1987 Developmental Stages In Human Embryos - Stage 7
| Embryology - 26 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
O'Rahilly R. and Müller F. Developmental Stages in Human Embryos. Contrib. Embryol., Carnegie Inst. Wash. 637 (1987).
| Online Editor Note |
|---|
| O'Rahilly R. and Müller F. Developmental Stages in Human Embryos. Contrib. Embryol., Carnegie Inst. Wash. 637 (1987).
The original 1987 publication text, figures and tables have been altered in formatting, addition of internal online links, and links to PubMed. Original Document - Copyright © 1987 Carnegie Institution of Washington.
|
- 1987 Stages: Introduction | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | References | Appendix 1 | Appendix 2 | Historic Papers | Embryonic Development
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
Stage 7
- Approximately 0.4 mm in length
- Approximately 16 postovulatory days
- Characteristic feature: notochordal process
Summary
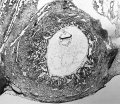
The appearance of the notochordal process immediately rostral to the primitive node and streak is used as the criterion for stage 7 (fig. 7-1).
In Streeter’s (1942) scheme, horizon VII was characterized by “branching villi, axis of germ disk defined.” Such specimens are here classified in stage 6b because (1) some branching of villi has been recorded even in certain stage 6a (horizon VI) embryos (e.g., No. 6734: “occasionally they [the villi] show dichotomous division...,” Ramsey, 1938), so that this feature is of scant use as a criterion, and (2) the embryonic axis is defined in some embryos already admitted to horizon VI (e.g., No. 7801, which shows “the primordium of the primitive streak,” Hertig, Rock, and Adams, 1956). Moreover, embryos already assigned to horizon VII (e.g., No. 7802) show additional differentiation in the presence of the notochordal process, which is not present in embryos of stage 6.
Size And Age
The maximum diameter of the chorion varies from 4 to 9 mm, that of the chorionic cavity from 1.5 to 8 mm. The embryonic disc is generally 0.3-0.7 mm in length but varies from 0.1 to 1 mm. The age is believed to be about 16 days.
External Form
The dorsal surface of the disc is generally slightly convex (fig. 7-9). It varies in shape from oval (Biggart specimen) to pyriform (Manchester 1285) but may be almost circular (Hugo, Schö).
Histological Features
Decidua
The epithelial layer is intact. The glands are markedly tortuous (fig. 7-7) and lined by secretory epithelium. The stroma is edematous and shows a decidual reaction.
Chorion
The villi, which may reach a length of 0.5 or even 1 mm, are either present on the entire surface of the chorion (No. 7802, fig. 40) or are absent superficially (Biggart specimen). In one specimen (Hugo), 942 villi were counted (Stieve, 1926). The mesoblastic cores of the villi contain vascular primordia which are said to be derived from (1) cells arising by (the now disputed) delamination from the cytotrophoblast lining the chorionic cavity and then migrating into the villi, and (2) angioblasts differentiating from the cytotrophoblastic cells of the trophoblastic columns during the formation of early villi (Hertig, Rock, and Adams, 1956).
At this stage (in the Missen embryo) “the human placenta clearly presents a combination of labyrinthine and villous characters. It must be stressed that the main trabeculae, which we will call primary villous stems, were never free villi. From this stage forwards, however, it would be pedantic to avoid calling the lacunar system the intervillous space” (Hamilton and Boyd, 1960).
Secondary villi, i.e., those “branching or arising secondarily from a villus or the chorionic membrane itself” (Hertig, 1968) are seen by stages 7 and 8. Their formation involves all the elements of previously formed chorion (trophoblast, stroma, and blood vessels). They lack maternal attachment, and their limited movement has been compared to that of seaweed waving in a sheltered tidal pool (ibid.). These free villi (in the Gar embryo) may branch as often as three or four times (Hamilton and Boyd, 1960). At the periphery of the primary villous stems, the cytotrophoblast has broken through the syncytial layer and, by lateral expansion and fusion, constitutes a thick trophoblastic shell (fig. 6-3).
