File:Human embryo thymus and parathyroid 01.jpg
Original file (639 × 1,000 pixels, file size: 58 KB, MIME type: image/jpeg)
Human Embryo Thymus and Parathyroid
Ectopic parathyroids are present from week 7 in the human embryo
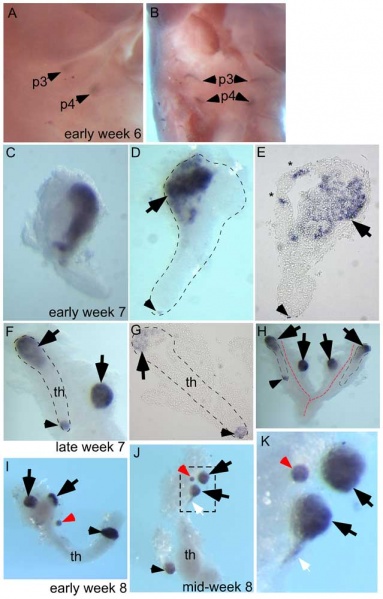
Whole-mount in situ hybridization for GCM2 (A–D, F, H–K) and whole-mounts embedded in paraffin and sectioned after in situ hybridization (E, G). Ages of embryos or dissected parathyroid/thymus primordia in the lower right corner of A, C, and F apply to the entire row; age in J also applies to K.
The entire thymus/parathyroid common primoridum is outlined in D, F, G, and H. In panels D–K, black arrows show presumptive primary parathyroids, small arrowheads indicate GCM2-positive clusters at the posterior end of the thymus, and red arrows show probable accessory parathyroids. White arrows in J and K show trailing GCM2-positive cells.
(A, B) Side (A) and frontal (B) views show GCM2 expression in the 3rd and 4th pharyngeal pouches in an early week 6 embryo (2/2 embryos).
(C–E) Images of whole-mount (C, D) and paraffin sectioned (E, section from D) early to mid week 7 dissected parathyroid/thymus common primordia showing GCM2 expressing cells present in the anterior (4/4 embryos) and posterior tip (1/4 embryos) of the common primordia. Small ectopic clusters away from the main cluster (*) are also often present by this stage.
(F) Image shows separation of parathyroids from the common primordia is occurring by late week 7 (right arrow; 1/1 embryo; the posterior parathyroid was present in only one of the two common primordia in this embryo).
(G) Paraffin section of whole mount shown in F. (H) Whole mount showing bilateral primordia (outlined) and carotid artery complex (red dashed line), with four main parathyroids and one small parathyroid cluster on the posterior tip of one thymic lobe.
(I, J) Thymic lobes and surrounding parathyroids from one side each of two separate early week 8 embryos show Gcm2 expression by three apparent primary parathyroids and a smaller accessory parathyroid (red arrow; 3/3 embryos; in each case the thymus/parathyroid primordium was examined from only one side of the embryo). The white arrow points to Gcm2 expressing cells that are still attached to the thymic domain.
(K) higher magnification of the region boxed in J. pt, parathyroid; th, thymus; p3, 3rd pharyngeal pouch; p4, 4th pharyngeal pouch.
Reference
<pubmed>21203493</pubmed>| PLoS Genet.
Copyright: © 2010 Liu et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Figure 4. Journal.pgen.1001251.g004.jpg
doi:info:doi/10.1371/journal.pgen.1001251.g004
Thymus-associated parathyroid hormone has two cellular origins with distinct endocrine and immunological functions
PLoS Genet. 2010 Dec 23;6(12):e1001251.
Liu Z, Farley A, Chen L, Kirby BJ, Kovacs CS, Blackburn CC, Manley NR. Source Department of Genetics, University of Georgia, Athens, Georgia, United States of America.
Abstract
In mammals, parathyroid hormone (PTH) is a key regulator of extracellular calcium and inorganic phosphorus homeostasis. Although the parathyroid glands were thought to be the only source of PTH, extra-parathyroid PTH production in the thymus, which shares a common origin with parathyroids during organogenesis, has been proposed to provide an auxiliary source of PTH, resulting in a higher than expected survival rate for aparathyroid Gcm2⁻/⁻ mutants. However, the developmental ontogeny and cellular identity of these "thymic" PTH-expressing cells is unknown. We found that the lethality of aparathyroid Gcm2⁻/⁻ mutants was affected by genetic background without relation to serum PTH levels, suggesting a need to reconsider the physiological function of thymic PTH. We identified two sources of extra-parathyroid PTH in wild-type mice. Incomplete separation of the parathyroid and thymus organs during organogenesis resulted in misplaced, isolated parathyroid cells that were often attached to the thymus; this was the major source of thymic PTH in normal mice. Analysis of thymus and parathyroid organogenesis in human embryos showed a broadly similar result, indicating that these results may provide insight into human parathyroid development. In addition, medullary thymic epithelial cells (mTECs) express PTH in a Gcm2-independent manner that requires TEC differentiation and is consistent with expression as a self-antigen for negative selection. Genetic or surgical removal of the thymus indicated that thymus-derived PTH in Gcm2⁻/⁻ mutants did not provide auxiliary endocrine function. Our data show conclusively that the thymus does not serve as an auxiliary source of either serum PTH or parathyroid function. We further show that the normal process of parathyroid organogenesis in both mice and humans leads to the generation of multiple small parathyroid clusters in addition to the main parathyroid glands, that are the likely source of physiologically relevant "thymic PTH."
PMID 21203493
http://www.plosgenetics.org/article/info%3Adoi%2F10.1371%2Fjournal.pgen.1001251
File history
Click on a date/time to view the file as it appeared at that time.
| Date/Time | Thumbnail | Dimensions | User | Comment | |
|---|---|---|---|---|---|
| current | 17:32, 21 May 2012 |  | 639 × 1,000 (58 KB) | Z8600021 (talk | contribs) | ==Human Embryo Thymus and Parathyroid== Ectopic parathyroids are present from week 7 in the human embryo Whole-mount in situ hybridization for GCM2 (A–D, F, H–K) and whole-mounts embedded in paraffin and sectioned after in situ hybridization (E, G). |
You cannot overwrite this file.
File usage
The following 2 pages use this file: