User talk:Z3251292: Difference between revisions
No edit summary |
No edit summary |
||
| Line 1: | Line 1: | ||
=Technical Progression= | |||
Three-person ''in-vitro'' fertilization is the process where gene replacement exerted to prevent mitochondrial disease passing through generations. Main aproaches to achieve this goal involve the replacement of mitochondrial genome between gametes or embryos <ref><pubmed> 24382342</pubmed></ref> <ref><pubmed> 25573721 </pubmed></ref>. | |||
*The first proposed treatment is cytoplasmic transfer, which transfers a small part of ooplasm from one oocyte to another. however, this approach were then considered to be inadequate to prevent the inheritance of diseased mitochondrial. because it adds in donor mitochondria without removing the mutated mtDNA, which will then generate a 'heteroplasmic oocyte' with both mitochondria haplotypes <ref><pubmed> 24382342</pubmed></ref>. | |||
*New emerged approaches of mitochondrial transmission are pronuclear transfer(PNT), spindle transfer (ST) and Polar body transfer (PBT). however, none of this techniques have been proved on generating healthy human offspring due to the technical difficulty, as well as the ethics issues being recognized worldwide <ref><pubmed>24373414</pubmed></ref>. | |||
*Major breakthroughs of these techniques rely on the practice on animal models (mice and primate) <ref><pubmed> 25229667 </pubmed></ref>, early stage human embryo and stem cell studies <ref><pubmed> 23103869 </pubmed></ref>. | |||
Image Source: http://www.popsci.com.au/science/medicine/what-3parent-babies-mean-for-the-future-of-reproductive-medicine,400376 | |||
===Cytoplasmic transfer=== | |||
Cytoplasmic transfer is also named Oooplasmic transfer. It is an in vitro fertilization (IVF) technique, which can be performed either as a repair of oocyte or repair of Emryo <ref><pubmed> 24382342</pubmed></ref> <ref><pubmed> 24382342</pubmed></ref>. In method one, the cytoplasm is withdrew from a donor’s oocyte, and then injected into a patient’s oocyte together with the sperm cells which will then fertilize the oocyte. In Method two, the cytoplasm from donor is injected into a patient’s fertilized oocyte. | |||
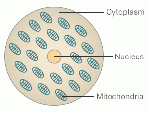
[[File:77260486_cell_structure_304.gif|150px|Simplified Cell Structure]] | |||
Simplified Cell Structure [http://www.bbc.com/news/magazine-28986843 (The girl with three biological parents)] | |||
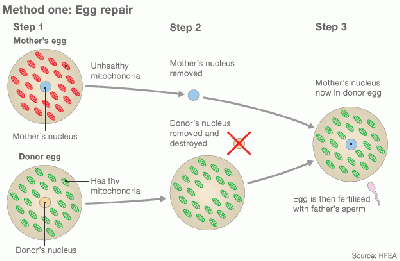
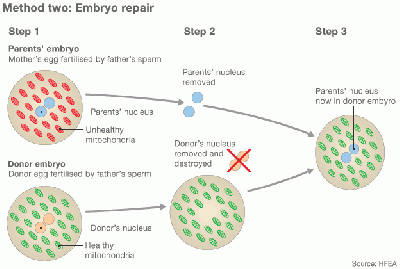
[[File:77266645 embryo repair 624 method 1.gif|400px| Egg repair by Cytoplasmic transfer]] [[File:77254175 embryo repair 624 method 2.gif|400px| Embryo repair by Cytoplasmic transfer]] | |||
Diagram of Cytoplasmic transfer [http://www.bbc.com/news/magazine-28986843 (The girl with three biological parents)] | |||
====Timeline ==== | |||
* '''1982, United Kingdom''' - Audrey Muggleton-Harris's group at MRC Laboratory Animals Center in Surrey, developed the technique and reported the first successful mammalian cytoplasmic transfer in mice. | |||
* '''1982 United Kingdon''' - Muggleton-Harris's group transferred cytoplasm from mice strains whose oocytes divide past the two-cell stage in vitro into mice to overcome the two-cell barrier | |||
* '''1997, United States''' - Jacques Cohen, Richard Scott, Tim Schimmel, Jacob Levron, and Steen Willadsen at the Institute for Reproductive Medicine and Science of St. Barnabas in West Orange, New Jersey, announced the birth of a baby girl after the first successful human cytoplasmic transfer <ref><pubmed> 9250192</pubmed></ref>. | |||
* '''1998 United States''' – The US Food and Drug Administration (FDA) banned the procedure. | |||
* '''2002 United States''' - one of the children conceived through ooplasmic transfer were diagnosed with pervasive developmental disorder, and indicated mild developmental delays to severe autism. | |||
* '''2014 United States''' - public meetings to discuss mitochondrial manipulation techniques were held by FDA. There was no formal decision made base on the efficacy of Cytoplasmic transfer, but agreements were made on further practice on animal models to provide scientific data. | |||
