2018 Group Project 4
| Projects 2018: 1 Adrenal Medulla | 3 Melanocytes | 4 Cardiac | 5 Dorsal Root Ganglion |
Project Pages are currently being updated (notice removed when completed)
Neural Crest and Cardiac Development
Introduction
The very first major system to develop its function within a vertebrate embryo is the cardiovascular system with the heart becoming active from the fourth week of development when the placenta is no longer able to sustain the requirements of the growing embryo by itself. The four major embryonic regions that are involved in the process of vertebrate heart development are the primary heart field, secondary heart field, cardiac neural crest, and proepicardium. Each region have important contributions to the overall cardiac development, which occurs with complex and precise developmental timing and regulation. Neural crests are a population of multipotent cells which arises during embryonic development at the dorsal neural tube and were first identified by Wilhelm His as “Zwischenstrang,” the intermediate cord, in 1868, the year of Meiji Ishin, the westernizing revolution of Japan. [1]. The first study showing the relationship of neural crest cells with heart development was published in 1983 where a specific subgroup of neural crest cells, known as cardiac neural crest cells, have been shown to be essential for the septation of the cardiac outflow track as well as the development of aortic arch artery. Since then, the role of cardiac neural crest cells in the development of heart have been explored extensively in various studies which allowed for the classification of neural crest associated human cardiac defects such as DiGeorge syndrome.
Development of the Cardiovascular System
Development of the cardiovascular system begins with the formation of two endocardial tubes that merge together to form the tubular heart. These loop together and separate into the four chambers and paired arterial trunks form the adult heart. The tubular heart differentiates into the truncus arterioles, bulbus cordis, primitive ventricle, primitive atrium and the sinus venosus. The truncus arteriosus splits into the ascending aorta and pulmonary artery. The bulbus cordis forms part of the ventricles. The sinus venosus connects to the fetal circulation. Septa form within the atria and ventricles to separate the left and right sides of the heart.
| Week 2 - 3 | *Bilateral cardiogenic areas form |
| Week 3 - 4 |
|
| Week 4 - 5 |
|
| Week 5-6 |
|
| Week 7 |
|
<html5media width="480" height="358">https://www.youtube.com/embed/5DIUk9IXUaI</html5media>
Cardiac Neural Crest Cells
Neural crest cells are a population of multipotent cells which arises during embryonic development at the dorsal neural tube. Neural crest cells originate from the dorsal-most region of the neural tube. These cells are capable of migrating and differentiating throughout the body to give rise to many different cell types. These cells, which originate from the ectoderm in a region lateral to the neural plate in the neural fold, give rise to neurons, glia, melanocytes, chondrocytes, smooth muscle cells, odontoblasts and neuroendocrine cells, among others[2]. Cardiac neural crest cells (CNCCs) are a subpopulation of the cranial neural crest cells and migrate ventrally from the dorsal neural tube during weeks 3–4 PubmedParser error: Invalid PMID, please check. (PMID: [1]). CNCCs will then proceed and fall in place into third, fourth and the sixth caudal pharyngeal arches as they develop during their migration to the cardiac outflow tract. They will form condensed mesenchymal cells of the aorticopulmonary septation complex and also differentiate into cardiac ganglia[3].NCCs are necessary for aortic arch artery remodeling and outflow tract septation (OFT). [4]
Cardiac neural Cells can develop into:
- Melanocytes near the heart region
- neurons associated with cardiac innervation
- cartilage
- connective tissue (they form the connective tissue wall of the large arteries from the heart, as well as the septum between the branches in the heart)
- provide signals required for the maintenance and differentiation of the other cell layers in the pharyngeal apparatus [4]
Early Development
Induction
Initially, NCCs are morphologically similar to other neuroepithelial cells and cannot be differentiated from them. With contact-mediated inductive signals from the surface ectoderm and underlying mesoderm through a process known as Induction where progenitor cells begin to differentiate PubmedParser error: Invalid PMID, please check. (PMID: [2]). Progenitor cells are found in the epiblast around Henson's node and are brought into the neural folds where signalling molecules will induce the progenitor cells to turn into CNCCs PubmedParser error: Invalid PMID, please check. (PMID: [3]). While key signalling regulators of neural crest cell formation such as bone morphogenetic protein (BMP) and fibroblast growth factor (FGF) have been identified in species such as fish and avians, there is currently no evidence that suggests the same factors play a role in mammalian neural crest cell induction, thus more studies have to be carried out to identify the signaling pathway for mammalian neural crest cell formation PubmedParser error: Invalid PMID, please check. (PMID: [4]). Studies have also shown that if BMP levels are too high or low, the progenitor cells will not be able to migrate, thus an intermediate level of BMP is ideal for the induction process.PubmedParser error: Invalid PMID, please check. (PMID: [5]) As for the other signaling cascades involved, little information is known.
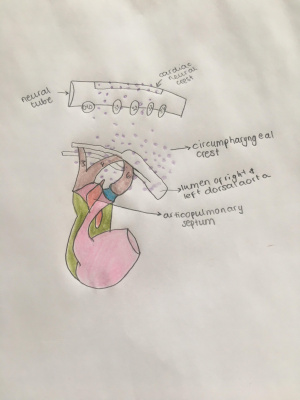
Migration From Neural Crest to Circumpharyngeal Ridge
After the Induction process, cranial neural crest cells undergo an epithelial-to-mesenchymal transition and emigrate from the neural tube to the circumpharyngeal ridge which is an arc-shaped ridge structure that is found dorsal to developing caudal pharyngeal arches PubmedParser error: Invalid PMID, please check. (PMID: [6]). In higher vertebrates, cranial neural crest cells will migrate in three clusters (cranial, middle and caudal) and eventually develop cranial nerve ganglia at even-numbered rhombomeres proximally and populate pharyngeal arches distally. The caudal stream comprises most of the CNCCs.
There are multiple signaling factors which control the migration of CNCCs.
- Snail2 inhibits the expression of cadherins and studies on avians and fish have shown that the presence of Snail2 helps to facilitate cell migration PubmedParser error: Invalid PMID, please check. (PMID: [7]). However, the expression of Snail does not seem to be needed for the neural crest induction in mammals PubmedParser error: Invalid PMID, please check. (PMID: [8]).
- RhoA/B, a GTPase protein, regulates and remodels the actin cytoskeleton of the cells to alter the planar cell polarity to allow neural crest migration PubmedParser error: Invalid PMID, please check. (PMID: [9]).
- CNCC expresses integrin receptors and MMP-2 to allow them to migrate on fibronectin in extracellular matrix which is believed to provide a permissive environment to allow the migration of crest cells to the circumpharyngeal ridge PubmedParser error: Invalid PMID, please check. (PMID: [10]).
Formation of Pharyngeal Arches and Cardiac Outflow Tract
The Cardiac Outflow Tract (OFT) is a transient embryonic structure located at the arterial pole of the heart that functions initially as a conduit for the blood flowing from the right ventricle into the aortic sac.[5].
CNCCs will initially pause their migration at the circumpharyngeal ridge as their destined pharyngeal arches have not been developed. The pericardial cavity will regress caudally, allowing pharyngeal pouches to indent the body wall and delineate the pharyngeal arches from the cranial to the caudal direction and generate arches (3, 4 and 6). As the arches develop, they will be populated by cardiac neural crest cells which migrates from the circumpharyngeal ridge.
CNCCs express different factors that target the cells to the pharyngeal arches. Slit cells can target cells to migrate to arch 3. FGF-8 targets for arch 4. EphA targets for arch 6. Rac1 and Sdf1 are both expressed in the cells, causing them to condensate around the arch arteries. Semaphorin is expressed and causes the cells to migrate further to the cardiac outflow tract. Notch and BMP are then expressed condensing the cells, forming the semilunar valve and aorticopulmonary septum.
- The 3rd arch is dedicated in the formation of the carotid system. It forms the left and right common carotid arteries which will sprout into internal and external carotid arteries by angiogenesis PubmedParser error: Invalid PMID, please check. (PMID: [11]).
- The 4th arch gives rise to the definitive aortic arch along with the pulmonary artery.
- The 6th arch initially develops into the pulmonary trunk that emerges from the right ventricles, but this structure will be remodeled asymmetrically and gives rise to the ductus arteriosus which is a crucial embryonic structure that connects the pulmonary artery with the descending aorta for blood circulation in the fetus. This shunt allows blood from the right ventricle to bypass the lungs because the fetal blood is oxygenated through the placenta. At birth, as the lungs start their function, the ductus arteriosus closes allowing circulation through to the lungs to oxygenate the blood that subsequently reaches the systemic circulation of the newborn PubmedParser error: Invalid PMID, please check. (PMID: [12]).
Ectomesenchyme that is derived from CNCCs in pharyngeal arches 3, 4 and 6 are critical for the repatterning of the bilaterally symmetrical pharyngeal arch arteries to form the asymmetric great arteries of the thorax.
Migration of cardiac neural crest cells from the neuroectoderm into the outflow tract cushions induces the formation of the aortopulmonary (AP) septum, which divides the common outflow tract at the cardiac to vascular border into an aortic and pulmonary orifice and more proximally intracardiac into a right and left ventricular outflow tract. [6]
Later Development
Outflow Septation
The septation of the outflow tract is tightly coordinated with the septation of both the ventricles and atria. After migrating into the pharynx, some CNCCs will remain in the pharyngeal arches while the rest would continue and migrate into cardiac outflow cushions which converges to separate blood flow from the embryonic left and right ventricles PubmedParser error: Invalid PMID, please check. (PMID: [13]).
Cushion formation and septation rely on the interaction of 3 distinct cell types, cardiac neural crest cells (NCCs), second heart field-derived (SHF-derived) cells, and endothelial cells (ECs) [5] .
Within the cushions, the CNCCs will condense and form the aorticopulmonary septation complex which is essentially two centrally placed columns and divides the common arterial outflow into the aorta and pulmonary trunks.
Studies have shown that the TGFbeta/BMP signaling family is involved in this condensation processPubmedParser error: Invalid PMID, please check. (PMID: [14]).
The cushions will be populated with three main types of mesenchymal cells, depending on their proximal-distal location in the outflow tract PubmedParser error: Invalid PMID, please check. (PMID: [15]).
The outflow septum has three main components:
- Aortico-Pulmonary Septum - The most cranial portion of the septum and forms in the aortic sac:
- Truncus Septum - Caudal of the aorticopulmonary septum and forms partition between aortic and pulmonary semilunar valves
- Conus Septum - Inferior portion of the outflow septum and is needed for the closure of the ventricular septum [7]
Failure of proper OFT septation during embryogenesis will result in inappropriate mixing of oxygenated and deoxygenated blood at birth and this may cause an unfavourable clinical prognosis.
Valvulogenesis
The majority of heart defects in live births arise from disruption of cardiac outflow tract development. The OFT is an embryonic structure that gives rise to the ascending aortic and pulmonary arteries as well as their respective tricuspid aortic (AV) and pulmonary (PV) valves.[8].The cNCC also contribute to the aortic and pulmonary valves, thereby connecting the heart to the vascular system. OFT endothelial cells that have undergone endoMT are thought to give rise to the bulk of the semilunar valves, which form within the aorta and pulmonary artery, to prevent the backflow of blood into the ventricles. In addition, cardiac NCCs also colonize the semilunar valves, where they mainly contribute to the two leaflets adjacent to the aorticopulmonary septum. Cells of the NCC have also been found to contribute to the atrioventricular valves, consisting of the bicuspid (mitral) valve and tricuspid valve, which is located between the upper atria and the lower ventricles.
