2009 Group Project 5: Difference between revisions
| Line 381: | Line 381: | ||
[The two figures reveal the development of structures deriving from the celluar components of the embryology of the frog].[Structures derived from Germ-layers of frog species ] | [The two figures reveal the development of structures deriving from the celluar components of the embryology of the frog].[Structures derived from Germ-layers of frog species ] | ||
==Life cycle of a frog== | ==Life cycle of a frog== | ||
Revision as of 09:27, 14 October 2009
The Embryology of Frogs
The Frog

Frogs are scientifically identified by the structure of their long posterior limbs, a petite framework, webbed fingers and feet, budged eyes and the lack of a tail. The majority of frog species are extensively recognized as outstanding jumpers, due to their long, dominant legs, which are adaptations to progress jumping performance during activities. The Frog is one of the most frequently studied species in experimental embryology and most anatomical sciences. The Frog is a practically effective model for human embryological development given that the Frog:
- Comprises of genes which can be effectively manipulated.
- Comprises of similar homogeny with humans species.
- Reproduces a large quantity of offspring in a short period of time.
- Are small species which can be simply sustained.
- Are not extremely luxurious and expensive.
The History of the uses in agriculture and research
There are numerous types of frog species that have been manipulated in many developmental experiments. The frog was traditionally used by countless of the early embryology investigators and presently there are several diverse molecular mechanisms regarding progression of the frog. Theses include:
1. Food source; frog legs are a delicacy and are eaten in European countries and in many parts of South American regions.
2. Frogs have the finest biochemical dissection of phenomena that take place in the egg and oocyte. Frogs are the evolutionarily closest to mammals, commonly used as a vertebrate model.
3. Frogs lay thousands of outsized eggs, from which cell extracts can be readily prepared that is capable of recapitulating most molecular phenomena in a test tube.
4. Frogs have been commonly used as a laboratory system for a very long period, and have an extensive history of producing crucial observations in countless fields of biology.
6. Frogs have a important historical connection to the study of epigenetics (John Gurdon-vertebrate cloning and reprogramming) which has been mostly performed on frog species. .
7. Frogs also have very strong evidence of pattern formation and early development, as the embryos are large in size and experimentally manipulative.
8. Recent research into the biochemistry of chromatin and epigenetics has been performed with frog species and mammalian cultured cells.
9. The biggest weak¬ness of the Frog model system is the difficulty of performing genetic experiments and analysis, as frogs are allotetraploid, meaning they take approximately a year to fully develop to sexual maturity, and the genome has not been completely sequenced. Nevertheless, biochemical manipulations of cell extracts, such as immunodepletions and application of heterologous DNAs and nuclei can avoid the need for genetic exploitation.
Growth and development of the Frog

- The frog is a well recognised species, abundant predominantly in ponds, swamps, though various species may also exist in damp or shady environments distant from moist habitats.
- Eggs of specific frogs are easily obtained and may be examined in from the beginning of fertilization onwards. The phases of embryonic development differs in various chordates, yet the typical phases are basically apparent in all frog species. The differences are associated principally to the amount of yolk particles present in an egg.
- The yolk particles offer nourishment of the developing embryo. The process of frog development will be discussed from the phases of gametogenesis to the adult stage.
The Egg
- The embryology of a frog egg is a vast cell; its dimensions are approximately 1.4-1.6 million times larger than a typical aquatic species egg cell. Throughout the frog’s embryonic maturation period, the egg will be transformed into a tadpole encompassing millions of cells but still remains with its constant volume of genetic material, the vast prodction of cell have been illusttrated in the figure on the right revealing the production of eggs from female frogs. [14]
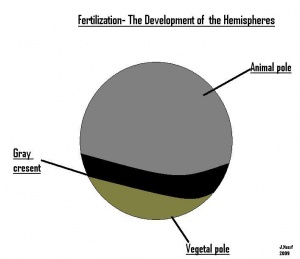
- The early embryonic frog structure consists of three main segments the superior hemisphere known as the animal pole which is usually visible as a grey coloured area. The innermost layer appears to be between the outer two sections known as the gray crescent represented in black. Inferiorly, represents the vegetal pole typically lighter than the superior compartment illustrated in gold.
