|
|
| Line 1: |
Line 1: |
| {{Header}}
| |
| =Muscle Development =
| |
|
| |
|
| == Introduction == | | == '''1. QUIZ''' == |
| [[File:Skeletal muscle structure cartoon.jpg|thumb|Skeletal Muscle]]
| |
| This laboratory concerns the development and differentiation of skeletal muscle, fibre type differentiation, muscle stem cells and muscle disease.
| |
|
| |
|
| The histology of skeletal muscle has been covered in ANAT2241.
| |
|
| |
|
| | == '''2. Guest Lecturer - Hongjun Shi (VCCRI) - "Somitogenesis and congenital vertebral malformation"''' == |
| | [[File:Hongjun_Shi_profile_photo.jpg]] |
|
| |
|
| ==Objectives==
| | Dr Shi’s research is focused on genetic regulation of the somitogenesis and identification of genetic and environmental factors that cause congenital vertebral malformation. |
|
| |
|
| # Understand the origin, differentiation and development of skeletal muscle tissue.
| | Introduction |
| # Know what is meant by patterning, conversion and adult plasticity of muscle fibre type.
| | The defining feature of vertebrates is the vertebral column which is composed of a series of structurally similar bone units – vertebrae along the body axis. The segmental pattern of the vertebral column is established during early embryo development when the somites are rhythmically produced from the paraxial mesoderm (Bailey and Dale, 2001). Somitogenesis requires the interaction of two components, referred to as the clock and wavefront (Aulehla and Pourquie, 2010; Pourquie, 2011). The wavefront (determination front) determines the future somite boundary and is created by opposing gradients of FGF and Wnt signaling (caudal-rostral) and retinoic acid signaling (rostral-caudal) in the presomitic mesoderm (PSM). At the same time, PSM cells express a number of genes in the FGF, Wnt and Notch signaling pathway in an oscillatory pattern (the clock), and bands of expression appear to move in a caudal-to-rostral direction. When the periodic signal reaches the determination front, a somite can form. Disruption of somitogenesis by genetic mutations results in vertebral defects. For example, in humans and mice, homozygous mutation of the DLL3, LFNG, MESP2, HES7 and RIPPLY2 (Pourquie, 2011) (McInerney-Leo et al., 2015) which are either components or downstream targets of Notch signalling leads to extensive vertebral malformation. |
| # Develop an understanding of research methods for studying skeletal muscle abnormalities.
| |
|
| |
|
| ==Muscle Contraction==
| |
| {|
| |
| ! Crossbridge animation
| |
| ! Component proteins
| |
| |-
| |
| | [[File:Actin_myosin_crossbridge_3D_animation.gif|link=File:Actin myosin crossbridge 3D animation.gif]]
| |
| | [[File:Actin_myosin_crossbridge_3D_animation.jpg|link=File:Actin myosin crossbridge 3D animation.gif]]
| |
| |-
| |
| ! Sarcomere Animation
| |
| ! Muscle Histology
| |
| |-
| |
| | [[File:Sarcomere_animation.gif]]
| |
| | [[File:Skeletal muscle histology 016.jpg|350px]]
| |
| |}
| |
|
| |
|
| ==Sarcomere - Electron Microscopy==
| | [[File:Hongjun_Shi_Research_photo01.jpg]] |
| {|
| |
| ! Virtual Slides
| |
| |-
| |
| | valign=bottom|{{SlideSkeletalMuscleEM01}}
| |
| | valign=bottom|{{SlideSkeletalMuscleEM02}}
| |
| | valign=bottom|{{SlideSkeletalMuscleEM03}}
| |
| |-
| |
| |}
| |
|
| |
|
| [http://www.lab.anhb.uwa.edu.au/mb140/CorePages/Muscle/Muscle.htm#SKELETAL Skeletal Muscle Histology]
| | ''Deletion of Dll3 gene casues failure of Notch1 signalling to restrict to a thin stripe of cells. In stead a broad rostral domain of Notch1 signalling is observed (Chapman et al., 2011).'' |
|
| |
|
| | In addition to genetic mutations, environmental insults during embryo development may also interfere with somitogenesis. For example, hypoxia during pregnancy can inhibit FGF signaling in the PSM and cause segmentation defects in mice. Heterozygous mutation in the Notch signaling genes increase the susceptibility to segmentation defects when the mice are exposed to mild hypoxia (Sparrow et al., 2012). |
|
| |
|
| ==Muscle Fibre Types==
| |
| {|
| |
| | width=410px|[[File:Muscle fiber types.jpg]]
| |
| | Muscle fibre types identified by ATPase staining. Myosin binds and hydrolyzes ATP during force generation.