Atrial and Ventricular Separation
The heart first transforms from the embryo into four parts: The atrial chamber, ventricular chamber, arterial trunks and great veins. The first part of cardiac development involves the separation into phases of formation of the primary myocardial tube, looping of the tube, additional parts for future topography compartments, and assembly of the components into arterial trunks and cardiac chambers subsequently. [9]
During the formation of the two arterial trunks, the cushions that divided them disintegrates. The parts that possess their own distinct walls, separated by extra-cardiac space are the definitive heart, the proximal parts of the aorta and pulmonary trunk, along with the sinuses of the arterial roots and the subpulmonary infundibulum.[10]
Formation of the Cardiac Ganglia
Cardiac ganglia are made entirely from cardiac crest cells.
"Virtually nothing is known about the factors that control their separation from the cardiac crest forming the aorticopulmonary septum or their condensation as ganglia. However, cardiac crest cells also participate in the formation of the nodose ganglion. This is the distal sensory ganglion of the vagus nerve. The nodose ganglion is formed from neurons derived from the nodose placode located dorsal to pharyngeal arches 4/6. Cells migrate from this placode to coalesce with cardiac crest to form the nodose ganglion. Condensation of this ganglion depends on N-cadherin and signaling by Slit/Robo signaling. In cranial crest Slit1/Robo signaling in conjunction with N-cadherin is important for coalescence of crest cells and placode-derived neurons into ganglia. N-cadherin and Robo2 are expressed by placodal neurons and Slit1 is on neural crest cells. If either N-cadherin or Robo2 is knocked down, the ganglia do not coalesce properly.115"
PubmedParser error: Invalid PMID, please check. (PMID: [16])
Signaling Molecules
- Wnt: extracellular growth factors that turn on intracellular signalling pathways. The Wnt pathway is needed to stabilize the expression of B-catenin by hindering proteasome degradation. If the Wnt pathway is inhibited, the decrease in B-catenin can result in a reduced proliferation of CNCCs which may result in the hindered development of the OFT. [11]
- Notch: a transmembrane protein whose signalling is required for differentiation of CNCCs to vascular smooth muscle cells and for proliferation of cardiac myocytes. Inhibition of the Notch pathway in neural crest cells via dominant-negative Notch inhibitor (DN-MAML) have been shown to cause various defects in outflow tract, cardiac and aortic arch defects. Neural crest cell migration, survival and contribution to the branchial arches are not affected by Notch inhibition thus suggesting that Notch signalling is essential for post-migratory differentiation of cardiac neural crest into smooth muscle.
- BMP (bone morphogenetic proteins): they are required for neural crest cell migration into the cardiac cushions (=precursors to heart valves and septa) and for differentiation of neural crest cells to smooth muscle cells of the aortic arch arteries.
- GATA: Transcription factors which are key factors in the restriction of cell lineage differentiation during the development of the heart. One important member of GATA is the GATA6 which regulates the morphogenetic patterning of the outflow tract and aortic arch. If GATA6 is inactivated in CNCCs, defects in the development of the cardiovascular system such as persistent truncus arteriorus would occur
- Meis2 PubmedParser error: Invalid PMID, please check. (PMID: [17])
Developmental Time Course
| Week 3-4 | Day 22-28 | Neural crest migration starts |
| Week 5-6 | Day 32-37 | Cardiac neural crest migrates through the aortic arches and enters the outflow tract of the heart |
| Week 9 | Day 57+ | Outflow tract and ventricular septation complete |
Human Congenital Heart Diseases associated with Neural Crest Cells
Cardiac neural crest ablation experiments in vertebrate models have demonstrated that upon removal of the pre-migratory cardiac neural crest cardiovascular abnormalities are induced such as:
- Defective development of the cardiac outflow tract;
- Abnormal myocardial function;
- Defective development of the derivatives of the caudal pharynx including arch arteries, pharyngeal glands and the secondary heart field.
The loss of neural crest cells or their dysfunction may not always directly cause abnormal cardiovascular development, but can also be involved secondarily because crest cells represent a major component in the complex tissue interactions in the head, pharynx and outflow tract. [12]
Even though there are various cardiac dysmorphologies caused by cardiac neural crest ablation, only those involving outflow or conotruncal defects are commonly seen, thus the majority of the studies have been focused on these cardiac defects which can include:
- Complete absence of outflow septation,
- Persistent truncus arteriosus,
- Overriding aorta
not yet edited----
that the quantity rather than the quality of neural crest cells is important in OFT septation.