====Human Model==== | |||
Alana Saarinen is one of only 30 to 50 people in the world who was conceived through a pioneering infertility treatment in the USA. Part of her mitochondria, and mitochondria DNA were from a third person by Cytoplasmic transfer. [[http://www.bbc.com/news/magazine-28986843|BBCNEWS]] | |||
====Heteroplasmy==== | |||
Heteroplasmy is one of the major concerns arise regarding cytoplasmic transfer in IVF procedure. Severe disease can occur due to a mixture of mtDNA in the offspring’s mitochondria. They may affect the development of the muscle, brain and endocrine system. | |||
===Spindle-chromosome transfer=== | |||
Transfer of the metaphase II spindle from the unfertilized oocyte of an affected woman to an enucleated donor oocyte, spindle transfer between human oocytes, resulting in blastocyst development and embryonic stem cell derivation, with very low levels of heteroplasmy. | |||
'''(reproduced diagrams to be uploaded)''' | |||
====Primate model==== | |||
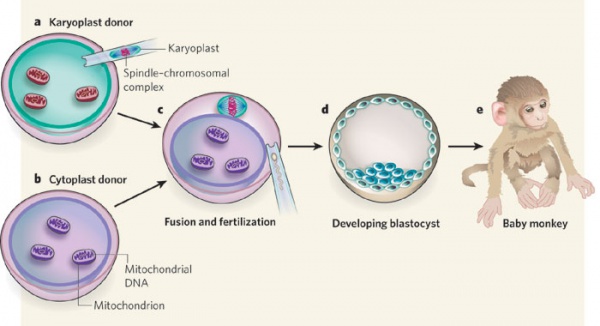
[[File:Swapping mitochondrial DNA mammalian oocytes.jpg|600px]] | |||
Spindle-chromosome transfer in primates supports normal development to adults and low mtDNA carryover.<ref><pubmed> 25573721 </pubmed></ref> | |||
====Current Research==== | |||
* PMID 23103867 '''Towards germline gene therapy of inherited mitochondrial diseases.''' | |||
Tachibana M, etc. investigated the spindle-chromosomal (ST) complex transfer on human oocytes. they concluded that the mtDNA can be efficiently replaced in human oocytes, although some ST oocytes displayed abnormal fertilization<ref><pubmed> 23103867</pubmed></ref>. | |||
* PMID 25973765 '''Extreme-Depth Re-sequencing of Mitochondrial DNA Finds No Evidence of Paternal Transmission in Humans''' | |||
Extreme-high depth mtDNA re-sequencing can perform up to ~1.2 million-fold coverage, Pyle A, etc. has proved the dogma that mammalian mitochondrial DNA (mtDNA) is strictly maternally inherited. It also indicated that an active mechanism eliminates paternal mtDNA which likely acts at the molecular level<ref><pubmed> 25973765 </pubmed></ref>. | |||
* PMID 19710649 '''Mitochondrial gene has been efficiently replaced by spindle-chromosomal complex transfer one egg to an enucleated, mitochondrial-replete egg in primate offspring and embryonic stem cells.''' | |||
None-human primate model were used for study the efficiency of spindle-chormosomal complex transfer.They reported that the reconstructed oocytes were capable of supporting normal fertilization, embryo development and produced healthy offspring. and there were no contribution of spindle donor mtDNA detected in offspring<ref><pubmed> 25973765 </pubmed></ref>. | |||
(Here are copy right and permission issues regarding reuse) | |||
===Pronuclear transfer=== | |||
Pronuclear transfer is performed as a repair of embryo. The nuclear genome from the pronuclear stage zygote of an affected woman is transferred to an enucleated donor zygote | |||
* It begins with creating an embryo using the parents’ sperm and eggs. | |||
* At the same time, a second embryo is created using a donor egg with healthy mitochondria and the father’s (or donor) sperm. | |||
* The pronuclei are removed from the single-cell stage embryo (day one). The leftover enucleated embryo with diseased mitochondria is discarded. | |||
* The pronuclei of the second embryo are removed and discarded. | |||
* The parents’ pronuclei can be placed into the second embryo for development. | |||
* The developed embryo will then be transferred into the mother. | |||
It is reported that pronuclear transfer is associated with high levels of mtDNA carryover in mice but low levels in human embryos, carries ethical issues secondary to donor embryo destruction <ref><pubmed> 25573721 </pubmed></ref> | |||
'''(reproduced diagrams to be uploaded)''' | |||
Diagram of Pronuclear transfer <ref><pubmed> 25377180</pubmed></ref> | |||
====Human Embryo Model==== | |||
PMID 20393463 '''Pronuclear transfer in human embryos to prevent transmission of mitochondrial DNA disease'''<ref><pubmed> 20393463 </pubmed></ref> | |||
As the name suggest this paper looks at pronuclear transfer as way to remove donor mitochondria measured by mt-DNA. And it effectiveness in doing so. And the processes that occur in the oocyte when this method is used | |||
====Current Research==== | |||
===Polar body transfer=== | |||
Polar bodies are small cells formed during the meiotic reductive division of the oocyte. they contains complementary choromosomes (to the mature oocyte) and small amount of cytoplasmic segregation<ref><pubmed> 24949971 </pubmed></ref>. | |||
* Polar body 1 is formed and released during ovulation. it contains a diploid set of chromosomes. | |||
* Polar body 2 is formed during fertilization and can be identified in the zygote. it contains a haploit set of chromosomes. | |||
* Both polar bodies are unable to be fertilized and disintegrate eventually | |||
It has been reported with mice modle that coupling Polar body transfer with Pronuclei transfer or Spindle-choromosome transfer may increase the yield of reconstructed embryos with low mtDNA carryover. <ref><pubmed> 25573721 </pubmed></ref> | |||
'''(reproduced diagrams to be uploaded)''' PMID 24949971 | |||
====Mice Model==== | |||
PMID 24949971 '''Polar body genome transfer for preventing the transmission of inherited mitochondrial diseases''' <ref><pubmed> 24949971 </pubmed></ref> | |||
The authur adopts polar body transfer to prevent the transmission of mtDNA variants. they also compare the effects of different types of germline genome transfer, including spindle-chromosome transfer, pronuclear transfer, and first and second polar body transfer, in mice. Their pre-clinical model indicate that polar body transfer has better potential in preventing the inheritance of mitochondrial diseases. | |||
====Current Research==== | |||
===Other approaches=== | |||
====germinal vesicle nuclear transfer==== | |||
=== endocrine organ=== | === endocrine organ=== | ||
Revision as of 16:18, 13 October 2015
Technical Progression
Three-person in-vitro fertilization is the process where gene replacement exerted to prevent mitochondrial disease passing through generations. Main aproaches to achieve this goal involve the replacement of mitochondrial genome between gametes or embryos [1] [2].
- The first proposed treatment is cytoplasmic transfer, which transfers a small part of ooplasm from one oocyte to another. however, this approach were then considered to be inadequate to prevent the inheritance of diseased mitochondrial. because it adds in donor mitochondria without removing the mutated mtDNA, which will then generate a 'heteroplasmic oocyte' with both mitochondria haplotypes [3].
- New emerged approaches of mitochondrial transmission are pronuclear transfer(PNT), spindle transfer (ST) and Polar body transfer (PBT). however, none of this techniques have been proved on generating healthy human offspring due to the technical difficulty, as well as the ethics issues being recognized worldwide [4].
- Major breakthroughs of these techniques rely on the practice on animal models (mice and primate) [5], early stage human embryo and stem cell studies [6].
Image Source: http://www.popsci.com.au/science/medicine/what-3parent-babies-mean-for-the-future-of-reproductive-medicine,400376
Cytoplasmic transfer
Cytoplasmic transfer is also named Oooplasmic transfer. It is an in vitro fertilization (IVF) technique, which can be performed either as a repair of oocyte or repair of Emryo [7] [8]. In method one, the cytoplasm is withdrew from a donor’s oocyte, and then injected into a patient’s oocyte together with the sperm cells which will then fertilize the oocyte. In Method two, the cytoplasm from donor is injected into a patient’s fertilized oocyte.
Simplified Cell Structure (The girl with three biological parents)
Diagram of Cytoplasmic transfer (The girl with three biological parents)
Timeline
- 1982, United Kingdom - Audrey Muggleton-Harris's group at MRC Laboratory Animals Center in Surrey, developed the technique and reported the first successful mammalian cytoplasmic transfer in mice.
- 1982 United Kingdon - Muggleton-Harris's group transferred cytoplasm from mice strains whose oocytes divide past the two-cell stage in vitro into mice to overcome the two-cell barrier
- 1997, United States - Jacques Cohen, Richard Scott, Tim Schimmel, Jacob Levron, and Steen Willadsen at the Institute for Reproductive Medicine and Science of St. Barnabas in West Orange, New Jersey, announced the birth of a baby girl after the first successful human cytoplasmic transfer [9].
- 1998 United States – The US Food and Drug Administration (FDA) banned the procedure.
- 2002 United States - one of the children conceived through ooplasmic transfer were diagnosed with pervasive developmental disorder, and indicated mild developmental delays to severe autism.
- 2014 United States - public meetings to discuss mitochondrial manipulation techniques were held by FDA. There was no formal decision made base on the efficacy of Cytoplasmic transfer, but agreements were made on further practice on animal models to provide scientific data.
Human Model
Alana Saarinen is one of only 30 to 50 people in the world who was conceived through a pioneering infertility treatment in the USA. Part of her mitochondria, and mitochondria DNA were from a third person by Cytoplasmic transfer. [[1]]
Heteroplasmy
Heteroplasmy is one of the major concerns arise regarding cytoplasmic transfer in IVF procedure. Severe disease can occur due to a mixture of mtDNA in the offspring’s mitochondria. They may affect the development of the muscle, brain and endocrine system.
Spindle-chromosome transfer
Transfer of the metaphase II spindle from the unfertilized oocyte of an affected woman to an enucleated donor oocyte, spindle transfer between human oocytes, resulting in blastocyst development and embryonic stem cell derivation, with very low levels of heteroplasmy.
(reproduced diagrams to be uploaded)
Primate model
Spindle-chromosome transfer in primates supports normal development to adults and low mtDNA carryover.[10]
Current Research
- PMID 23103867 Towards germline gene therapy of inherited mitochondrial diseases.
Tachibana M, etc. investigated the spindle-chromosomal (ST) complex transfer on human oocytes. they concluded that the mtDNA can be efficiently replaced in human oocytes, although some ST oocytes displayed abnormal fertilization[11].
- PMID 25973765 Extreme-Depth Re-sequencing of Mitochondrial DNA Finds No Evidence of Paternal Transmission in Humans
Extreme-high depth mtDNA re-sequencing can perform up to ~1.2 million-fold coverage, Pyle A, etc. has proved the dogma that mammalian mitochondrial DNA (mtDNA) is strictly maternally inherited. It also indicated that an active mechanism eliminates paternal mtDNA which likely acts at the molecular level[12].
- PMID 19710649 Mitochondrial gene has been efficiently replaced by spindle-chromosomal complex transfer one egg to an enucleated, mitochondrial-replete egg in primate offspring and embryonic stem cells.
None-human primate model were used for study the efficiency of spindle-chormosomal complex transfer.They reported that the reconstructed oocytes were capable of supporting normal fertilization, embryo development and produced healthy offspring. and there were no contribution of spindle donor mtDNA detected in offspring[13]. (Here are copy right and permission issues regarding reuse)
Pronuclear transfer
Pronuclear transfer is performed as a repair of embryo. The nuclear genome from the pronuclear stage zygote of an affected woman is transferred to an enucleated donor zygote
- It begins with creating an embryo using the parents’ sperm and eggs.
- At the same time, a second embryo is created using a donor egg with healthy mitochondria and the father’s (or donor) sperm.
- The pronuclei are removed from the single-cell stage embryo (day one). The leftover enucleated embryo with diseased mitochondria is discarded.
- The pronuclei of the second embryo are removed and discarded.
- The parents’ pronuclei can be placed into the second embryo for development.
- The developed embryo will then be transferred into the mother.
It is reported that pronuclear transfer is associated with high levels of mtDNA carryover in mice but low levels in human embryos, carries ethical issues secondary to donor embryo destruction [14]
(reproduced diagrams to be uploaded)
Diagram of Pronuclear transfer [15]
Human Embryo Model
PMID 20393463 Pronuclear transfer in human embryos to prevent transmission of mitochondrial DNA disease[16] As the name suggest this paper looks at pronuclear transfer as way to remove donor mitochondria measured by mt-DNA. And it effectiveness in doing so. And the processes that occur in the oocyte when this method is used
Current Research
Polar body transfer
Polar bodies are small cells formed during the meiotic reductive division of the oocyte. they contains complementary choromosomes (to the mature oocyte) and small amount of cytoplasmic segregation[17].