| |
| '''Type I fibres'''
| |
| * Red muscles contain predominantly (but not exclusively) red muscle cells. Red muscle fibres are comparatively thin and contain large amounts of myoglobin and mitochondria.
| |
| * Red fibres contain an isoform of myosin with low ATPase activity, i.e. the speed with which myosin is able to use up ATP. Contraction is therefore '''slow'''.
| |
| * Red muscles are used when sustained production of force is necessary, e.g. in the control of posture.
| |
|
| |
|
| '''Type II fibres'''
| | [[File:Hongjun_Shi_Research_photo02.jpg]] |
| * White muscle cells, which are predominantly found in white muscles, are thicker and contain less myoglobin. ATPase activity of the myosin isoform in white fibres is high, and contraction is '''fast'''.
| |
| ** '''Type IIA''' fibres (red) contain many mitochondria and are available for both sustained activity and short-lasting, intense contractions.
| |
| ** '''Type IIB/IIX''' fibres (white) contain only few mitochondria. They are recruited in the case of rapid accelerations and short lasting maximal contraction. Type IIB/IIX fibres rely on anaerobic glycolysis to generate the ATP needed for contraction.
| |
| |}
| |
|
| |
|
| | ''Oscillatory pattern of Hes7 expression and Notch signaling in PSM under normal conditions. Under the hypoxic condition, Hes7 protein expression is lost and a broader expression domain of Notch signaling is observed in PSM.'' |
|
| |
|
| ==Muscle Embryology==
| |
|
| |
|
| * Determined - when cells are located within the somite myotome.
| |
| * Migrate - small cell population moves to location of adult muscle.
| |
| * Proliferate - as myoblasts, single nuclei cells.
| |
| * Fuse - to form myotubes, the primitive skeletal muscle fibre not yet showing sarcomeres.
| |
| * Differentiate - express contractile proteins
| |
|
| |
|
| [[File:Somite cartoon5.png|400px]][[File:Stage11 sem100.jpg|400px]] | | [[File:Hongjun_Shi_Research_photo03.jpg]] |
|
| |
|
| ==Muscle Stem Cells==
| | ''Vertebral defects induced by hypoxia (Sparrow et al., 2012).'' |
|
| |
|
| {|
| |
| ! Satellite cells
| |
| |-
| |
| |
| |
| * These cells remain as muscle stem cells and lie under the basal lamina around each skeletal muscle fibre.
| |
| * They have a role in postnatal growth and also regeneration of muscle fibres.
| |
| * Derived from committed myogenic progenitors.