Furthermore, cardiac malformations associated with partial ablation of the cardiac neural crest, show normal formation of the aorticopulmonary septum and as a result an aorta and a pulmonary trunk. However, the aorta and pulmonary trunk are malaligned with respect to the ventricles. One might argue that the neural crest-derived cells are not only crucial in the regulation of septation but also in the alignment of the great arteries with respect to the ventricles. On the other hand, one might argue that the malalignment is due to an indirect effect of neural crest ablation. https://academic.oup.com/cardiovascres/article/47/2/212/363634
End of unedited part----
Disruptions to outflow tract septation
In the human, at Carnegie stage 14, the myocardial wall of the outflow tract extends to the border of the pericardial cavity where it joins the aortic sac, giving rise to the arteries that feed the pharyngeal arches [13].OFT remodeling is a process whereby the embryonic outflow tract undergoes a series of developmental transitions that involves extra cardiac cells recruitment and transformations that when disrupted, can result in Persistent Truncus Arteriosus (PTA), a rare congenital heart anomaly in humans. [8]. PTA occurs when there is a deficiency in the number of neural crest cells that reach the cardiac outflow tract, thus the truncus arteriosus will not be developed properly and will not be able to properly divide the common truncal outflow vessel into the pulmonary trunk and aorta, resulting in only a single arterial trunk arising from the heart [14]. This will cause the oxygenated and deoxygenated blood to be mixed and the mixed blood will be pumped through the coronary and pulmonary arteries as well as the systemic circulation. This can result in various defects such as cyanosis at birth, cardiomegaly and even heart failure in the fetus. Failure of outflow tract septation may also be responsible for other forms of congenital heart disease, including transposition of the great vessels, high ventricular septal defects, and tetralogy of Fallot. Anomalies involving the outflow tract and its valves make up a significant proportion of congenital cardiac defects, with a prevalence of at least 4 per 10,000 births [13].
DiGeorge Syndrome
DiGeorge syndrome (DGS), also known as Velocardiofacial Syndrome, is a congenital condition which affects the development of many tissues that are patterned by or derived from NCCs. People suffering from DGS display symptoms such as craniofacial defects, aplasia or hypoplasia of the thymus and parathyroid glands, mental disorders and cardiovascular defects. [1]. DGS results primarily due to defective development of cranial and cardiac NCCs which invades the first four pharyngeal arches that contribute to the development of the lower jaw, neck and cardiac structures. Studies have shown in chicks that the ablation of the pharyngeal NCCs population produces cardio-craniofacial anomalies which can be found in DGS patients. [15]
DGS
- Caused by a chromosomal 22q11.2 deletion.
- a hemizygous deletion within chromosome band 22q11.2 has been found in 25% of DGS patients.
- Characterized by interrupted aortic arch type B, outflow tract malformations that include xxx
Research in the pathway/mechanism of why chromosome 22q11.2 deletion occurs happens due to a recent discovery in T-Box transcription factor (TBX1). TBX1 is involved in the regulation of neural crest cell migratory pathways. Haploinsufficiency of the T-box transcription factor TBX1 is responsible for the many features of 22q11.2 deletion syndrome [16]. Tbx1 controls cardiac innervation by modulating NCC and nerve migratory pathways in a non-cell autonomous fashion [16].
Models and Research
Animal Models
In the past century, various vertebrates have been utilised for the study of neural crest biology, such as: amphibians, fish, avians, mice, lamprey and even hagfish. [17][18][19] Interestingly enough, Zebrafish, Xenopus and chick embryos largely display consistent requirements for specific genes in early steps of neural crest development. However, knockout of homologous genes in the mouse often do not exhibit comparable early neural crest phenotypes, suggesting that there might be major differences between vertebrate species.[20] It is important to note that the absence of an obvious phenotype in an animal model does not necessarily mean that the gene of interest is not normally involved in the process for humans. Rather, it could be that the gene has a redundant function in the animal model, which could differ amongst species.