Amnion
(figs. 7-9 to 7-11). The amnion consists of two layers of squamous cells: internally the amniotic ectoderm, and externally an interrupted stratum of cells resembling mesothelium. Between the two layers, some mesenchyme may be seen in places. An amniotic duct may be present (e.g., in No. 7802).
Primitive node and streak (figs. 7-10 and 7-11). At the rostral end of the primitive streak, “the ectodermal cells are loosely arranged and are disposed in a ventrolateral direction to form the primitive node” although a surface swelling is not necessarily present (Heuser, Rock, and Hertig, 1945). The primitive node (fig. 7-10), first described as a Knoten by Hensen in 1876, has been recorded as present in practically all stage 7 embryos, and varies in length from 0.02 to 0.1 mm, In some instances (e.g., Manchester 1285) the node appears to be separated from the streak by a slight constriction, or neck. From his studies of the pig embryo, Streeter (1927a) regarded the node as an additional, specialized center and not merely as the rostral end of the primitive streak. The idea that the node appears before the streak has been discussed under stage 6. Intercellular vacuoles in the primitive node may presage the appearance of the notochordal canal (Jirásek, personal communication, 1970).
In the chick embryo, the primitive node has been shown to furnish simultaneously the axially located mesoblast and endoblast. At the stage of the notochordal process, the streak contains material destined for the formation of only the notochord, somites, lateral plates, and a small quantity of extra-embryonic mesoblast, Invagination remains active in the entire streak until at least the stage of the notochordal process, except in the node, where invagination has terminated already at the stage of the definitive streak.
Although the primitive streak (including the node) attains its maximum length (about 0.7 mm) in stage 8, its greatest relative length (about 50 percent of the total length of the embryonic disc) is probably reached during stage 7 (fig. 7-2). In the chick embryo, where the length of the streak is employed as a criterion of staging, the definitive streak extends over two-thirds to three-fourths of the area pellucida, whereas, in the human, it scarcely exceeds one-half of the length of the disc even at the height of its development.
The primitive streak is not necessarily straight, as has been shown in Hugo, Bi 24, and Manchester 1285 (Hill and Florian, 1963).
The presence of a primitive groove (which has already been seen in the previous stage) has been recorded in a number of embryos of stage 7: e.g., No. 7802, P.M., Robertson et al., H. Schm. 10, and No. 1399 (Mateer). Its occurrence was used by Streeter (1920a) and by von Möllendorff (Meyer, 1924) in the grouping of human embryos. A primitive groove is never found in the absence of a primitive streak (Stieve, 1926a,b), although the converse does not hold.
Embryonic mesoblast
Mesoblastic cells accumulate ventral and caudal to the caudal end of the primitive streak, so that a temporary end node (Endknoten or Sichelknoten) may be formed: e.g., in Hugo (Stieve, 1926a,b) and in Bi 24 (Florian, 1933).
The embryonic mesoblast spreads laterally and rostrally from the primitive streak. Contributions to it are probably being made also by the gut endoderm and by the extra-embryonic mesoblast (Heuser, Rock, and Hertig, 1945). The limits between the primitive streak mesoblast and the endoderm of the umbilical vesicle are indistinct, and the two layers appear to be fused (Stieve, 1926a,b; Florian, 1933). In the Biggart specimen, according to Morton (1949), “in the angle between the anterior end of the shield and the yolk-sac there is a more loosely arranged mass of mesoderm which may well be the protocardiac area.” As in the previous stage, localized mesoblastic proliferations directly from the disc epiblast have been recorded, e.g., in Bi I and in Hugo (Florian, 1945).
Notochordal process (fig. 7-9). The notochordal process gives an appearance of being a prolongation of the primitive streak in the direction of the future head region of the embryo. The unsatisfactory term “head process” (Kopffortsatz), objected to by Waldeyer (1929a) who proposed der kraniale Mesoblastfortsatz des Primitivknotens, is better handled by the French authors, who write of the prolongement céphalique of the primitive streak. However, because the cell column “is without question primarily concerned with the formation of the notochord... it seems therefore appropriate to refer to it as the notochordal process.” (Heuser, 1932b).