| |
|
| |
|
| | '''References:''' |
| | Aulehla, A., Pourquie, O., 2010. Signaling gradients during paraxial mesoderm development. Cold Spring Harbor perspectives in biology 2, a000869. |
| | Bailey, C., Dale, K., 2001. Somitogenesis in Vertebrate Development, eLS. John Wiley & Sons, Ltd. |
|
| |
|
| Muscle injury -> satellite cells enter the cell cycle -> express muscle regulatory factors (MyoD and Myf-5) -> undergo myogenic program -> eventually restoring muscle
| | Chapman, G., Sparrow, D.B., Kremmer, E., Dunwoodie, S.L., 2011. Notch inhibition by the ligand DELTA-LIKE 3 defines the mechanism of abnormal vertebral segmentation in spondylocostal dysostosis. Human molecular genetics 20, 905-916. |
|
| |
|
| | McInerney-Leo, A.M., Sparrow, D.B., Harris, J.E., Gardiner, B.B., Marshall, M.S., O'Reilly, V.C., Shi, H., Brown, M.A., Leo, P.J., Zankl, A., Dunwoodie, S.L., Duncan, E.L., 2015. Compound heterozygous mutations in RIPPLY2 associated with vertebral segmentation defects. Human molecular genetics 24, 1234-1242. |
|
| |
|
| Search term: [http://www.ncbi.nlm.nih.gov/pubmed/?term=Muscle+Satellite+Cell ''Muscle Satellite Cell'']
| | Pourquie, O., 2011. Vertebrate segmentation: from cyclic gene networks to scoliosis. Cell 145, 650-663. |
|
| |
|
| :'''Links:''' [[Stem Cells]] | [http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3859734/figure/F2 Muscle cell regeneration following damage]
| | Sparrow, D.B., Chapman, G., Smith, A.J., Mattar, M.Z., Major, J.A., O'Reilly, V.C., Saga, Y., Zackai, E.H., Dormans, J.P., Alman, B.A., McGregor, L., Kageyama, R., Kusumi, K., Dunwoodie, S.L., 2012. A mechanism for gene-environment interaction in the etiology of congenital scoliosis. Cell 149, 295-306. |
| | [[File:Muscle satellite cell EM02.jpg|300px]]
| |
| |}
| |
| | |
| {| class="wikitable mw-collapsible mw-collapsed"
| |
| ! Satellite cell molecular markers
| |
| |-
| |
| | [[File:Muscle satellite cell markers.jpg|600px]]
| |
| | |
| Muscle satellite cell markers<ref name="PMID25364710"><pubmed>25364710</pubmed></ref> (Note - for this class you do not need to know these markers).
| |
| |}
| |
| | |
| ==Muscle Innervation==
| |
| | |
| Mouse Limb Tissue Development (muscle, nerve, skeletal)
| |
| | |
| [[File:Mouse limb tissue development.jpg|500px]]
| |
| | |
| Basal lamina control of innervation site
| |
| | |
| [[File:Skeletal muscle basal lamina exp01.jpg|500px]]
| |
| | |
| ==Muscular Dystrophies==
| |
| [[File:X-Linked recessive (carrier mother).jpg|thumb|X-linked recessive (carrier mother)]]
| |
| Dystrophy refers to the degeneration of a tissue, muscular dystrophy is degeneration of skeletal muscle.
| |
| | |
| * '''Duchenne Muscular Dystrophy''' - (DMD) The most common occuring in boys and in Duchenne Muscular Dystrophy (DMD). PMID 3319190 PMID 2447503
| |
| * '''Becker Muscular Dystrophy''' - (BMD) Similar to DMD but allows muscles to function better than in DMD, slower progression, make a shortened form of the mutated protein.
| |
| * '''Autosomal Recessive Muscular Dystrophy''' - Dystroglycan, a protein that associates with both dystrophin and membrane molecules, is a candidate gene for the site of the mutation in autosomal recessive muscular dystrophies. A knockout mouse has been generated that has early developmental abnormalities.
| |
| * '''Myotonic Dystrophy''' - An inherited disorder in which the muscles contract but have decreasing power to relax. With this condition, the muscles also become weak and waste away. The myotonic dystrophy gene, found on chromosome 19, codes for a protein kinase that is found in skeletal muscle, where it likely plays a regulatory role. The disease is "amplified" through generations probably by a similar GC expansion associated with Huntington disease.
| |
| * '''Facioscapulohumeral Muscular Dystrophy''' - (FSHD) This form of muscular dystrophy is an autosomal dominant genetic disorder characterized by progressive muscle weakness and wasting that typically begins in the face, shoulder-girdle and upper arm. Currently thought to relate to an impaired muscle regeneration process.