Quail-Chick Chimeras
For CNCCs, the main experimental animal model is the chick embryo, specifically quail-chick chimeras, where premigratory presumptive arch neural crest cells from quail embryos were grafted onto early chick embryos.[21] Majority of what was learnt about the importance of CNCCs in cardiovascular development were derived from studies done on the Quail-Chick Chimeras. For example, ablation studies on quail-chick chimeras have shown that CNCCs are absolutely required to form the aorticopulmonary septum dividing the cardiac arterial pole into systemic and pulmonary circulations. They support the normal development and patterning of derivatives of the caudal pharyngeal arches and pouches, including the great arteries and the thymus, thyroid and parathyroids.
Mouse
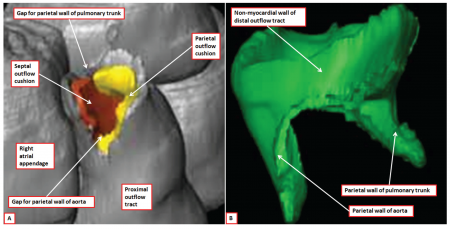
Another key animal model that is commonly used for CNCC studies is the mouse. The mouse offers a powerful genetic model for as many mouse mutants exhibit various neural crest phenotypes that lead on to important discoveries related to the importance of the proper development of neural crest cells. For example, knockout studies on mouse models have shown the importance of a ubiquitously expressed nonreceptor tyrosine phosphatase, known as SHP-2 which plays a role in the cardiac outflow tract development and semilunar valvulogenesis.[22] Mouse models also allow for the digital reconstruction of the aspects of the outflow tract in a developing embryo as shown in the figure on the right.
Zebrafish model
Using the zebrafish model, studies have demonstrated that MEIS2 (myeloid ecotropic viral integration site 2 homolog) is critical for the proper development of the heart and cardiac looping. Embryos that received splice-blocking morpholino oligonucleotide directed against meis2b were shown to have defective cardiac morphogenesis. [23] MEIS transcription factors are known to interact with HOX and Pre-B cell leukemia transcription factors (PBX) to regulate downstream targets in multiple cellular processes. In situ time course experiments in developing zebrafish embryos have shown that MEIS2b expression in the heart field resembled that of Gata4, a known cardiac transcription factor. [23]
Current Research
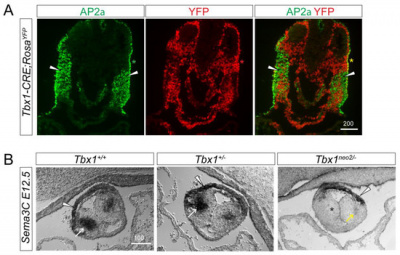
Importance of Semaphorin 3c in the proper septation of OFT
Semaphorin 3c (Sema3c) is a neurovascular signalling guide factor which is necessary for the proper development of the OFT. Recent studies have shown that Sema3c mediates the interaction between cNCCs and the SHF during the development of the OFT to allow proper septation of the OFT and establish the separate systemic and pulmonary circulation systems.[24] During the initial stages of heart development, Sema3c is expressed in the OFT as well as in the pharyngeal arch region which also contains cardiac progenitor niches composed of SHF progenitor cells and CNCCs.[24] Sema3c expression can be regulated positively and negatively by Foxc1/Foxc2 and Tbx1-Fgf8 signalling respectively. Changes in expression levels of Sema3c can alter the development and migration of CNCCs for OFT formation during embryogenesis. For example, the inhibition of Sema3c expression in mouse models have caused disruption in the aortic arch and also led to persistent truncus arteriosus. [24]
Defective Parasympathetic Innervation in Tbx1 Mutant Hearts