In the chick embryo, autoradiographic analysis has led to the conclusion that, at the definitive streak stage, all of the chordal cells are massed in the primitive node, and that the presumptive notochord may be responsible for somite formation. According to carbon-marking experiments, the notochordal process does not form out of nodal epiblast, although it does arise in part from the rostral end of the nodal mesoblast and also receives contributions from the endoderm. Removal of the primitive node results in complete absence of the notochord and in an apparent loss of control in the process of neurulation. In the mouse, X-ray destruction of the primitive node area before the appearance of the notochordal process results in absence of the notochord, although a certain degree of cerebral neurulation occurs.
The notochordal process, which is the characteristic feature of stage 7, varies in length from 0.03 to about 0.3 mm in the recorded specimens, In addition to a median cord, the notochordal process may also (in Bi 24 and Manchester 1285) possess lateral mesoblastic wings (Hill and Florian, 1931b). Moreover, the notochordal process “very early becomes intercalated in, or fused with, the endoderm.”
Although the notochordal process appears to develop from the rostral end of the primitive node, Hill and Florian (1963) had no hesitation in identifying the notochordal process in Tarsius before the appearance of the recognizably differentiated primitive node. This again raises the question whether the node may not actually be present before the streak.
The notochordal process comprises “not only the primordium of a part of the mesoderm (which does not seem to be very extensive), but also that of the chorda” (Hill and Florian, 1931b). The notochordal process, however, is not synonymous with the notochord sensu stricto, which does not appear until stage 11.
Prechordal plate
This localized thickening of the endoderm, situated rostral to the notochordal process, has been recorded in certain embryos of stage 7, such as Bi 24 and Manchester 1285. It has already been mentioned under stage 6 and will be discussed under stage 8.
Umbilical vesicle
The cavity of the umbilical vesicle tends to be slightly larger than that of the amnion. The vesicle may project beyond the rostral limit of the embryonic disc (No. 1399), be approximately flush with it (Bi 24, Manchester 1285) or be receding in its relationship to it (No. 7802), although these variations may be caused, at least in part, by distortion.
A diverticulum of the umbilical vesicle may be present (e.g., in the Biggart specimen). In No. 7802, “detached vesicles observed in the chorionic cavity are regarded as transient parts of the earlier primary yolk sac” (Heuser, Rock, and Hertig, 1945).
Further spread of extra-embryonic mesoblast envelops the umbilical vesicle. The wall of the vesicle (figs. 7-9 to 7-12), like that of the amniotic cavity, may be said to comprise, at least in many areas, three layers: mesothelium, mesoblast, and endoderm, from external to internal. Clumps of cells, especially in the ventral wall of the umbilical vesicle, indicate that angioblastic tissue is differentiating. From their investigation of the Missen embryo, Gladstone and Hamilton (1941) conclude:
“The vascular spaces are partly developed by the fusion of small vacuoles, which are formed in solid angioblastic cords (intracellular spaces), and partly by direct transformation of mesodermal cells into flattened endothelium, which may either enclose the blood islands of the yolk sac, or form the walls of vascular spaces, which at first empty and incomplete, become secondarily filled with blood cells and enclosed by a continuous membrane.”
Hematopoietic foci develop in the wall of the secondary umbilical vesicle, although it is not clear whether they are derived from the endoderm or from the embryonic mesoblast (Hertig, 1968). It seems that they are definitely seen only after such foci are already present in the chorion and the body stalk (ibid.). Moreover, “it appears certain that blood vessel precedes blood cell formation” (Gilmour, 1941). From their study of the Missen embryo, Gladstone and Hamilton (1941) conclude:
“The earliest generation of blood cells (haemocytoblasts and primitive erythroblasts) are formed in the wall of the yolk sac and in the umbilical segment of the connecting stalk in close connexion with the entoderm of the allanto-enteric diverticulum, and in the situation of the future umbilical vessels. A few rounded cells of endothelial origin were, however, found in the mesenchyme at the base, or amnio-embryonic segment, of the connecting stalk. These differ in type from the former cells which arise in close association with the entoderm of the yolk sac and its diverticulum.”
Thus, although a number of workers have “suggested that the endoderm is the site of origin of the blood cells,” nevertheless the blood cells may “depend for their normal development and haemoglobinisation upon their early release into the mesenchyme” (Hoyes, 1969).
At stage 7, three types of hemopoietic cells have been recognized in the blood islands of the umbilical vesicle (Robertson, O’Neill, and Chappell, 1948): modified mesenchymal cells, hemocytoblasts, and primitive erythroblasts.
A low incidence of sex chromatin has been recorded in the umbilical vesicle of No. 7802 (Park, 1957).
Cloacal membrane (Figs, 7-4 and 7-12). The cloacal membrane is larger and better defined than in stage 6. It varies from 0.03 to 0.085 mm in length.
Allantoic diverticulum (Figs. 7-4 and 7-12). Because of the considerable difficulty in finding a convincing example of an allantoic diverticulum at stage 6, it is safer to assume for the present that the allantoic primordium first appears during stage 7, where it can be identified with a reasonable degree of certainty in such embryos as No. 7802, Bi 24, and Manchester 1285. In his discussion of the diverticulum of the umbilical vesicle found in Peh. l-Hochstetter (stage 8), Florian (1930a) referred to “the allanto-enteric diverticulum since its proximal part represents the later hind-gut, its distal portion the entodermal allantoic canal.” In other words, “the very early primordium of the allantois does not arise directly from the hind-gut... but from an allanto-enteric diverticulum. The orifice of this diverticulum later comes to form a part of the hindgut. It is only in the stage [10] when the insertion of the umbilical stalk has reached the ventral wall of the embryonic body that the allantois can be said to arise directly from the hind-gut...” (ibid.). This distinction was based largely on the relationship of the cloacal membrane to the orifice of the diverticulum. A comparison, however, of the wider choice of specimens now available would seem to indicate that, in stages 7-10, the diverticulum in question may be either allantoic or allanto-enteric at any given stage, as indicated in Table 7-1 and figure 7-3.
Table 7-1. Examples of Embryos of Stages 7-10 Showing either an Allanto-Enteric or an Allantoic Diverticulum
n the chick embryo, it has been maintained that the dorsal wall of the allantois is situated close to the epiblast, and the combination appears to be the cloacal membrane. These relationships do not appear to hold in No. 7802 or in Manchester 1285, although embryo Bi 24 (Florian, 1945, plate 5, fig. 43) does seem to resemble somewhat the conditions found in the chick, except that the hindgut has not been seen at such early stages in the human embryo. Embryo Bi I (stage 6) also bears some resemblance to the arrangement depicted in the chick but the diverticulum of its umbilical vesicle is not regarded as the allantois. It would seem that the above interpretation of the chick embryo is comparable to Florian’s concept of an allanto-enteric rather than an allantoic diverticulum.
Primordial germ cells have been noted in the region of the allanto-enteric diverticulum in Bi 24 (Politzer, 1933).
“The essential part of the mammalian allantois from the physiological standpoint is its vascular mesoderm” (Mossman, 1937), and the question has been raised (George, 1942) whether “the precociously formed allantois in man may not have some inductor function in the origin and differentiation of the blood islands and blood channels of the body stalk.” This would require the presence of the allantoic primordium at latest by stage 6b.
Specimens of Stage 7 Already Described
(listed in order of length of notochordal process)
HEB-37. Summarized by Mazanec (1959). Chorionic cavity, 2.25 x 1.29 x 0.4 mm. Embryonic disc, 0.4 mm. Primitive streak, 0.104 mm, and node, 0.04 mm. Notochordal process, 0.032 mm. Stalk of umbilical vesicle (ibid., fig. 77). Median projection published (ibid., fig. 45).
H. R. 1. Described by Johnston (1940), who believed that a notochordal process (0.04 mm) and a prechordal plate (0.075 mm) were present. Florian in an appendix to the article disagreed, and his interpretation is followed here (see stage 6).
Biggart. Described by Morton (1949). Curettage. Embryonic disc (narrow type), 0.27 x 0.16 mm. Primitive streak and node, 0.059 mm. A notochordal process is not referred to in the text but is mapped on a dorsal projection of the embryo (ibid., fig. 2) and is approximately 0.04 mm in length. The specimen is said to resemble the Yale embryo.
Guá (Guálberto). Described by Lordy (1931). Hysterectomy. Chorionic cavity, 8 x 7.5 mm. Embryonic disc, 0.776 x 0.0465 mm. Primitive streak, 0.09 mm. Notochordal process, 0.045 mm. Possible notochordal canal. Said to resemble Hugo embryo. Probably belongs either to stage 7 or to stage 8.
Carnegie No. 7802 (figs. 7-4 to 7-12). An important specimen described and illustrated by Heuser, Rock, and Hertig (1945). Hysterectomy. Chorion, 3.75 x 2.35 x 2.2 mm. Chorionic cavity, 2.3 x 1.4 x 1.1 mm. Embryonic disc (broad type), 0.42 x 0.35 x 0.05 mm. Primitive streak, 0.11 mm, and node, 0.03 mm. Notochordal process, 0.048 mm. Presumed age, 16 days. Median projection published (ibid., plate 6; Mazanec, 1959, fig. 46) and dorsal projection has been prepared by the present writers.
P.M. Described by Meyer (1924). Curettage. Measurements have been criticized by Stieve (1926) but defended by Mazanec (1959). Chorion, 3.9 x 3.77 x 2.5 mm. Chorionic cavity, 2.7 x 2.6 x 2.1 mm. Embryonic disc (circular), 0.41 x 0.41 mm. Primitive streak, 0.12 mm, and node, 0.02 mm. Notochordal process, 0.06 mm, acknowledged by Mazanec (1959) although denied by Fahrenholz (1927). No notochordal canal. Median projection published (Mazanec, 1959, fig. 47).
Hugo. Described by Stieve (1926), who reproduced a photomicrograph of every second section. Hysterectomy. Surrounded by 942 chorionic villi ranging in length from 0.3 to 1 mm. Chorion, 6.4 x 5.9 x 5.6 mm. Chorionic cavity, 4.7 x 4.4 x 3.8 mm. Embryonic disc (broad type), 0.635 (Florian, 1931) x 0.63 mm. Primitive streak, 0.245 mm, and node, 0.05 mm. Notochordal process, 0.07 (0.11?) mm. (Florian, 1934c). No notochordal canal. Prechordal plate probably not yet developed (Hill and Florian, 1931a). Dorsal and median projections published (Florian, 1934c, fig. 1; Hill and Florian, 1931b, figs. 44 and 11; Mazanec, 1959, fig. 49).
Robertson, O’Neill, and Chappell (1948) described a hysterectomy specimen that possessed a chorion of 3.816 x 3.639 x 2.687 mm. Chorionic cavity, 2.718 x 2.239 x 1.679 mm. Embryonic disc (broad type), 0.462 x 0.485 mm. Primitive streak, 0.138 mm, and node (situated halfway), 0.03 mm. Notochordal process, 0.072 mm. Suggestion of notochordal canal in one or two sections. Assigned to horizon VIII by authors but probably belongs to stage 7. Median projection published (ibid., fig. 12).
D’Arrigo (1961) described an embryonic disc of 0.47 mm, which showed a notochordal process of 0.075 mm. Canalization of the process is “doubtful,” but the presence of a prechordal plate is “probable.” The specimen “could be recorded in Streeter’s horizon VII.”
Goodwin. Described by Kindred (1933). Tubal. Chorion, 5.8 x 2.72 x 2.25 mm. Chorionic cavity, 2.44 x 2.25 x 0.75 mm. Embryonic disc, 0.588 mm in width. Primitive streak, 0.215 mm, and node, 0.078 mm. Notochordal process, 0.078 mm. No notochordal canal and no prechordal plate.
Pha I. Described by Mazanec (1949). Chorionic cavity, 7.872 x 5.475 x 2.032 mm. Embryonic disc, 0.66 x 0.52 mm. Primitive streak, 0.145 mm, and node, 0.06 mm. Notochordal process, 0.09 mm. No prechordal plate. Median projection published (ibid., fig. 51).
H. Schm. 10 (H. Schmid). Described briefly by Grosser (1931c). Embryonic disc (almost circular), 0.51 x 0.58 mm. Primitive streak, 0.14 mm, and node, 0.1 mm. Notochordal process, 0.1 mm. Probably belongs to stage 7, although a cavity in one section was thought to represent “Lieberkühn’s canal.”
Bi 24 (Bittmann). Described by Hill and Florian (1931b). Chorionic cavity, 3.05 x 3.036 x 3.029 mm. Embryonic disc (narrow type), 0.62 x 0.39 mm. Primitive streak, 0.28 mm, and node (Mazanec, 1959, fig. 105), 0.05 mm. Notochordal process, 0.105 mm, consists of median chord and lateral mesoblastic wings. Prechordal plate, 0.03 mm. Possible primordial germ cells in endoderm of region of cloacal membrane and in endoderm of umbilical vesicle caudally (Florian, 1931). Politzer (1933) counted 41 germ cells in the region of the allanto-enteric diverticulum in this embryo, and 19 such cells in another presomite specimen (Bi 25). Dorsal and median projections published (Hill and Florian, 1931b, figs. 4 and 12; Florian, 1945, plate 5, fig. 43; Mazanec, 1959, fig. 50).
Manchester No. 1285 (fig. 7-1). Described by Florian and Hill (1935). Hysterectomy. Chorionic cavity, 4.28 x 3.28 mm. Embryonic disc (narrow type), 0.87 x 0.625 mm. Primitive streak, 0.39 mm, and node, 0.05 mm. Notochordal process, 0.125 mm. Prechordal plate, 0.03 mm. Connecting stalk attached to chorion at decidua capsularis (suggesting polar variety of velamentous insertion of umbilical cord). Dorsal and median projections published (Hill and Florian, 1931b, figs. 5 and 13; Florian and Hill, 1935, figs. 1-3; Mazanec, 1959, fig. 52). Specimen is housed in Department of Anatomy, University of Manchester.
Pha II. Summarized by Mazanec (1959). Chorionic cavity, 4.985 x 3.882 x 3.52 mm. Embryonic disc, 0.895 x 0.62 mm. Primitive streak, 0.37 mm, and node, 0.06 mm. Notochordal process, 0.13 mm. No prechordal plate. Median projection published (ibid., fig. 53).
Thompson and Brash (1923) described a specimen that showed a notochordal process of 0.3 mm. It is described in the present work under stage 8.
Additional Specimens
Precise measurements of the notochordal process have not been provided in the accounts of the following embryos. The specimens are listed in order of year of publication.
Debeyre (1912) described in detail a specimen that possessed a chorionic cavity of 5.6 x 2.1 mm. Embryonic disc, 0.9 x 0.6 x 0.95 mm. Primitive streak stated to be 0.54 mm in length. Chorionic villi (0.4-1.6 mm) showed some branching. Unsuitable plane of section makes it impossible to assess the specimen precisely.
Carnegie No. 1399, Mateer. Described by Streeter (1920). Hysterectomy. Angiogenesis in villi described by Hertig (1935). Chorion, 9 x 8 x 3.5 mm. Chorionic cavity, 6.1 x 5.6 x 2.5 mm. Embryonic disc, 1 x 0.75 mm. Primitive streak and groove present. Although the notochordal process was originally thought probably to be absent, Hill and Florian (1931b) have no doubt that it is present. More advanced than Hugo (Florian and Völker, 1929). A very small twin embryo was originally described but that “interpretation has become open to doubt” (Corner, 1955). Drawing of every section reproduced by Turner (1920). A median drawing (Davis, 1927, fig. 5A) and a projection have been published (Mazanec, 1959, fig. 56).