| |
| | |
| | |
| | |
| == Lab 7 Assessment Questions==
| |
| | |
| | |
| TBA
| |
| | |
| | |
| ==Search ==
| |
| | |
| [http://www.ncbi.nlm.nih.gov/sites/entrez?db=Books&cmd=search&term=muscle_development Bookshelf - Muscle Development]
| |
| | |
| [http://www.ncbi.nlm.nih.gov/sites/gquery?itool=toolbar&cmd=search&term=muscle_fiber_type Pubmed - muscle fiber type]
| |
| | |
| | |
| | |
| | |
| == Terms ==
| |
| | |
| * '''anterior tibialis''' - (tibialis anterior) skeletal muscle situated on the lateral side of the tibia and is a direct flexor of the foot at the ankle-joint.
| |
| * '''cardiomyopathy''' - heart muscle begins to dilate, stretching and becomes thinner, associated with DMD boys and some DMD carrier girls.
| |
| * '''carrier females''' - (carrier mother, carrier) X-linked recessive inheritance seen with DMD and a range of other inherited disorders where the gene is recessive and located on the X chromosome.
| |
| * '''cis-acting elements''' - DNA sequences that through transcription factors or other trans-acting elements or factors, regulate the expression of genes on the same chromosome.
| |
| * '''corticosteroids''' - used as a pharmacologic agents in DMD treatment these are the synthetic glucocorticoids; prednisone and its active metabolite prednisolone and
| |
| deflazacort an inactive prodrug.
| |
| * '''dystrophy''' - Refers to the degeneration of a tissue, a muscular dystrophy is the degeneration of skeletal muscle.
| |
| * '''enhancer''' - A cis-regulatory sequence that can regulate levels of transcription from an adjacent promoter. Many tissue-specific enhancers can determine spatial patterns of gene expression in higher eukaryotes. Enhancers can act on promoters over many tens of kilobases of DNA and can be 5' or 3' to the promoter they regulate.
| |
| * '''extensor digitorum longus''' - (EDL) skeletal muscle situated at the lateral part of the front of the leg and extend the phalanges of the toes, and, continuing their action, flex the foot upon the leg.
| |
| * '''Gtf2ird1''' - General Transcription Factor 2 -I Repeat domain-containing protein 1. [http://www.ncbi.nlm.nih.gov/entrez/dispomim.cgi?id=604318 OMIM: Gtf2ird1]
| |
| * '''multiplex ligation-dependent probe amplification''' - (MLPA) a genetic test used to identify disorders including DMD.
| |
| * '''muscle biopsy''' - a clinical test where a small part of the muscle is removed to analyse structure, fibre type and muscle protein abnormalities.
| |
| * '''MusTRD1''' - muscle TFII-I repeat domain-containing protein 1.
| |
| * '''MyHC''' - acronym for myosin heavy chain.
| |
| * '''myoblast''' - the undifferentiated mononucleated muscle cell progenitor, which in skeletal muscle fuses to form a myotube, that in turn expresses contractile proteins to form a muscle fibre.
| |
| * '''myosin heavy chain''' - protein forming the thick filament of the sarcomere and the motor for actin-myosin contraction. There are 17 different myosin classes.
| |
| * '''myosin ATPase''' - the enzyme activity of myosin head that moves along actin filaments by coupling the hydrolysis of ATP to conformational changes.
| |
| * '''myotube''' - the initial multinucleated cell formed by fusion of myoblasts during skeletal muscle development.
| |
| * '''promoter''' - A regulatory region a short distance upstream from the 5' end of a transcription start site that acts as the binding site for RNA polymerase II. A region of DNA to which RNA polymerase IIbinds in order to initiate transcription.
| |
| * '''regulatory sequence''' - (regulatory region, regulatory area) is a segment of DNA where regulatory proteins such as transcription factors bind preferentially.
