1987 Developmental Stages In Human Embryos - Stage 15
| Embryology - 26 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
O'Rahilly R. and Müller F. Developmental Stages in Human Embryos. Contrib. Embryol., Carnegie Inst. Wash. 637 (1987).
| Online Editor Note |
|---|
| O'Rahilly R. and Müller F. Developmental Stages in Human Embryos. Contrib. Embryol., Carnegie Inst. Wash. 637 (1987).
The original 1987 publication text, figures and tables have been altered in formatting, addition of internal online links, and links to PubMed. Original Document - Copyright © 1987 Carnegie Institution of Washington.
|
- 1987 Stages: Introduction | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | References | Appendix 1 | Appendix 2 | Historic Papers | Embryonic Development
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
Stage 15
- Approximately 7–9 mm
- Approximately 33 postovulatory days
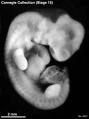
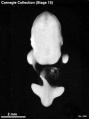
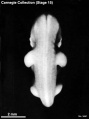
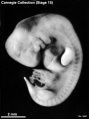
Stage 15 (3512)
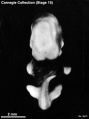
Stage 15 (3512)
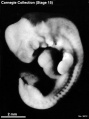
Stage 15 (3512)
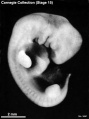
Stage 15 (3512)
Stage 15 (3441)
Stage 15 (3441)
Stage 15 (3441)
Stage 15 (3441)
Summary
External: lens vesicles are closed; nasal pits are appearing; hand plates are forming.
Internal: foramen secundum begins to develop in the heart; a definite intestinal loop and caecum are present; lobar buds appear in the bronchial tree; the pelvis of the ureter develops, and the primary urogenital sinus forms; the future cerebral hemispheres are better delineated, the future paleostriatum and neostriatum become distinguishable, and the primordium of the epiphysis cerebri becomes recognizable; retinal pigment appears.
Size and Age
Approximately 80 percent of the embryos after fixation range from 6.5 to 8.5 mm. This range may be taken as the expected length at this stage, and many of these embryos are from 7 to 8 mm. A few, however, are smaller (6 mm) or larger (11 mm).
The size of the chorion is even more variable. Its greatest diameter is generally 30–40 mm.
The age of the embryos of stage 15 is believed to be approximately 33 postovulatory days (Olivier and Pineau, 1962) but may extend to 38 days Jirásek, 1971). One specimen with known coital history was 36½ days in age (Windle, 1970).
External Form
(figs. 15-1 to 15-3, and 16-4) Up to this time the central nervous system appears to have played the principal role in determining the contours of the head and trunk of the embryo. Other details in its form are provided by the heart, the limb buds, and the condensed masses that are to form the mandibular and hyoid regions (the pharyngeal arches). As in stage 14, the embryo resulting from these influences is bilaterally flattened, having a curved or partially spiral axis. In the embryos of stage 15, the relative width of the trunk region (fig. 15-1) has become greater because of growth of the spinal ganglia, the muscular plates, and the mesenchymal tissues associated with them, all of which add to the bulk of the trunk. Thus when viewed from the back, embryos of this stage appear wider from side to side than those of stage 14. This increase in relative width of the trunk becomes still greater in the succeeding groups.
The result is that the greatest transverse diameter, which originally is in the dorsoventral axis, eventually coincides with the side-to-side width of the embryo. In stages 15–18 this increase in relative width of the trunk is rapid enough to serve as one of the criteria for determining the developmental status attained by a given embryo. Allowance must be made, however, for the fact that the occipitocervical region is more advanced and is consequently wider than the lumbosacral because of the rostrocaudal gradient in growth. New factors greatly complicate the picture in more-advanced stages, where special modifications occur in the relative diameters of the trunk in its different regions and where its width varies correspondingly.
