2018 Group Project 3
| Projects 2018: 1 Adrenal Medulla | 3 Melanocytes | 4 Cardiac | 5 Dorsal Root Ganglion |
Project Pages are currently being updated (notice removed when completed)
Melanocytes
Introduction
Melanocytes are a type of neural crest-derived cells in the body [1], most commonly found in the stratum basale, the bottom layer, of the epidermis of the skin, eyes [2], and to a lesser known degree, the meninges (membrane around the brain), the heart [3] and inner ear [4].
Their most commonly known function is the production of melanin, melanogenesis, of which there are two types, eumelanin (black) and pheomelanin (reddish yellow) [5], and are responsible for the pigmentation of various parts of the human body, including the skin, hair and irises, etc. Melanogenesis has different levels, basal and activated. The basal level of melanogenesis determines an individual's skin colour and is determined by genetics, i.e. a light-skinned individual have low levels of basal melanogenesis. Activated levels of melanogenesis are usually due to external factors, such as exposure to UV-B radiation, resulting in increased levels of melanogenesis [6], which can be commonly seen as sunburn or tan after long exposure to sunlight.
History
The history of the discovery of the melanocyte spans about 4000 years, from when a pigmentation disorder of skin was first documented in 2200 bc, to when the melanocyte was confirmed as a pigment synthesising cell in 1917[2]. Melanocytes were first described and named ‘chromatophores’ by Giosué Sangiovanni in 1819, who identified them in squid [7] . Later, in 1837, Friedrich Henle identified melanocytes to be in the human skin epidermis and eye[2]. In 1910, Ross Granville Harrison, an American anatomist, proposed that melanocytes originated from the neural crest [8]. But it wasn’t until 1917, that Bruno Bloch identified and confirmed the enzyme tyrosinase within melanocytes to be responsible for producing melanin pigment [9].
Tissue Organ Structure and Function
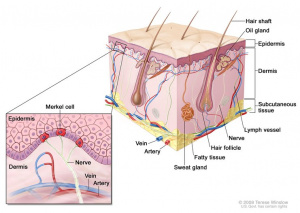
Skin
Ears
Melanocytes are mainly present in the cochlea, vestibular organ and endolymphatic sac:
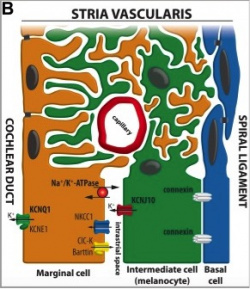
The cochlea of the inner ear functions as a transducer, by converting sound vibrations to electrical potentials in the auditory nerve via hair cells [10]. In the cochlea of humans, melanocytes are found in the vascularised epithelial tissue in the intermediate layer of the stria vascularis and the modiolus, between the marginal and basal cell layers [11](Figure 1). During development, marginal layer cuboidal epithelium develops processes which interdigitate with the intermediate melanocytes and basal cells [12].Based on studies performed on mice models, it is thought that melanocytes are important for the development of the endocochlear potential (EP), produced by strial cells. Intermediate melanocytes conduct K+, which plays an important role in sound conductance, as it flows from the endolymph into the ciliated epithelial cells of the ear via mechano-electrical channels. This influx of K+ is driven by a combination of the membrane potential of these ciliated epithelial cells, and the EP. A study on guinea pig models showed that blocking the K+ channels of melanocytes produces a lower EP [13]. This study agrees with evidence showing that mice deficient in cochlea melanocytes have a lower EP, requiring a greater sound stimulus to produce an action potential [14].
Figure 1: Diagram of the Stria Vascularis, containing the marginal cells, intermediate melanocytes and basal cells. It shows marginal cell extensions intercalating with melanocytes, and the K+ ion channels involved in generating the EP [15].
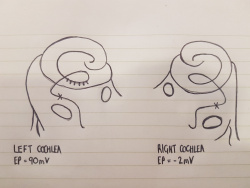
Figure 2: The cochlea of a Wv/Wv viable dominant spotting mutant, showing the distribution of melanocytes as dots within the Stria Vascularis of the left cochlea [16].
Eyes
The uveal tract (iris, ciliary body and choroid) and conjunctiva normally contains melanocytes derived from the neuro-ectodermal neural crest. Peri-orbital soft tissue is also derived from the embryonic neural crest.[17] On the other hand, pigmented melanocytes (such as those found on the retina) were derived from the neuroepithelium or from different layers of the optic cup. [18] Eye melanocytes (like all other melanocytes) differentiate into melanin-producing cells, which in this case determines a person's eye colour, protect the eye from UV radiation [19] and many ocular diseases that can cause blindness, including age-related macular degeneration. [20] Melanocytes found in the eye are not able to participate in regeneration unlike epidermal melanocytes. [21]
Heart
Central Nervous System
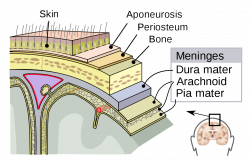
Melanocytes can also be found within the Central Nervous System, primarily in the leptomeninges [23], which consists of the inner two layers membranes of the meninges, the pia mater and arachnoid, which encapsulate the brain and the spinal cord. While its function within the meninges is unknown, they are essential to the health of the meninges, with their removal increases the risk of aseptic meningitis [24]. Furthermore, due to its receptivity to the same signalling molecules as neurons, scientists can study diseases afflicting the central nervous system using it as a model [25].
Embryonic Origins
Development Time Course
Molecular Mechanisms/ Factors/ Genes
Animal Models
Potential Animal Models= Opossum, Platyfish, Zebrafish, Genetically Engineered Mice (GEM)- developments for understanding the biological events in naves and melanoma formation
Current Research
Potential Current Research= Using pluripotent stem cells to generate melanocytes, Regulating melanocytes for neural trans-differentiation
Abnormalities
Skin
Ears
Eyes
Melanoma that occurs in the eye is known as uveal melanoma. This is an uncommon form of cancer that only accounts for about 3% of all melanomas. The risk factors of this cancer are light skin colour, red or blonde hair and blue or light irises. [17]
Heart
Central Nervous System
Glossary
References
- ↑ RAWLES ME. (1947). Origin of pigment cells from the neural crest in the mouse embryo. Physiol. Zool. , 20, 248-66. PMID: 20256541
- ↑ 2.0 2.1 2.2 Westerhof W. (2006). The discovery of the human melanocyte. Pigment Cell Res. , 19, 183-93. PMID: 16704452 DOI.
- ↑ Theriault LL & Hurley LS. (1970). Ultrastructure of developing melanosomes in C57 black and pallid mice. Dev. Biol. , 23, 261-75. PMID: 5476812
- ↑ Markert CL & Silvers WK. (1956). The Effects of Genotype and Cell Environment on Melanoblast Differentiation in the House Mouse. Genetics , 41, 429-50. PMID: 17247639
- ↑ Thody AJ, Higgins EM, Wakamatsu K, Ito S, Burchill SA & Marks JM. (1991). Pheomelanin as well as eumelanin is present in human epidermis. J. Invest. Dermatol. , 97, 340-4. PMID: 2071942
- ↑ Agar N & Young AR. (2005). Melanogenesis: a photoprotective response to DNA damage?. Mutat. Res. , 571, 121-32. PMID: 15748643 DOI.