| |
| * '''serum creatine kinase''' - CK is an intracellular enzyme found in tissues with high ATP requirement, like muscle. Serum levels are raised in DMD 10–20 times (or higher 50–200 times) the upper limit of normal level before the age of 5 years.
| |
| * '''soleus''' - slow skeletal muscle situated immediately in front of the gastrocnemius and when standing taking its fixed point from below, steadies the leg upon the foot and prevents the body from falling forward.
| |
| * '''Troponin''' - striated muscle contraction is regulated by the calcium-ion-sensitive, multiprotein complex troponin and the fibrous protein tropomyosin. Troponin has 3 subunits (TnC, TnI, TnT) and is located on the actin filament. [http://www.ncbi.nlm.nih.gov/entrez/dispomim.cgi?id=191040 OMIM: Troponin I slow]
| |
| | |
| == External Links ==
| |
| {{External Links}}
| |
| | |
| * Blue Histology [http://www.lab.anhb.uwa.edu.au/mb140/CorePages/Muscle/Muscle.htm#SKELETAL Skeletal Muscle histology]
| |
| * [http://currents.plos.org/md/ PLOS Currents - Muscular Dystrophy]
| |
| * [http://omim.org/entry/310200 OMIM]
| |
| * Primer [http://www.plosbiology.org/article/info%3Adoi%2F10.1371%2Fjournal.pbio.0020348 Skeletal Muscle Fiber Type: Influence on Contractile and Metabolic Properties]
| |
| | |
| | |
| {{Glossary}}
| |
| | |
| {{Footer}}
| |
| | |
| | |
| ==References==
| |
| | |
| <references/>
| |
| | |
| {{2016ANAT2341}}
| |
1. QUIZ
2. Guest Lecturer - Hongjun Shi (VCCRI) - "Somitogenesis and congenital vertebral malformation"

Dr Shi’s research is focused on genetic regulation of the somitogenesis and identification of genetic and environmental factors that cause congenital vertebral malformation.
Introduction
The defining feature of vertebrates is the vertebral column which is composed of a series of structurally similar bone units – vertebrae along the body axis. The segmental pattern of the vertebral column is established during early embryo development when the somites are rhythmically produced from the paraxial mesoderm (Bailey and Dale, 2001). Somitogenesis requires the interaction of two components, referred to as the clock and wavefront (Aulehla and Pourquie, 2010; Pourquie, 2011). The wavefront (determination front) determines the future somite boundary and is created by opposing gradients of FGF and Wnt signaling (caudal-rostral) and retinoic acid signaling (rostral-caudal) in the presomitic mesoderm (PSM). At the same time, PSM cells express a number of genes in the FGF, Wnt and Notch signaling pathway in an oscillatory pattern (the clock), and bands of expression appear to move in a caudal-to-rostral direction. When the periodic signal reaches the determination front, a somite can form. Disruption of somitogenesis by genetic mutations results in vertebral defects. For example, in humans and mice, homozygous mutation of the DLL3, LFNG, MESP2, HES7 and RIPPLY2 (Pourquie, 2011) (McInerney-Leo et al., 2015) which are either components or downstream targets of Notch signalling leads to extensive vertebral malformation.
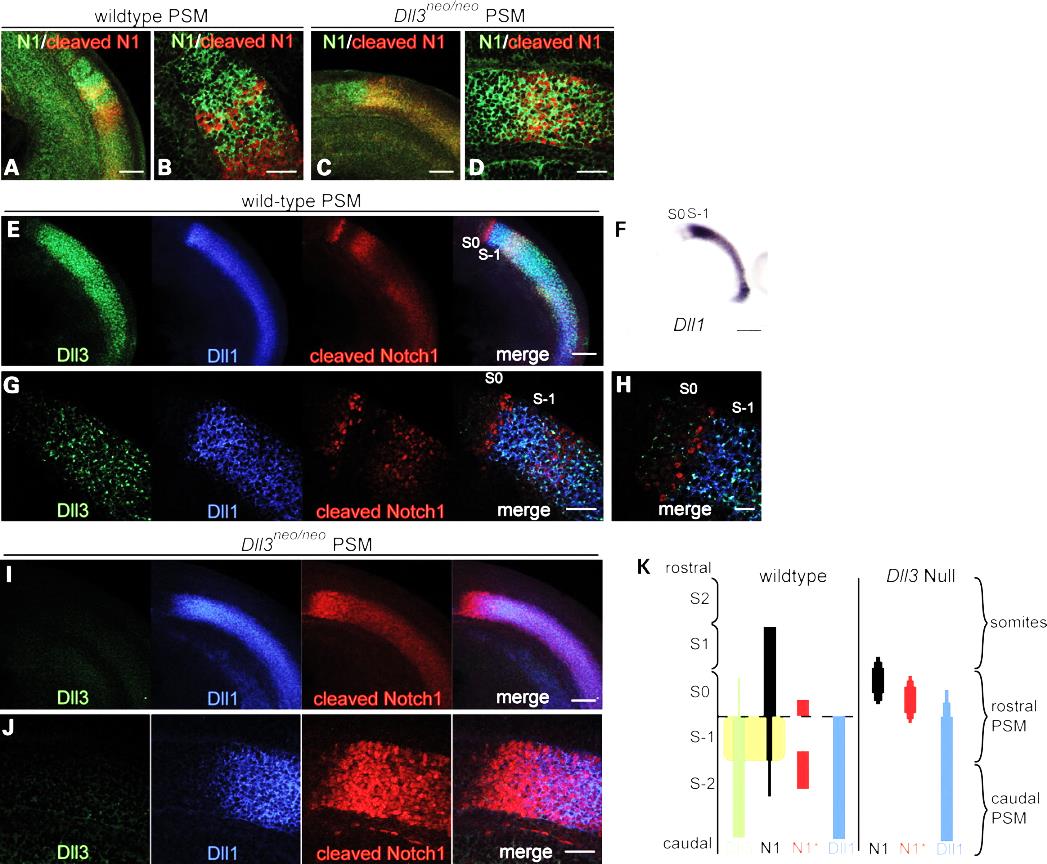
Deletion of Dll3 gene casues failure of Notch1 signalling to restrict to a thin stripe of cells. In stead a broad rostral domain of Notch1 signalling is observed (Chapman et al., 2011).
In addition to genetic mutations, environmental insults during embryo development may also interfere with somitogenesis. For example, hypoxia during pregnancy can inhibit FGF signaling in the PSM and cause segmentation defects in mice. Heterozygous mutation in the Notch signaling genes increase the susceptibility to segmentation defects when the mice are exposed to mild hypoxia (Sparrow et al., 2012).
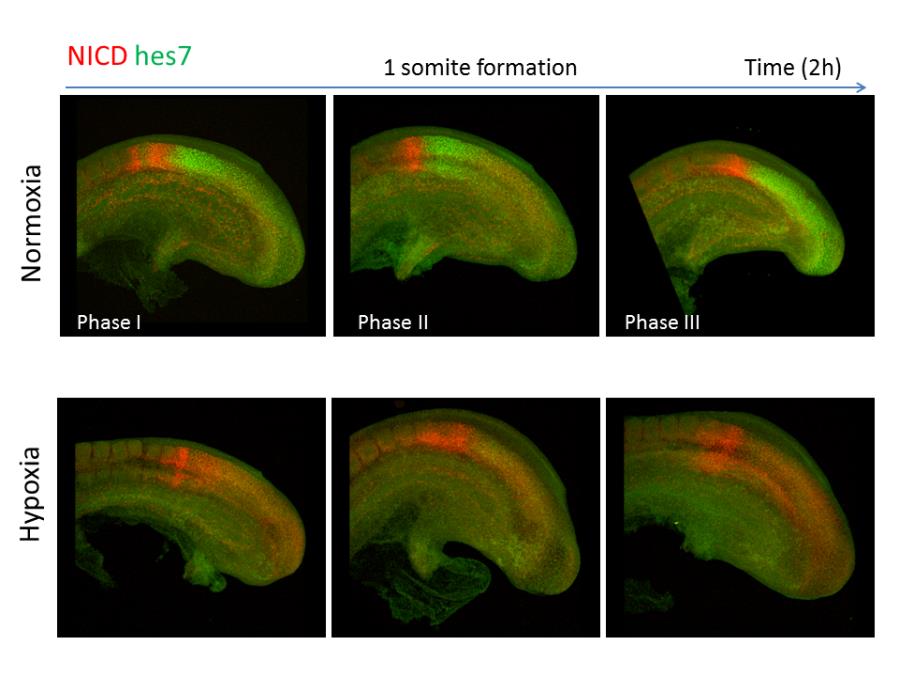
Oscillatory pattern of Hes7 expression and Notch signaling in PSM under normal conditions. Under the hypoxic condition, Hes7 protein expression is lost and a broader expression domain of Notch signaling is observed in PSM.