Ho (Hodiesne). Described by Fahrenholz (1927). Abortion. Chorionic cavity, 6.5 x 6 x 3 mm. Embryonic disc (deformed), 0.6 mm (0.725 mm by flexible scale). Primitive streak, 0.22 mm (0.345 mm by flexible scale). Notochordal process just beginning (“undoubtedly present,” Hill and Florian, 1931b). Possible prechordal plate claimed (disputed by Waldeyer, 1929a, but supported by Hill and Florian, 1963). “Lieberkühn’s canal” (0.065 mm) is an artificial folding of the embryonic disc (Hill and Florian, 1931b). Dorsal and median projections published (Fahrenholz, 1927, figs. 32, 6 and 7; Mazanec, 1959, fig. 54).
Debeyre (1933) described a specimen (0.9 mm) that possessed a primitive streak and probably belonged to stage 6 or stage 7. Large cells near the opening of the allantois were identified as primordial germ cells.
Falkiner. Described by Martin and Falkiner (1938). Curettage. Embryo damaged and not in good condition. Measurements seem too small (see Mazanec, 1959). Chorionic cavity, 1.5 x 1.4 mm. Embryonic disc, 0.15 x 0.29 mm. Primitive streak, 0.07 mm. Notochordal process contains “no definite lumen.” Cells rostral to notochordal process are “probably” the prechordal plate. Development “agrees most closely” with that of Bi I. Median projection published (Martin and Falkiner, 1938, fig. 8; Mazanec, 1959, fig. 39).
Gar (Green-Armytage). Described by West (1952). Hysterectomy. Chorionic cavity, 3 x 2.6 x 2 mm. Embryonic disc (broad type), 0.56 x 0.69 mm. Primitive streak, node, and groove present. Short notochordal process. No notochordal plate. Said to resemble Hugo embryo. Trophoblast described by Hamilton and Boyd (1960).
Mal (Maliphant). Described by West (1952). Hysterectomy. Chorionic cavity, 3 x 1.8 mm. Embryonic disc (broad type), 0.45 x 0.6 mm. Primitive streak, node, and groove present. Notochordal process present (Mazanec, 1959); small cavity in node (West, 1952) or in notochordal process (Mazanec, 1959) “hardly sufficient to warrant the name chorda canal.” No prechordal plate. Belongs either to stage 7 or to stage 8 (said to resemble Jones-Brewer I, which is in stage 8).
Carnegie No. 8602. Photomicrograph reproduced by Hertig, Rock, and Adams (1956, plate 10, fig. 53). Chorion, 2.73 x 2.43 mm. Chorionic cavity, 1.83 x 1.33 mm. Embryonic disc, 0.3 x 0.06 mm. Presumed age, 16-17 days.
Missen. Trophoblast described by Hamilton and Boyd (1960). Curettage. Chorion, 1.66 x 1.43 mm. Embryonic disc, 0.28 x 0.214 mm. Primitive streak and node. Notochordal process. Said to resemble No. 7801 and Edwards Jones-Brewer (stage 6). Presumed age, about 14 days.
Certain other embryos that probably belong to stage 7 but that have not been described in detail will not be referred to here. These include Fitzgerald, Fitzgerald-Brewer II, and Jones-Brewer II (Brewer and Fitzgerald, 1937).
References
| Online Editor Note |
|---|
| O'Rahilly R. and Müller F. Developmental Stages in Human Embryos. Contrib. Embryol., Carnegie Inst. Wash. 637 (1987).
The original 1987 publication text, figures and tables have been altered in formatting, addition of internal online links, and links to PubMed. Original Document - Copyright © 1987 Carnegie Institution of Washington.
|
Search Pubmed
- 1987 Stages: Introduction | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | References | Appendix 1 | Appendix 2 | Historic Papers | Embryonic Development
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
Cite this page: Hill, M.A. (2024, April 26) Embryology 1987 Developmental Stages In Human Embryos - Stage 7. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/1987_Developmental_Stages_In_Human_Embryos_-_Stage_7
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G