Five characteristics are present in stage 15. (l) The lens vesicles have closed, and on each side the pores by which the vesicles had communicated with the surface have disappeared. (2) The nasal discs, because of the relatively greater growth of the surrounding tissues, begin to recede from the surface, acquiring the form of large oval depressions (i.e., the nasal pits). In the less advanced members a low ridge marks the frontal and lateral borders of the depression. In the more advanced members this ridge has become a slightly overhanging lip. It is this rapidly growing ridge that will subsequently form the nostril on its side of the head. (3) Before this period the hyoid arch has already been differentiated into a dorsal and a ventral segment, but now its ventral segment acquires a subsegment, or ventralmost segment, which is the primordium of the antitragus. (4) The active transformations occurring in the upper limb buds contribute to the precision with which embryos can be identified as belonging, or not belonging, in stage 15. In stage 14 one could speak of an elongating upper limb bud. In stage 15, as shown in figures 15-1 and 15-2, one can recognize the developmental steps by which the upper limb bud becomes regionally subdivided into a distal hand plate and a proximal forearm, arm, and shoulder region. The less precocious lower limb bud exhibits a beginning differentiation into a rounded rostral half and a more tapering caudal half (fig. 16-4). It is the tip of the latter that will form the foot. (5) As in less-advanced embryos, in all the members of this group the muscular plates, somites, and underlying spinal ganglia produce characteristic elevations which can be seen sufficiently well for fairly accurate counting, throughout the length of the cord, from the occipital region to the coccygeal levels. In transparent specimens they appear as white opaque condensations. In subsequent stages, the ganglia and somites will become covered over with mesenchymal tissues so that they can no longer be seen from the surface. This occurs first in the occipitospinal region, whence it spreads caudal ward.
The developing facial region at three successive stages is shown in figure 15-3. The mandibular processes are more prominent than the maxillary. In stage 13 the nasal discs consist merely of areas of thickened surface ectoderm. In stage 14 the nasal disc has undergone active cellular proliferation, with the result that it is larger and thicker. It has also acquired a shallow central depression and its margins are elevated. In stage 15 a nasal pit is produced, and a dorsolateral lip known as the lateral nasal process develops. The two nasal pits are relatively wide apart at the outset.
The lateral and caudal portions of the mandibular arch contribute to the formation of the external ear, shown by stippling in the figure.
Cardiovascular System
(figs. 15-4, 15-5, and 16-7) The vascular system is highly adaptable. Streeter was impressed by the circumstance that the venous drainage of the enlarging brain undergoes a continuous series of modifications, and that many of the constituent channels of a blood pattern have ephemeral importance. On the other hand, some of them persist as adult vessels with only minor modifications. During this same time, however, the heart itself undergoes marked alterations, changing from a relatively simple bulbous pump to a two-current pump with valves. These changes are sufficiently marked to be detected between successive stages in ordinary serial sections. Thus the structure of the heart can be included with advantage in listing the syndromic features that characterize developmental stages.
In earlier stages (compare figs. 11-7, 12-6, 12-7, 13- 3, and 13-4) the origin of the venous end of the heart (i.e., the sinus venosus and atria) has been traced from specialized parts of the vitelline plexus which form a vascular saddle astride the neck of the umbilical vesicle. From the very outset the arterial part of the heart, the part from which the ventricles are derived, is clearly demarcated from the venous part coming from the vitelline plexus. This is shown in figure 12-7. The arterial part of the heart is further illustrated in figure 15-4. Comparison of the four levels shown in the latter figure reveals that throughout that period one can speak of an arterial part that starts abruptly at the atrioventricular junction and curves rostrally to terminate in the aortic sac. The cardiac jelly is found mostly in the arterial part and fills the myo-endocardial interval. This layer appears to act as a closure cushion that facilitates the emptying of the tube and at the same time checks regurgitation from the aortic sac, biding the time when specialized cardiac valves will become established. In arriving at more-advanced stages the cardiac jelly acquires more cells, and one can then speak of it as a gelatinous reticulum or cardiac mesenchyme. In more-condensed form this may become converted into the semilunar valves, and it finally disappears in the formation of the atrioventricular valves and the membranous part of the interventricular septum.
At stage 15 the distribution of the thick layer of cardiac mesenchyme can be seen in typical sections selected from a sagittal series shown in figure 15-4. It still exists as a continuous sleeve around the arterial part of the tube, beginning at the atrioventricular canal and stopping before reaching the aortic sac. In figure 15-5, this part of the tube is shown at four levels of development. These drawings were made from three-dimensional reconstructions of the endocardial surfaces and therefore correspond to casts of the cavities of the heart. The form of the atria is largely influenced by the state of their contraction and consequent distention by the contained blood. The variation in the arterial part is less marked, and, with the gelatinous reticulum removed, its form is fairly constant for each developmental level. In passing from stage 13 to stage 15 it becomes relatively more voluminous, and its distended parts mark what are becoming the aortic and pulmonary channels.
A major feature of the heart at stage 15 is that the flow of blood through the atrioventricular canal is already divided into left and right streams (de Vries and Saunders, 1962), and continues as separate streams through the outflow tract and aortic sac (McBride, Moore, and Hutchins, 1981).
The aorticopulmonary septum, a controversial feature, is regarded by Los (1978) as “an artifact of two-dimensional histology” and merely a section through the condensed mesenchyme that forms the dorsal wall of the aortic sac.