VIDEO RESOURCE: [15]
History of frog embryology use
- 1851 - Henby Nelson(MD): He identified a remarkable fact through the frog embryo. Henby observed the first cleavage of the yolk, in the egg of the frog. And cleaved structure corresponds in line of direction to the longitudinal axis of the body of the embryo of the frog species.

- 1888 - Wilhelm Roux: Wilhelm Roux attempted to solve the above observation by damaging one cell of a two-cell frog embryo with a hot needle. The cell stayed in place. However, it did not develop further. Its partner developed into a left or right half-embryo;
- 1907 - John Hopkins hospital: In order to identify the cellular source of neuronal fibres. Scientist placed small portions of frog embryo spinal cords in lymph on a microscope slide and was able to observe clear cut neuronal sprouting.
- 1951 - Robert Briggs: Robert Briggs was able to clone a frog embryo by substituting the nucleus of an unfertilized frog egg cell with the nucleus of a frog embryo cell. This process is known as nuclear transplant, has formed the basis for all cloning.
- 1952 - Robert Briggs and T.J. King: Robert Briggs and T.J. King used frog for test experiment. Because the size of the eggs in the frogs are enormous compared with those of mammals, which make them easier to manipulate.
- 1976- Using the technique that had been successful in cloning frog embryos, the doctor transferred the nucleus of one cells into a donated egg cell. As an embryo began to develop, it was implanted into the uterus of a young woman.
- 1997 - Wilmut and Campbell: Utilizing the cloning technique from frog embryo, Drs. Wilmut and Campbell tried the starvation technique on embryo cells to produce Megan and Morag, the world's first cloned sheep and, until now, the most famous sheep in history.
- 2000 - Tokyo University: Scientists at Tokyo University have grown artificial eyeballs. Scientists formed them in tadpoles by using frog embryo cells.
- 2001 - Advanced Cell Technology: Scientists from Advanced Cell Technology announced production of a human embryo clone. This is significant as its parthenogenesis has been artificially induced in frogs.

- 2002 - John Gurdon: John Gurdon from Wellcome Cancer Research Institute in Cambridge experimented on cloned frog embryo.
Gametogenesis
- The sexual reproduction occurs through the fusion of either mature reproductive cells or germ cells known as gametes, which include the sperm from the male frog and ova from female frog species so as to form a single cell, the fertilized zygote. The gametes are typically developed in parents of different sexes . [16]
- Males gametes is a spermatozoon , the female frog gamete is an ovum. Each gamete is formed by a process, recognised as maturation or gametogenesis in gonads. The typical male frog gonads are testies in male, while the female gonads are ovaries. The synthesis of both gonads is associated with the process of fertilization. [17]
- The zygote changes into a mature frog through the process of embryology and metamorphosis. Gametogenesis is a in progression which frog gametes are established from germ cells. Initial germ cells are called primordial germ cells, which can be recognised extremely early in the life of frog species.
The Egg & Fertilization
- This occurs once the sperm cell has inserted, following the insertion of the sperm cells meiosis II is completed, there is a 30 degree position change of the cytoplasm, gray crescent allows this change to be visible in some amphibians, the gray crescent is able what determines the expect ted outline of how the frog will form. The sperm cell joins with the nuclei of the egg which forms the diploid zygote nucleus. --Sando Rashed 10:09, 24 September 2009 (EST)
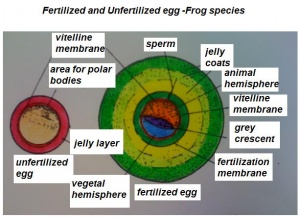
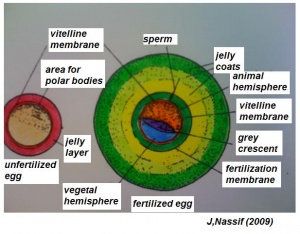
- The early phases in the development of the egg is visible, but must be studied in tadpoles throughout maturation. In embryonic tadpoles of about 10 millimetres in length, soon after the opening of the oral cavity, a pair of longitudinal ridge-like thickenings of peritoneum becomes apparent along the posterior surface of the body cavity situated near to the mesentery and along the inner boundaries of the kidneys. Genital ridges are established in all tadpoles of this age, sex is not distinct until a later period. The development of the egg is illustrated in the figure on the left revealing the structure of a developed egg and a mature of egg.

Maturation of the Egg
- The eggs have currently accomplished their full size, and develop from the exterior of the ovaries like a small shot, but they still have to pass through the course of maturation before they are prepared to be fertilised. This progression of maturation relates to the nucleus almost completely.
- The nucleus component, containing the nuclear fluid that excludes through the nuclear membrane into the substance of the egg, a great segment of the nuclear reticulum vanishes and becomes degraded into separate globules known as nucleoli, but a extremely small division remains in the midpoint as a slender intricately thread recognised as the nuclear skets. Relative to the egg getting discharged from the ovary, the follicles shatter allowing the eggs to fall into the abdominal cavity of the frog species, the egg then passes forwards, directly by the contraction of the muscular wall, somewhat by the movement of the cilia of the peritoneum, to the apex of the oviduct, which positioned at the anterior compartment of the body cavity opposite to the roots of the respiratory organs.
- The terminal part of the oviduct establishes a thin-walled pouch capable of great swelling, inside which the eggs gathers in large numbers. In conclusion, the eggs are migrated out through the cloaca into water which the albuminous investments of the eggs rapidly augment to form the gelatinous mass of the frogs spawn.
Maturation phases
1. Nuclear skein, moves from the midpoint of the egg to its outer surface, which it reaches opposite the midpoint of the black pole. The skein, subsequently an unevenly twisted thread, now presumes the specific arrangement of a nuclear spindle, for instance may be visible in the nucleus of an epithelial or additional cell instantly prior to division of the cell occurs.[18]
2. The first Polar Body, regarding the instance the egg is laid, but prior to its fertilistion, the egg develops a considerably flattened appearance at its upper or black pole, a definite sum of fluid being exuded among the egg and the vitelline membrane. The nuclear spindle currently separate into two identical segments, one of which remains with the egg, and the supplementary is extruded as the first polar body, a small ovoidal white globule, which is situated on the surface of the egg surrounding the exuded peri-vitellline fluid.
3. The Second Polar Body, half of the nuclear spindle that stay behind then splits into two equivalent divisions, one of which remains inside the egg as the female pronucleus, whereas the other segment is extruded as the second polar body, a small white globule extremely related to the first polar body, and like this positioned in the perivitelline fluid on the superior portion of the egg.
4. Fertilisation of the egg, includes the synthesis of the spermatozoon with to egg, specifically, synthesis of the nuclei of these two bodies. The spermatozoa, subsequent to being shed over the seed by the male, distribute vigorously by means of their extended tails, break through the gelatinous investment of the female eggs, bore their way through the vitelline membrane and so go through into the eggs themselves, which they penetrate relative to the superior or black hemispheres.
- Subsequently, an hour following the spermatozoon has entered; a progression may be visible projecting within from the exterior segement of the egg, with a liquid spot in the mid point. This liquid centre is the nucleus of the spermatozoon, and is said to be the male pronucleus, it break through beyond into the female egg, transporting the specialised pigment into it, so that it appears bounded by a pigmented capsule linked with the exterior of the egg by a pigmented stalk.
- By this instance, the second polar body has been established and extruded, and the female pronucleus is merely the only component of the primary egg nucleus still present. Both the male and female pronuceli, which are at initially distance separate to each other, merge and after having enlarged significantly in size then fuse mutually about two and half hours after fertilisation has originated to produce the segmentation nucleus.