Previous studies have shown that T-box transcription factor (Tbx1) is expressed dynamically in the pharyngeal during the development of mouse and that Tbx1 homozygous mutants display various neural crest cell defects. This led to further investigations on whether parasympathetic (vagal) innervation of the heart will be affected by mutations in Tbx1. Recent studies have shown that Tbx1 plays a role in regulating epibranchial ganglion positioning, migratory paths of CNCCs and subsequent vagal nerve projections to the heart. Tbx1 mutants show reduced expression of Sema3c which results in a disrupted CNCC migration pattern. Sema3C mutant embryos display a cardiac innervation phenotype similar to those observed in Tbx1 mutant embryos.[25]
Glossary
- Number | A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z
Bulbus cordis DiGeorge Syndrome Primitive atrium Primitive ventricle Persistent Truncus Arteriosus RhoA/B Sinus venosus Snail2 Truncus arterioles Valvulogenesis
References
Nakanishi T, Markwald RR, Baldwin HS, Keller BB, Srivastava D, Yamagishi H, Miyagawa-Tomita S, Arima Y & Kurihara H. (2016). The “Cardiac Neural Crest” Concept Revisited. , , . PMID: 29787146 DOI. Odelin G, Faure E, Coulpier F, Di Bonito M, Bajolle F, Studer M, Avierinos JF, Charnay P, Topilko P & Zaffran S. (2018). Krox20 defines a subpopulation of cardiac neural crest cells contributing to arterial valves and bicuspid aortic valve. Development , 145, . PMID: 29158447 DOI. Pauli S, Bajpai R & Borchers A. (2017). CHARGEd with neural crest defects. Am J Med Genet C Semin Med Genet , 175, 478-486. PMID: 29082625 DOI.
- ↑ 1.0 1.1 Nakanishi T, Markwald RR, Baldwin HS, Keller BB, Srivastava D, Yamagishi H, Miyagawa-Tomita S, Arima Y & Kurihara H. (2016). The “Cardiac Neural Crest” Concept Revisited. , , . PMID: 29787146 DOI.
- ↑ Vega-Lopez GA, Cerrizuela S, Tribulo C & Aybar MJ. (2018). Neurocristopathies: New insights 150 years after the neural crest discovery. Dev. Biol. , , . PMID: 29802835 DOI.
- ↑ Taneyhill LA & Schiffmacher AT. (2013). Cadherin dynamics during neural crest cell ontogeny. Prog Mol Biol Transl Sci , 116, 291-315. PMID: 23481200 DOI.
- ↑ 4.0 4.1 Odelin G, Faure E, Coulpier F, Di Bonito M, Bajolle F, Studer M, Avierinos JF, Charnay P, Topilko P & Zaffran S. (2018). Krox20 defines a subpopulation of cardiac neural crest cells contributing to arterial valves and bicuspid aortic valve. Development , 145, . PMID: 29158447 DOI.
- ↑ 5.0 5.1 Plein A, Calmont A, Fantin A, Denti L, Anderson NA, Scambler PJ & Ruhrberg C. (2015). Neural crest-derived SEMA3C activates endothelial NRP1 for cardiac outflow tract septation. J. Clin. Invest. , 125, 2661-76. PMID: 26053665 DOI.
- ↑ Peterson JC, Chughtai M, Wisse LJ, Gittenberger-de Groot AC, Feng Q, Goumans MTH, VanMunsteren JC, Jongbloed MRM & DeRuiter MC. (2018). Nos3 mutation leads to abnormal neural crest cell and second heart field lineage patterning in bicuspid aortic valve formation. Dis Model Mech , , . PMID: 30242109 DOI.
- ↑ Creazzo TL, Godt RE, Leatherbury L, Conway SJ & Kirby ML. (1998). Role of cardiac neural crest cells in cardiovascular development. Annu. Rev. Physiol. , 60, 267-86. PMID: 9558464 DOI.
- ↑ 8.0 8.1 Mifflin JJ, Dupuis LE, Alcala NE, Russell LG & Kern CB. (2018). Intercalated cushion cells within the cardiac outflow tract are derived from the myocardial troponin T type 2 (Tnnt2) Cre lineage. Dev. Dyn. , 247, 1005-1017. PMID: 29920846 DOI.