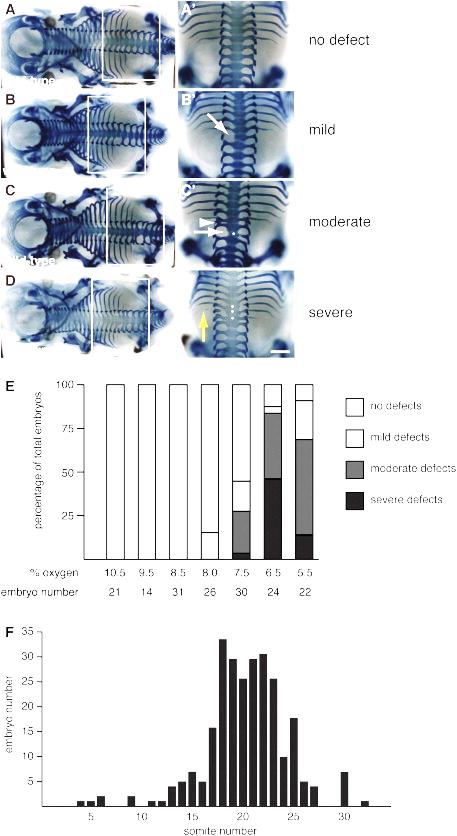
Vertebral defects induced by hypoxia (Sparrow et al., 2012).
References:
Aulehla, A., Pourquie, O., 2010. Signaling gradients during paraxial mesoderm development. Cold Spring Harbor perspectives in biology 2, a000869.
Bailey, C., Dale, K., 2001. Somitogenesis in Vertebrate Development, eLS. John Wiley & Sons, Ltd.
Chapman, G., Sparrow, D.B., Kremmer, E., Dunwoodie, S.L., 2011. Notch inhibition by the ligand DELTA-LIKE 3 defines the mechanism of abnormal vertebral segmentation in spondylocostal dysostosis. Human molecular genetics 20, 905-916.
McInerney-Leo, A.M., Sparrow, D.B., Harris, J.E., Gardiner, B.B., Marshall, M.S., O'Reilly, V.C., Shi, H., Brown, M.A., Leo, P.J., Zankl, A., Dunwoodie, S.L., Duncan, E.L., 2015. Compound heterozygous mutations in RIPPLY2 associated with vertebral segmentation defects. Human molecular genetics 24, 1234-1242.
Pourquie, O., 2011. Vertebrate segmentation: from cyclic gene networks to scoliosis. Cell 145, 650-663.
Sparrow, D.B., Chapman, G., Smith, A.J., Mattar, M.Z., Major, J.A., O'Reilly, V.C., Saga, Y., Zackai, E.H., Dormans, J.P., Alman, B.A., McGregor, L., Kageyama, R., Kusumi, K., Dunwoodie, S.L., 2012. A mechanism for gene-environment interaction in the etiology of congenital scoliosis. Cell 149, 295-306.