- The segmentation nucleus is a huge sphere-shaped vesicle embedded in delicately granular protoplasm, and bounded by a distinct capsule of pigment, its arrangement by the synthesis of the male and female pronuclei completes the action of fertilisation. Specifically female pronucleus may be observe as an imperfect nucleus, and consequently result in the course of fertilisation. The nucleus of the spermatozoon or male pronucleus, replaces the component of the egg- nucleus which has been misplaced as the polar bodies.
5. Segmentation of the Egg, the initial phase of growth consist in constant separation of the egg, whereby it becomes transformed from the unicellular state, which is everlasting only in the lowest species, to muliticelluar state charactertics of all higher species. To these early processes of growth the names segmentation is specified. Shorty subsequent to the competition of fertilisation and arrangement of the segmentation nucleus this later misplaces its sphere-shaped appearance and develops to be spindle-shaped, the yolk granules at the equivalent instance showing a tendency to develop into lines distributing outwards from the distal segment of the spindle.
- The nucleus now split into two halves, which shift away from one another, the yolk granules are likely to combined themselves around the two nuclei, and a slender vertical plate of delicately granular substance is left, dividing the egg.[19]
- Superiorly the eggs depression now becomes visible, initially as a small cavity and then develops as a groove, which almost immediately extends all round, and speedily deepening, splits the egg into two entirely separate halves along a plane equivalent with the vertical plane.
- Shorty after, the two nuclei soon separate again into two, and therefore a second cleft is created in the same state as before, its additionally in the vertical plane, however in a plane at 90 degrees to the initial one, and on its finishing point the egg comprises of four accurately similar segments, each containing a nucleus. The third cleft is horizontal in shape, but not equal, lying closer to the superior than the lower pole, it segregates each of the four cells into, an superior smaller and a inferior larger pole
Cleavage
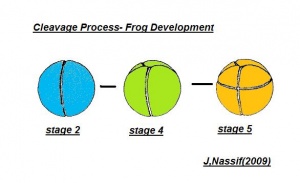
- The egg of the frog is scientifically identified as’ telolecithal’ meaning there is a outsized amount of yolk concentrated at a single pole, in contrast to the concentration of cytoplasm and the site of the nucleus. The cleavage phases are holoblastic, therefore the total and after the second cleavage they are asymmetrical .The initial cleavage stage appears about two and half to three hours after fertilization. It commences as a minor depression in close proximity to the centre of the animal hemisphere. It appears as if some interior force is pushing the surface the egg towards the centre. This small upturned fold steadily continues in the form of a channel until it surrounds the egg. This groove is shallow in the commencement, but develop into deeper eventually separating the fertilized egg into two halves recognised as the blastomeres. [The figure on the left symbolise the development and growth of the frog embryo during cleavage].
- Internally the separation is mitotic, consequently each daughter cell contains a nucleus resulting from the copulation nucleus of the fertilised egg. This cleavage is vertical, the two cells are indistinguishable in respect of cytoplasm, pigment and yolk. The subsequent cleavage appears about an hour after the first. The channel of this cleavage begins at the centre of the animal hemisphere, is at right angles to the first and is vertical. This divides the egg into four blastomeres. The fourth blastomere so produced are not qualitatively equal, since of these only two contain the material from the gray crescent. The cleavage begins about thirty minutes after the second is completed or four hours after fertilisation. the cleavage plane of the third furrow is horizontal and slightly above the equator. Thus the four upper cells are a little smaller than the four lower cells. The smaller blastomeres are called micromeres and the larger blastomeres are called macromeres. The fourth cleavages follow 20 minutes after the third and tend to be vertical. This is usually a double furrow.
- The cleavage rate is accelerated with each of the early divisions and since the blastomeres are of unequal size and have varying amounts of cytoplasm and yolk, synchronous cleavage is lost and there is an obvious overlapping of the division. The upper most cells divide more rapidly than the lowermost cells. From this point onwards perfect symmetry in cleavage and in blastomere is very rare, although the embryo developed perfectly. The fifth cleavage is also doubled, appearing first in the upper hemisphere and then in the lower.