- ↑ Moorman A, Webb S, Brown NA, Lamers W & Anderson RH. (2003). Development of the heart: (1) formation of the cardiac chambers and arterial trunks. Heart , 89, 806-14. PMID: 12807866
- ↑ Anderson RH, Webb S, Brown NA, Lamers W & Moorman A. (2003). Development of the heart: (2) Septation of the atriums and ventricles. Heart , 89, 949-58. PMID: 12860885
- ↑ Gessert S & Kühl M. (2010). The multiple phases and faces of wnt signaling during cardiac differentiation and development. Circ. Res. , 107, 186-99. PMID: 20651295 DOI.
- ↑ 12.0 12.1 Keyte A & Hutson MR. (2012). The neural crest in cardiac congenital anomalies. Differentiation , 84, 25-40. PMID: 22595346 DOI.
- ↑ 13.0 13.1 Webb S, Qayyum SR, Anderson RH, Lamers WH & Richardson MK. (2003). Septation and separation within the outflow tract of the developing heart. J. Anat. , 202, 327-42. PMID: 12739611
- ↑ Nishibatake M, Kirby ML & Van Mierop LH. (1987). Pathogenesis of persistent truncus arteriosus and dextroposed aorta in the chick embryo after neural crest ablation. Circulation , 75, 255-64. PMID: 3791607
- ↑ Escot S, Blavet C, Faure E, Zaffran S, Duband JL & Fournier-Thibault C. (2016). Disruption of CXCR4 signaling in pharyngeal neural crest cells causes DiGeorge syndrome-like malformations. Development , 143, 582-8. PMID: 26755698 DOI.
- ↑ 16.0 16.1 Calmont A, Anderson N, Suntharalingham JP, Ang R, Tinker A & Scambler PJ. (2018). Defective Vagal Innervation in Murine Tbx1 Mutant Hearts. J Cardiovasc Dev Dis , 5, . PMID: 30249045 DOI.
- ↑ Aybar MJ & Mayor R. (2002). Early induction of neural crest cells: lessons learned from frog, fish and chick. Curr. Opin. Genet. Dev. , 12, 452-8. PMID: 12100892
- ↑ Trainor PA. (2005). Specification of neural crest cell formation and migration in mouse embryos. Semin. Cell Dev. Biol. , 16, 683-93. PMID: 16043371 DOI.
- ↑ Sauka-Spengler T, Meulemans D, Jones M & Bronner-Fraser M. (2007). Ancient evolutionary origin of the neural crest gene regulatory network. Dev. Cell , 13, 405-20. PMID: 17765683 DOI.
- ↑ Barriga EH, Trainor PA, Bronner M & Mayor R. (2015). Animal models for studying neural crest development: is the mouse different?. Development , 142, 1555-60. PMID: 25922521 DOI.
- ↑ Stevens T, Kahn JK, McCallister BD, Ligon RW, Spaude S, Rutherford BD, McConahay DR, Johnson WL, Giorgi LV & Shimshak TM. (1991). Safety and efficacy of percutaneous transluminal coronary angioplasty in patients with left ventricular dysfunction. Am. J. Cardiol. , 68, 313-9. PMID: 1858673
- ↑ Dunn AM, Darvell F & Wilson RS. (1985). An evaluation of the foot pump as a driving source for nebulizer solutions. Br J Dis Chest , 79, 172-6. PMID: 3986121
- ↑ 23.0 23.1 Bhatt AP. (1986). Case of the month. Lichen planus of the lower lip and buccal mucosa. J Indian Dent Assoc , 58, inside front cover. PMID: 3462257
- ↑ 24.0 24.1 24.2 Reuling FH & Schwartz JT. (1970). Heritability of the effect of corticosteroids on intraocular pressure. Acta Genet Med Gemellol (Roma) , 19, 264-7. PMID: 5533775
- ↑ Calmont A, Anderson N, Suntharalingham JP, Ang R, Tinker A & Scambler PJ. (2018). Defective Vagal Innervation in Murine Tbx1 Mutant Hearts. J Cardiovasc Dev Dis , 5, . PMID: 30249045 DOI.