Foramen secundum appears in septum primum from stage 15 to stage 17, and the semilunar cusps appear at the same time (McBride, Moore, and Hutchins, 1981). The level of the semilunar valves (at the dorsal bend of the outflow tract, according to de Vries and Saunders, 1962) has been disputed by some workers. The semilunar valves are said to be derived from the truncal ridges (Los, 1978) or cushions (Pexieder, 1982).
Other features of the heart at stage 15 are that the left ventricle is more voluminous and thicker-walled than the right (de Vries and Saunders, 1962). The lumen of the outflow tract is H-shaped. The atrioventricular cushions are opposed. The atrioventricular bundle has been detected (Mall, 1912).
Digestive System
(figs. 15-7 and 15-8) The thyroid primordium may be detached from the pharyngeal epithelium in some instances. The gland rests near the rostral border of the aortic sac.
The esophagus is longer and is straddled in front by the primary bronchi (fig. 15-8). From one stage to the next the intestine appears to become more slender. At least, the epithelial tube becomes steadily longer relative to its diameter.
The trabeculated parts of the ventricles show endocardial diverticula along the peripheral edge of the cardiac tube. These diverticula interlock with corresponding slender trabeculae from the overlying myocardium, as was illustrated in figures 13-6 and 13-7B, D. Thus in these two areas, instead of having a thick covering of gelatinous reticulum, the cardiac tube extends peripherally in the form of two trabeculated side-pouches which are the primordia of the right and left ventricles. These side pouches can be recognized as early as stage 11 while the cardiac tube is a simple channel. Contractions of the right ventricular sac would result in a current directed along the pulmonary border of the cardiac tube, whereas the left ventricular sac would favor a cross current toward the aortic border of the tube. It therefore seems possible that these two ventricular sacs may be factors in producing respectively the pulmonary and aortic currents.
The elongation of the ileum has produced a definite intestinal loop, which is more marked than the slight ventral deflection of the previous stage. A definite caecum marks the junction of ileum and colon. The separation of bladder from rectum has passed caudal to the ureters. It is to be stressed that the epithelial tube shown in figure 15-8 is surrounded throughout its length by a zone of condensed visceral mesenchyme, derived from the overlying coelomic epithelium.
The ventral pancreas, which, according to some reports, may be distinguishable as early as stage 13, is now seen as an evagination from the bile duct, and the dorsal pancreas is an elongated sac (fig. 15-8).
Respiratory System
(figs. 15-7, 15-8, and 15-10) The form of the trachea and primary bronchi at stage 15 is shown in figure 15-10. Lobar buds are developing and are becoming marked by focal swellings at the sites of the future secondary bronchi.
The close proximity of the caudal end of the trachea to the front wall of the esophagus at this time may be important in the elucidation of congenital anomalies (O'Rahilly and Müller, 1984c).
To determine the developmental status of the lung where no reconstructions have been made, one resorts to the microscopic appearance of the sections, and important advances can be recognized in that way. The progress in histogenesis is sufficiently rapid to enable one to distinguish between stages 13, 14, and 15, for instance, as is illustrated in figure 15-6. Comparing these three stages, one finds that in the least developed (stage 13) the coelomic epithelium is still actively shedding cells which are moving in toward the alimentary epithelium, forming a layer that envelops the esophagus and lung bud in a common coat of visceral mesenchyme, the primordium of the vascular, muscular, and supporting tissues of these structures. In stage 14 the shower of visceral mesenchyme has ceased and the residual coelomic epithelium is becoming a unicellular layer. Those cells that moved in as visceral mesenchyme begin to exhibit angiogenesis, and as this occurs the investment of the esophagus becomes marked off from the investment of the primary bronchi. The delineation of the esophageal field is aided by the circumstance that angiogenesis first occurs around its margins. It will be seen that a definite advance in differentiation of both pulmonary and esophageal epithelia is present in this group compared with the preceding one. Passing to stage 15, both the esophageal and the bronchial capillary plexuses mark off their respective fields. Forming basket-like networks around the primary bronchi, there is now a conspicuous pulmonary plexus with especially wide meshes between the bronchial tips and the atrial walls. In favorable specimens this can be seen to drain into the left atrium through a main channel, the common pulmonary vein, as shown by an arrow in specimen No. 721, figure 15-6. What is now left of the coelomic epithelium bordering the lung surface will be the source of the visceral pleura.
Urinary System
The ureteric bud is longer, and its tip is expanded as the pelvis of the ureter (fig. 15-10). The primary urogenital sinus is distinguishable.
Reproductive System
The gonadal ridges contain numerous germ cells. A basement membrane is lacking.
Nervous System
The nerve fibers of the dorsal funiculus extend rostral to C1. The dorsal roots of the cervical nerves consist predominantly of nerve fibers rather than cells.
