File:Listeria maternal-fetal barrier.jpg: Difference between revisions
No edit summary |
No edit summary |
||
| Line 1: | Line 1: | ||
Model of L. monocytogenes mechanisms for breaching the maternal-fetal barrier. | '''Model of ''L. monocytogenes'' mechanisms for breaching the maternal-fetal barrier.''' | ||
L. monocytogenes is subjected to multiple bottlenecks when infecting the placenta. Our data support extravillous cytotrophoblasts (EVT) as the primary portal of entry. | L. monocytogenes is subjected to multiple bottlenecks when infecting the placenta. Our data support extravillous cytotrophoblasts (EVT) as the primary portal of entry. | ||
(1), Relatively few ''L. monocytogenes'' reach the maternal decidua, carried by phagocytes. These can infect extravillous cytotrophoblasts by cell-to-cell spread, or by lysing the leukocyte and subsequently infecting extravillous cytotrophoblasts via InlA-E-cadherin interactions. | |||
(2) Extravillous cytotrophoblasts further winnow bacterial numbers by delaying the L. monocytogenes intracellular life cycle. If infection progresses, bacteria spread through subsyncytial cytotrophoblasts. | (2) Extravillous cytotrophoblasts further winnow bacterial numbers by delaying the ''L. monocytogenes'' intracellular life cycle. If infection progresses, bacteria spread through subsyncytial cytotrophoblasts. | ||
(3) The basement membrane underlying these cells presents a third barrier, which few bacteria cross to invade the fetal stroma (STR). On the other hand, L. monocytogenes in the blood contacts only the syncytiotrophoblast (SYN), which is highly resistant to both internalin-mediated infection and intercellular spread. However, it is possible that sites of syncytial damage provide access to subsyncytial cytotrophoblasts. In vivo, such sites are rapidly covered by fibrinoid clots [28] that may present yet another physical barrier to infection. | (3) The basement membrane underlying these cells presents a third barrier, which few bacteria cross to invade the fetal stroma (STR). On the other hand, ''L. monocytogenes'' in the blood contacts only the syncytiotrophoblast (SYN), which is highly resistant to both internalin-mediated infection and intercellular spread. However, it is possible that sites of syncytial damage provide access to subsyncytial cytotrophoblasts. In vivo, such sites are rapidly covered by fibrinoid clots [28] that may present yet another physical barrier to infection. | ||
Placental syncytiotrophoblast constitutes a major barrier to vertical transmission of Listeria monocytogenes. Robbins JR, Skrzypczynska KM, Zeldovich VB, Kapidzic M, Bakardjiev AI. PLoS Pathog. 2010 Jan 22;6(1):e1000732. [http://www.ncbi.nlm.nih.gov/pubmed/20107601 PMID: 20107601] | [http://www.plospathogens.org/article/info%3Adoi%2F10.1371%2Fjournal.ppat.1000732 PLoS] | Placental syncytiotrophoblast constitutes a major barrier to vertical transmission of Listeria monocytogenes. Robbins JR, Skrzypczynska KM, Zeldovich VB, Kapidzic M, Bakardjiev AI. PLoS Pathog. 2010 Jan 22;6(1):e1000732. [http://www.ncbi.nlm.nih.gov/pubmed/20107601 PMID: 20107601] | [http://www.plospathogens.org/article/info%3Adoi%2F10.1371%2Fjournal.ppat.1000732 PLoS] | ||
Revision as of 00:15, 22 April 2010
Model of L. monocytogenes mechanisms for breaching the maternal-fetal barrier.
L. monocytogenes is subjected to multiple bottlenecks when infecting the placenta. Our data support extravillous cytotrophoblasts (EVT) as the primary portal of entry.
(1), Relatively few L. monocytogenes reach the maternal decidua, carried by phagocytes. These can infect extravillous cytotrophoblasts by cell-to-cell spread, or by lysing the leukocyte and subsequently infecting extravillous cytotrophoblasts via InlA-E-cadherin interactions.
(2) Extravillous cytotrophoblasts further winnow bacterial numbers by delaying the L. monocytogenes intracellular life cycle. If infection progresses, bacteria spread through subsyncytial cytotrophoblasts.
(3) The basement membrane underlying these cells presents a third barrier, which few bacteria cross to invade the fetal stroma (STR). On the other hand, L. monocytogenes in the blood contacts only the syncytiotrophoblast (SYN), which is highly resistant to both internalin-mediated infection and intercellular spread. However, it is possible that sites of syncytial damage provide access to subsyncytial cytotrophoblasts. In vivo, such sites are rapidly covered by fibrinoid clots [28] that may present yet another physical barrier to infection.
Placental syncytiotrophoblast constitutes a major barrier to vertical transmission of Listeria monocytogenes. Robbins JR, Skrzypczynska KM, Zeldovich VB, Kapidzic M, Bakardjiev AI. PLoS Pathog. 2010 Jan 22;6(1):e1000732. PMID: 20107601 | PLoS
Citation: Robbins JR, Skrzypczynska KM, Zeldovich VB, Kapidzic M, Bakardjiev AI (2010) Placental Syncytiotrophoblast Constitutes a Major Barrier to Vertical Transmission of Listeria monocytogenes. PLoS Pathog 6(1): e1000732. doi:10.1371/journal.ppat.1000732
Editor: David S. Schneider, Stanford University, United States of America
Received: September 29, 2009; Accepted: December 18, 2009; Published: January 22, 2010
Copyright: © 2010 Robbins et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
File history
Click on a date/time to view the file as it appeared at that time.
| Date/Time | Thumbnail | Dimensions | User | Comment | |
|---|---|---|---|---|---|
| current | 00:03, 22 April 2010 | 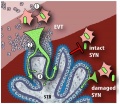 | 600 × 522 (49 KB) | S8600021 (talk | contribs) | Model of L. monocytogenes mechanisms for breaching the maternal-fetal barrier. L. monocytogenes is subjected to multiple bottlenecks when infecting the placenta. Our data support extravillous cytotrophoblasts (EVT) as the primary portal of entry. First ( |
You cannot overwrite this file.
File usage
The following 4 pages use this file: