User:Z3418981: Difference between revisions
| Line 62: | Line 62: | ||
<pubmed>25128525</pubmed> | <pubmed>25128525</pubmed> | ||
<pubmed>24397701</pubmed> | <pubmed>24397701</pubmed> | ||
===LAB 4 ASSESSMENT=== | |||
1. Identify a paper that uses cord stem cells therapeutically and write a brief (2-3 paragraph) description of the paper's findings. | |||
<pubmed>24104453</pubmed> | |||
The inhibitory effect of human umbilical cord-derived mesenchymal stem cells (hUC-MSCs) on the growth of C6 glioma cells was investigated in this study. Initially C6 cells were cultured with different concentrations of hUC-MSCs in order to examine whether the hUC-MSCs inhibition of glioma cell growth was mediated by soluble factors. It was found that hUCMSCs-CM exhibited a concentration-dependent inhibitory effect on C6 cell growth which in turn suggested that soluble factors in the conditioned media from hUC-MSCs were responsible for the inhibition of C6 glioma cells. Subsequently, flow cytometric analysis was used to test the effect of the soluble factors derived from hUCMSCs-CM on the cell cycle of glioma cells. Using this method, cell cycle status of C6 cells treated with different concentrations of hUCMSCs-CM was identified. It was observed that C6 cells treated with hUCMSCs-CM showed increases in the G0/G1 phase and reductions in the S phase compared to the control group (0 % hUCMSCs). These results suggested that hUC-MSCs could potentially inhibit the growth of C6 glioma cells and stop the cell cycle at the G0/G1 phase by secreting some soluble factors. | |||
Western blot analysis was then performed and it was observed that the expression levels of β-catenin and c-Myc in C6 cells were reduced in the conditioned media derived from hUC-MSCs. These results indicated that some soluble factors secreted from hUCMSCs-CM may play a role in the inhibition of Wnt signaling pathway in C6 cells. Further investigations demonstrated that the secretion levels of dickkopf-1 (DKK1) were positively correlated with the concentrations of hUCMSCs-CM. Subsequently, the hypothesis that stem cells secreted Wnt inhibitors, such as DKK1, which could inhibit the Wnt signaling in tumor cells, was made. | |||
Neutralizing antibody against DKK1 was added to the hUCMSCs-CM in order to further confirm that DKK1 is a key factor in the inhibitory effect of hUCMSCs on C6 cell proliferation. It was observed that the inhibitory effect of hUC-MSCs on C6 cells was weakened when DKK1 was neutralized by anti-DKK1 antibody. Moreover, it was found that conditioned media from hUC-MSCs transfection with siRNA targeting DKK1 mRNA altered the regulation of the Wnt signaling in C6 cells. Therefore, it was concluded that hUC-MSCs inhibited C6 glioma cell growth by secreting DKK1, an inhibitor of Wnt pathway. This finding may introduce a novel therapeutic strategy for malignant glioma. | |||
Revision as of 01:13, 3 September 2014
Lab Attendance
Lab 1 --Z3418981 (talk) 12:45, 6 August 2014 (EST) http://www.ncbi.nlm.nih.gov/pubmed PubMed PMID25084016 <pubmed>25084016</pubmed> Lab 2 --Z3418981 (talk) 12:17, 13 August 2014 (EST) Lab 3--Z3418981 (talk) 12:58, 20 August 2014 (EST) Lab 4--Z3418981 (talk) 11:48, 27 August 2014 (EST)
Individual Assessments
LAB 1 ASSESSMENT
Reference: PMID24726222
<pubmed>24726222</pubmed>
Summary of the Method
In this study, genomic DNA was extracted from the umbilical cord blood of a total of 185 newborn females. Patients included 60 infants conceived by intracytoplasmic sperm injection (ICSI) and 73 infants conceived by in vitro fertilization (IVF) all recruited from a number of IVF centers across Canada. In addition, 52 naturally conceived patients were recruited from hospitals across the Lower Mainland in British Columbia, Canada. A karyotype or comparative genomic hybridization (CGH) analysis of the chromosomes was performed for all newborn cases. Cases were not included if congenital and/or chromosome abnormalities were present.
The X-chromosome inactivation (XCI) assay was performed to determine the XCI skewing of different tissues in different parts of the placenta by assaying allelic ratio of methylated alleles at the androgen receptor(AR), fragile X mental retardation 1 (FMR1), and DXS6673E loci. Fisher's exact test was a statistical method used to compare the frequency of mildly skewed (≥75%) and extremely skewed (≥90%) XCI in the patients. The parental nature of the skewed allele was determined by automated fluorescence analysis which was used to measure the AR alleles of the maternal decidua of the placenta.
Summary of the Results
There was no statistically significant difference between the ICSI, IVF and NC populations in the frequency of skewing ≥75% (7.0% vs. 5.7% vs. 2.0%, respectively; P=.523) or ≥ 90% (0 vs. 1.4% vs. 2.0%, respectively; P=.747). The mean level of skewing between the ICSI, IVF, and ICSI groups also was not significantly different (63.7% vs. 61.8% vs. 60.7%, respectively). Only two samples were found to have extremely skewed cases (≥90% skewing): one IVF (89.6%) and one NC (90.6%). The parental origin of the preferentially inactivated X chromosome in these extremely skewed cases was maternal for IVF and paternal for NC case.