- ↑ Template:Sangiovanni, G. (1819) Descrizione di un particolare sistema di organi cromoforo espansivo-dermoideo e dei fenomeni che esso produce, scoperto nei molluschi cefaloso. G. Enciclopedico Napoli, 9, 1-13
- ↑ Template:Granville Harrison, R. Archiv für Entwicklungsmechanik der Organismen (1910) 30: 15. https://doi.org/10.1007/BF02263801
- ↑ Template:Boissy RF. Histopathology of vitiliginous skin. In: Hann SK, Nordlund JJ, editors. Vitiligo. Oxford: Blackwell Science Ltd; 2000. pp. 23–34: https://doi.org/10.1002/9780470760116.ch5
- ↑ Template:Purves D, Augustine GJ, Fitzpatrick D, et al., editors. Neuroscience. 2nd edition. Sunderland (MA): Sinauer Associates; 2001. The Inner Ear. Available from: https://www.ncbi.nlm.nih.gov/books/NBK10946/
- ↑ Meyer zum Gottesberge AM. (1988). Physiology and pathophysiology of inner ear melanin. Pigment Cell Res. , 1, 238-49. PMID: 3070525
- ↑ Steel KP & Barkway C. (1989). Another role for melanocytes: their importance for normal stria vascularis development in the mammalian inner ear. Development , 107, 453-63. PMID: 2612372
- ↑ Takeuchi S, Kakigi A, Takeda T, Saito H & Irimajiri A. (1996). Intravascularly applied K(+)-channel blockers suppress differently the positive endocochlear potential maintained by vascular perfusion. Hear. Res. , 101, 181-5. PMID: 8951443
- ↑ Cable J, Huszar D, Jaenisch R & Steel KP. (1994). Effects of mutations at the W locus (c-kit) on inner ear pigmentation and function in the mouse. Pigment Cell Res. , 7, 17-32. PMID: 7521050
- ↑ Locher H, de Groot JC, van Iperen L, Huisman MA, Frijns JH & Chuva de Sousa Lopes SM. (2015). Development of the stria vascularis and potassium regulation in the human fetal cochlea: Insights into hereditary sensorineural hearing loss. Dev Neurobiol , 75, 1219-40. PMID: 25663387 DOI.
- ↑ DENIER A. (1951). [Microwaves of 12 cm; their biological and therapeutic effect]. J Radiol Electrol Arch Electr Medicale , 32, 664-5. PMID: 14909014
- ↑ 17.0 17.1 Schoenfield L. (2014). Uveal melanoma: A pathologist's perspective and review of translational developments. Adv Anat Pathol , 21, 138-43. PMID: 24508696 DOI.
- ↑ Baderca F, Solovan C & Boghian L. (2013). Epidemiological and morphological data of ocular melanocytic lesions. Rom J Morphol Embryol , 54, 77-83. PMID: 23529312
- ↑ Hou L & Pavan WJ. (2008). Transcriptional and signaling regulation in neural crest stem cell-derived melanocyte development: do all roads lead to Mitf?. Cell Res. , 18, 1163-76. PMID: 19002157 DOI.
- ↑ Hu DN, Simon JD & Sarna T. (2008). Role of ocular melanin in ophthalmic physiology and pathology. Photochem. Photobiol. , 84, 639-44. PMID: 18346089 DOI.
- ↑ Yamaguchi Y & Hearing VJ. (2014). Melanocytes and their diseases. Cold Spring Harb Perspect Med , 4, . PMID: 24789876 DOI.
- ↑ Hwang H, Liu F, Levin MD & Patel VV. (2014). Isolating primary melanocyte-like cells from the mouse heart. J Vis Exp , , 4357. PMID: 25285608 DOI.
- ↑ Gudjohnsen SA, Atacho DA, Gesbert F, Raposo G, Hurbain I, Larue L, Steingrimsson E & Petersen PH. (2015). Meningeal Melanocytes in the Mouse: Distribution and Dependence on Mitf. Front Neuroanat , 9, 149. PMID: 26635543 DOI.
- ↑ Goldgeier MH, Klein LE, Klein-Angerer S, Moellmann G & Nordlund JJ. (1984). The distribution of melanocytes in the leptomeninges of the human brain. J. Invest. Dermatol. , 82, 235-8. PMID: 6699426
- ↑ Yaar M & Park HY. (2012). Melanocytes: a window into the nervous system. J. Invest. Dermatol. , 132, 835-45. PMID: 22158549 DOI.