- Polar body 1 is formed and released during ovulation. it contains a diploid set of chromosomes.
- Polar body 2 is formed during fertilization and can be identified in the zygote. it contains a haploit set of chromosomes.
- Both polar bodies are unable to be fertilized and disintegrate eventually
It has been reported with mice modle that coupling Polar body transfer with Pronuclei transfer or Spindle-choromosome transfer may increase the yield of reconstructed embryos with low mtDNA carryover. [18]
(reproduced diagrams to be uploaded) PMID 24949971
Mice Model
PMID 24949971 Polar body genome transfer for preventing the transmission of inherited mitochondrial diseases [19] The authur adopts polar body transfer to prevent the transmission of mtDNA variants. they also compare the effects of different types of germline genome transfer, including spindle-chromosome transfer, pronuclear transfer, and first and second polar body transfer, in mice. Their pre-clinical model indicate that polar body transfer has better potential in preventing the inheritance of mitochondrial diseases.
Current Research
Other approaches
germinal vesicle nuclear transfer
endocrine organ
PMID 26362096
ETM transition
PMID 20943648 PMID 21559368 PMID 21447558
Talk
- Timeline of The artificial reproductive technology revolution
- 1984 donor egg pregnancies
- 1984 frozen embryos
- 1960 Ovulation induction
- 1960 Donor sperm
- 1978 IVF
- 1988 Pre-implantation genetic diagnosis
- 1992 Sperm injection
- 1994 Blastocyst development
- 1998 Reliable sperm sex selection
- 2000 Stem cells and cloning and gene transfer
- 2005 Ovarian reserve testing
- 2008 Oocyte freezing, social freezing
- 2013 Whole genome screening of embryos
- Process of IVF
- Ovarian hyperstimulation
- two phase during menstrual cycle: lacteal phase and follicular phase
- About 30 Follicles grows to large size in every mentrual cycle, but only one survived to the ovulation.
- FSH drove the growth of follicles, also control the growth of follicles (pituitary down regulation), used to stimulate the mature of multiple follicles (10-30).
- Estrogen....
- Monitoring by transvirgena ultrasound.
- LH containing hormones injected to women to stimulate ovulation.
- Ovarian hyperstimulation
- Ooocyte Pick-Up(OPU)
- Sperm retrieval via ejaculation or surgery
- In Vitro Fertilisation
- intra-cytoplasmic sperm injection (ICSI)
- Embryo culture
- day1- form Zygote
- day3- divide to 8 blastomeres
- day5- frozen, and transfer to uterus
- Transfer of the embryo(s)
- New technologies
- Preimplantation genetic diagnosis(PGD) of embryo. eg, fibrosis-premature death
- Embryo Biopsy for PGD
- analysis of blastomeres for PGD
- FISH
- PCR
- What to be detected in an embryo
- Aneuploidy
- trisomy 21
- translocations
- single gene defects(eg cystic fibrosis)
- Ovarian reserve and the natural cycle
- blood test measure hormones(AMH, AFC)
- Egg banking ('social freezing')
- Fertility preservation for cancer
- Oocyte in vitro maturation (IVM)
- putting oocytes in vitro and culture 24 hours before IVF (Complex medium , protein, FSH, EGF, LH, E2 cysteamine)
- comparing to conventional IVF, no Hormones required to give to mother for the maturation of oocyte.
- Preimplantation genetic diagnosis(PGD) of embryo. eg, fibrosis-premature death
- ↑ <pubmed> 24382342</pubmed>
- ↑ <pubmed> 25573721 </pubmed>
- ↑ <pubmed> 24382342</pubmed>
- ↑ <pubmed>24373414</pubmed>
- ↑ <pubmed> 25229667 </pubmed>
- ↑ <pubmed> 23103869 </pubmed>
- ↑ <pubmed> 24382342</pubmed>
- ↑ <pubmed> 24382342</pubmed>
- ↑ <pubmed> 9250192</pubmed>
- ↑ <pubmed> 25573721 </pubmed>
- ↑ <pubmed> 23103867</pubmed>
- ↑ <pubmed> 25973765 </pubmed>
- ↑ <pubmed> 25973765 </pubmed>
- ↑ <pubmed> 25573721 </pubmed>
- ↑ <pubmed> 25377180</pubmed>
- ↑ <pubmed> 20393463 </pubmed>
- ↑ <pubmed> 24949971 </pubmed>
- ↑ <pubmed> 25573721 </pubmed>
- ↑ <pubmed> 24949971 </pubmed>