Reference: PMID24399508
<pubmed>24399508</pubmed>
Summary of the Method
All of the pregnancies conceived by in vitro fertilization in Denmark from 1995 to 2005 (n = 18 787) was included in this study using the data reported to the National In Vitro Fertilisation register (IVF register). Information about the pregnancy outcomes as well as cycle-specific information on the type and date of treatment, and the occurrence of pregnancy, abortions and deliveries was also obtained from IVF.
A study published by Virkus et al. on venous thromboembolism in pregnant and puerperal women in Denmark was used as a reference (Virkus et al., 2011). This study was used as a reference since the population used in this study (727 VTE patients among the 805 464 pregnancies recorded in the Danish National Patient Registry from 1995 to 2005) is ideal and comparable to the present study. Consequently, venous thrombosis incidence rates in pregnancies conceived by in vitro fertilization were compared with venous thrombosis incidence rates in reference pregnancies, by calculating incidence rate ratios.
Summary of the Results
The venous thrombosis incidence was significantly increased in pregnancies after in vitro fertilization. The overall ratio of venous thrombosis incidence rate during in vitro fertilization pregnancies to reference pregnancies was 3.0 (95% CI 2.1–4.3). The overall venous thrombosis incidence rate was 28.6 per 10 000 pregnancy-years (95% confidence interval (CI) 20.6–39.6) for pregnancies after in vitro fertilization compared to 10.7 per 10 000 woman-years in reference pregnancies.
Reference used in the "Summary of the Method" section:
<pubmed>21713323</pubmed>
LAB 2 ASSESSMENT
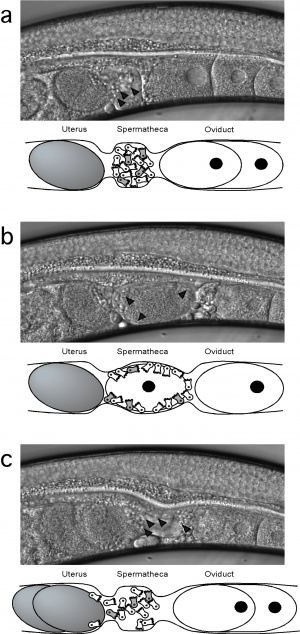
Image showing the process of fertilisation in mutant C. elegans[1]
--Mark Hill (talk) 16:19, 21 August 2014 (EST) This is all correct. The image is very large (1.15 MB), perhaps a smaller image version could have been uploaded. You can adjust the resolution and size in most image editing programs.
- ↑ <pubmed>15086962</pubmed>|BMC Developmental Biology
LAB 3 ASSESSMENT
Abnormalities associated with neural development
<pubmed>12454899</pubmed> <pubmed>25007063</pubmed> <pubmed>16530991</pubmed> <pubmed>7504639</pubmed> <pubmed>19651588</pubmed> <pubmed>25135350</pubmed> <pubmed>25128525</pubmed> <pubmed>24397701</pubmed>
LAB 4 ASSESSMENT
1. Identify a paper that uses cord stem cells therapeutically and write a brief (2-3 paragraph) description of the paper's findings. <pubmed>24104453</pubmed> The inhibitory effect of human umbilical cord-derived mesenchymal stem cells (hUC-MSCs) on the growth of C6 glioma cells was investigated in this study. Initially C6 cells were cultured with different concentrations of hUC-MSCs in order to examine whether the hUC-MSCs inhibition of glioma cell growth was mediated by soluble factors. It was found that hUCMSCs-CM exhibited a concentration-dependent inhibitory effect on C6 cell growth which in turn suggested that soluble factors in the conditioned media from hUC-MSCs were responsible for the inhibition of C6 glioma cells. Subsequently, flow cytometric analysis was used to test the effect of the soluble factors derived from hUCMSCs-CM on the cell cycle of glioma cells. Using this method, cell cycle status of C6 cells treated with different concentrations of hUCMSCs-CM was identified. It was observed that C6 cells treated with hUCMSCs-CM showed increases in the G0/G1 phase and reductions in the S phase compared to the control group (0 % hUCMSCs). These results suggested that hUC-MSCs could potentially inhibit the growth of C6 glioma cells and stop the cell cycle at the G0/G1 phase by secreting some soluble factors. Western blot analysis was then performed and it was observed that the expression levels of β-catenin and c-Myc in C6 cells were reduced in the conditioned media derived from hUC-MSCs. These results indicated that some soluble factors secreted from hUCMSCs-CM may play a role in the inhibition of Wnt signaling pathway in C6 cells. Further investigations demonstrated that the secretion levels of dickkopf-1 (DKK1) were positively correlated with the concentrations of hUCMSCs-CM. Subsequently, the hypothesis that stem cells secreted Wnt inhibitors, such as DKK1, which could inhibit the Wnt signaling in tumor cells, was made. Neutralizing antibody against DKK1 was added to the hUCMSCs-CM in order to further confirm that DKK1 is a key factor in the inhibitory effect of hUCMSCs on C6 cell proliferation. It was observed that the inhibitory effect of hUC-MSCs on C6 cells was weakened when DKK1 was neutralized by anti-DKK1 antibody. Moreover, it was found that conditioned media from hUC-MSCs transfection with siRNA targeting DKK1 mRNA altered the regulation of the Wnt signaling in C6 cells. Therefore, it was concluded that hUC-MSCs inhibited C6 glioma cell growth by secreting DKK1, an inhibitor of Wnt pathway. This finding may introduce a novel therapeutic strategy for malignant glioma.