies
The zebrafish has been utilized to express the gene of lissencephaly LIS1; a severe disease associated to brain malformation such as Miller-Dieker syndrome. The Institute of Biochemistry and Cell Biology, China found that the zebrafish exhibits two LIS1 genes; LIS1a and LIS1b. Proliferation and migration deficiencies in neural and brain development arise when the LIS1 gene is mutated or depleted. It was determined that the LIS1 genes were present within the protein and genetic structures and were expressed during embryonic development within mature tissue samples. The LIS1 gene was predominately evident within brain tissue and when the LIS1 gene was destroyed, it resulted in developmental deficiencies such as brain malformation, circulation and structural abnormalities. This study determined that the LIS1 gene plays a vital role in embryonic development especially in relation to brain formation.
Embryonic Growth & Development
An insulin-like growth factor (IGF) has been identified by the Department of Biochemistry, Ireland as playing an essential role in normal embryonic growth and development throughout vertebrate evolution. However the specific role that the IGF family plays in embryonic development has previously been unclear. The National University of Ireland focused on the role of IGF2 in the development of the zebrafish embryo. Two genes, IGF2a & IGF2b, were identified in the zebrafish genome and modified by the introduction of antisense morpholinos. It was identified that neural and cardiovascular abnormalities arose from defects in IGF2 embryos, where embryos were ventralized and subsequently resulted in reduced growth, compact eyes and disruptions to the brain structure and cardiovascular system, especially in relation to the cardiac outflow tract, cardiac looping and cardiac valve development. There were also substantial affects to the development of anterior neural structures and the regulation of genes for dorsal-ventral patterning. It was determined through the utilization of the zebrafish embryo, that IGF2a & IGF2b played a vital role in embryonic development, especially in relation to neural and cardiovascular development.
Glossary
Antisense morpholinos – a molecule used to modify genetic expression
Blastula – the early embryological developmental stage consisting of a single layer of cells enclosing a cavity
Cleavage – a series of mitotic divisions by a fertilized ovum
Contig – a fragment of a DNA sequence
Diploid – a cell with double the number of chromosomes
Ectoderm – the outermost germ cell layer of an embryo
Endoderm – the innermost germ cell layer of an embryo
Epiboly – the rapid proliferation of cells
Gastrula – the early stage of germ cell formation consisting to ectoderm, mesoderm and endoderm
Genotype – the genetic makeup of an organism
Haploid – a cell with a complete set (half the number of a diploid cell) of chromosomes
Heterozygous – an organism with different alleles
Homozygous – an organism with identical alleles
Lissencephaly – an abnormality of brain formation
Meroblastic – the partial cleavage of a fertilized egg
Mesoderm – the middle germ cell layer of an embryo
Mutagenesis – the occurrence and the development of mutation
Nephronophthisis – the genetic abnormality of the kidneys
N-Ethyl-N-Nitrosourea – a mutagen
Oxidative Phosphorylation – the metabolic pathways for the production of ATP
Phenotype – the expression of specific characteristics of an organism based upon its genetic and environmental influences
Progeny – the resulting offspring
Retinitis Pigmentosa – the degeneration of the retina, resulting in visual loss
Situs Inversus – a congenital abnormality where visceral organs are inverted
Zygote – a cell the develops as a result of fertilization
References
Videos
(Video) Eye Disorders and Zebrafish
(Video) First Digital Blueprint of Zebrafish Embryo
Website Links
Ensembl Zebrafish: Zebrafish Danio rerio sequence
EU Science Education Media: Retinitis Pigmentosa
National Center for Biotechnology Information: Zebrafish Genome Resources
Sanger Institute: The Danio Rerio Sequencing Project
The Zebrafish Model Organism Database: Stages of Zebrafish Development
UCSC Genome Bioinformatics: Zebrafish (Danio rerio) Genome Browser Gateway
Articles
Boore J (1999) Animal mitochondrial genomes Nucleic Acids Research, 27:1767-1780.
Bradbury J (2004) Small Fish, Big Science, PLoS Biology 2(5):148-149. PMID 15138510
Broughton R, Milam J & Roe B (2001) The Complete Sequence of the Zebrafish (Danio rerio) Mitochondrial Genome and Evolutionary Patterns in Vertebrate Mitochondrial DNA Genome Research, 11:1958-1967. PMID 11691861
Crown, Elizabeth (2005) Tiny Zebrafish teaches Researchers how to Fight off a Deadly Cancer Bio-Medicine, Northwestern University.
Glickman N & Yelon D (2002) Cardiac development in zebrafish:coordination of form and function Cell and Developmental Biology, 13:507-513. PMID 12468254
Green D & Reed J (1998) Mitochondria and Apoptosis Science, 281:1309-1312.
Grunwald DJ, Eisen JS (2002) Timeline: Headwaters of the Zebrafish emergence of a new water vertebrae, Nature Reviews Genetics, 3:717-724. PMID 12209146
Hartnett L, Glynn C, Nolan CM, Grealy M and Byrnes L. (2009) Insulin-like Growth factor-2 Regulates early Neural and Cardiovascular system development in Zebrafish embryos Department of Biochemistry, National University of Ireland, Ireland. PMID 19757379
Heidelberg (2008) Digital zebrafish embryo provides the first complete developmental blueprint of a vertebrate European Molecular Biology Laboratory
Keller P, Schmidt A, Wittbrodt J, Stelzer E. (2008) Reconstruction of Zebrafish Early Embryonic Development by Scanned Light Sheet Microscopy Science, 322:1065-1069.
Kendrick C, Zhang L, Jülich D and Holley AS Cell cycle progression is required for zebrafish somite morphogenesis but not segmentation clock function Department of Molecular, Cellular and Developmental Biology, Yale University, USA.
Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF (1995)Stages of Embryonic Development of the Zebrafish Institute of Neuroscience, University of Oregon, Eugene USA. PMID 8589427
Postlethwait JH (2006) The Zebrafish Genome: A review and msx Gene Case Study. In J. N. Volff Vertebrate Genomes, 2:183-197
Schafer T, Putz M, Lienkamp S, Ganner A, Bergbreiter A, Ramachandran H, Gieloff V, Gerner M, Mattonet C, Czarnecki PG, Sayer JA, Otto EA, Hildebrandt F, Kramer-Zucker A and Walz G (2008) Genetic & Physical Interaction between NPHP5 and NPHP6 gene products Renal Division, University Hospital Freiburg, Germany, 17(23):3655-62. PMID 18723859
Stainier D (2001) Zebrafish Genetics and Vertebrate Heart Formation Nature Reviews, 2:39-48.
Sun C, Xu M, Xing Z, Wu Z, Li Y, Li T and Zhao M (2009) Expression and Function on Embryonic Development of Lissencephaly-1 genes in Zebrafish State Key Laboratory of Molecular Biology, Institute of Biochemistry and Cell Biology, Shanghai Institutes for Biological Science, Shanghai, China, 41(8):677-88. PMID 19657569
Zhou Y, Xu Y, Liu Y, Zhang Z and Deng F. (2009) Znrg, a novel gene expressed mainly in the developing Notochord of Zebrafish The Laboratory of Molecular Genetics and Development Biology, College of Life Science, Wuhan University, China, 41(8):677-88. PMID 19693699
ANAT2341 group projects
Project 1 - Rabbit | Project 2 - Fly | Project 3 - Zebrafish | Group Project 4 - Mouse | Project 5 - Frog | Students Page | Animal Development