The brain shown in figure 15-9 is about one-third larger in its overall dimensions than that illustrated for the previous stage.
The rhombomeres are still distinguishable. Between the rhombomeres, the rhombic grooves are crowded laterally and disappear in the sulcus limitans. The abducent nerves are constantly present. The geniculate ganglion begins to separate from the vestibulocochlear ganglion, and the latter now contains small dark cells which belong to the cochlear part. The cerebellum presents a marginal layer. In some embryos, nerve fibers are identifiable in the cerebellum. The area between rhombomere 2 and the mesencephalon increases greatly in length.
The mesencephalon still consists of two parts: M2 and M1. Between the two parts, a median button-like formation may be found in the roof. The roof of M1 is thinner than that of M2. The intracerebral root of the trochlear nerve is always present, and so is its decussation. The peripheral (extracerebral) part of the nerve may be very short. The commissure of the superior colliculi is present, whereas the posterior commissure is found in only the most advanced embryos of this stage.
The diencephalon exhibits a marginal layer almost throughout. The least advanced portions of the diencephalon are the future neurohypophysis and the dorsal thalamus. The primordium of the epiphysis can be distinguished as a slight median thickening. The habenular nucleus is the most developed part of the diencephalic roof. The nerve fibers it contains represent the beginning of the fasciculus retroflexus (habenulo-interpeduncular tract).
The future cerebral hemispheres are now better delineated. The medial striatal ridge (paleostriatum), of diencephalic origin, and the lateral striatal ridge (mainly neostriatum), telencephalic in source, can be distinguished.
The site of the optic evagination is becoming compressed as a slit between the corpus striatum and the optic chiasma. From this slit a thin strip of wall extends to the median plane, forming the optic groove (or preoptic recess), which constitutes a landmark between the striatal commissural thickening and the thickened ridge through which the chiasmatic fibers will pass when they arrive at the median plane. It is to be noted that in the design of the brain wall the pathway for crossing is laid down both for the optic decussation and for the anterior commissure before the nerve fibers themselves are present. In the most advanced embryos olfactory fibers reach the brain.
Most parts of the brain are surrounded by primary meninx (O'Rahilly and Müller, 1986b). The cellular material for the future sympathetic trunks is diffusely present in the cervical region.
Eye
(fig. 15-10) The lens pit has closed so that the lens vesicle is formed, although it may still be connected to the surface ectoderm. The lens is surrounded by its capsule. The lens body appears and consists of early lens fibers. The restored surface ectoderm constitutes the anterior epithelium of the future cornea, which possesses its own basement membrane. Retinal pigment appears in the external layer of the optic cup (O'Rahilly, 1966, fig. 29).
Ear
(fig. 15-10) The endolymphatic duct is more slender and the utriculo-endolymphatic fold is pronounced. In the most advanced embryos, slight grooves on the exterior of the membranous labryinth indicate the sites of the future semicircular ducts. Distinct nerve bundles in the areas of the ampullae of at least the anterior and posterior ducts are distinguishable. The otic capsule is represented by condensed mesenchyme.
Specimens of Stage 15 Already Described
- Carnegie No. 2, 7 mm. Described in detail by Mall (1891).
- Hochstetter's embryo I, 7 mm. Described in monographic form by Elze (1907). Includes attractive illustrations of reconstructions.
- Legg embryo, 7 mm. Described in detail by Thompson (1915). Includes illustrations of reconstructions.
- 8-mm embryo. The peripheral nervous system was described by Volcher (1963) in this embryo of stage 15.
- Keibel No. 1495, 8.5 mm. This advanced example of stage 15 was well described in detail by Barniville (1914)[1].
References
| Online Editor Note |
|---|
| O'Rahilly R. and Müller F. Developmental Stages in Human Embryos. Contrib. Embryol., Carnegie Inst. Wash. 637 (1987).
The original 1987 publication text, figures and tables have been altered in formatting, addition of internal online links, and links to PubMed. Original Document - Copyright © 1987 Carnegie Institution of Washington.
|
See also Müller F & O'Rahilly R. (1988). The development of the human brain, including the longitudinal zoning in the diencephalon at stage 15. Anat. Embryol. , 179, 55-71. PMID: 3213956
Search Pubmed
- 1987 Stages: Introduction | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | References | Appendix 1 | Appendix 2 | Historic Papers | Embryonic Development
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
Cite this page: Hill, M.A. (2024, April 26) Embryology 1987 Developmental Stages In Human Embryos - Stage 15. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/1987_Developmental_Stages_In_Human_Embryos_-_Stage_15
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G