- The cleavage thus far follows the rule that each cleavage plane comes in at right angles to the previous one. The subsequent divisions become so irregular that it is previous one. The subsequent division become so irregular that it is impossible to trace out any plan or procedure. The segmentation continues more rapidly in the pigmented regions, since at that place the protoplasm is most dense, whereas, yolk which is very abundant in the vegetal side delays cell division. The multicelluar embryo at this stage is called morula by some biologists.
Gastrulation
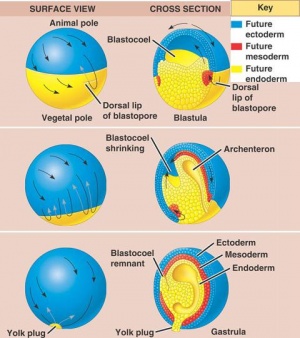
The phase of Blastulation is followed by the unique developmental progression of gastrulation. The process of frog gastrulation involves the following processes.
1. Epiboly Phase
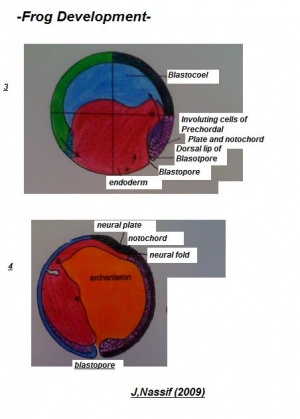
The pigmented cellular materials of blastula have the propensity to overgrow the yolk cells within the developing content. This course which is known as epiboly commences gradually in the final blastula and rapidly accelerates with advancing gastrulation development. The cellular matter within the animal pole reproduces and matures on all sides except in area surrounding the dorsal lip of the blastopore has been established.
2. Convergence
Scientists suggested that the unfolding was thought to result in spreading of the superficial cells over a substrate with suitable absorption properties. Consequently epiboly and convergence are an outcome to this spreading tendency, which is actually increased by a reduction in surface tension of the distributing cells.
3. Rotation
The epiboly phase is maintained until the region of the dorsal lip has migrated and is a to some extent greater than 90 degrees and the region of the white blastopore is reduced to small circular rings. This section will be positioned away from the developing vegetal pole. Laterally the complete developing gastrula has been rotated to a horizontal axis, allowing it to lie at right angles to the original median plane of the egg. Therefore, the course of rotation is such that the dorsal lip is practically pushed backwards in one direction as rapid or quicker than epiboly moves it forward in the other. The outcome will be that the blastopore produced at roughly the vegetal pole is posterior, and the dorsal and ventral lips are essentially dorsal and ventral. This in addition allows the eggs to form the antero-ventral side of the potential embryo whilst the area marked by the grey crescent is to develop the dorsal segment.
4. Invagination
Visible is a small fissure- like invagination which is apparent in the middle between the equator and the vegetal segment. The most superior or dorsal border of the cleft produce the dorsal lip of the blastopore. This imput move back and forth on the dorsal plane near the dorsal lip and migrates around the boundaries of the blastocoel in company with the extension of the lateral lips. This adapted invagination is maintained until the blastocoel cavity has been almost abolished; excluding the slender opening separating epiblasts from the hypoblast, the new segment formed is known as the archenteron cavity.
5. Involution
Additional to previous course of action a distinct involution also occurs at the blastoporal borders. This is mainly dynamic at the median dorsal lip and gradually more less as it shifts across either side until it reaches the ventral lip where it constant.Throughout this progression, cells positioned beside the superior boundaries of the blastoporal lip migrate over the lip to the interior portion of the lip. These cells are remained within the embryo along the internal edge of the blastopore. This indicates the root of archenteron is composed of involuted cells and beyond this the external layer known as the ectoderm. The base and lateral sides are enclosed mainly of endodermal cellular material, which have been established from larger yolk cells, situated in the vegetal pole of the blastula. In the final phases of the gastrula development, the cells contributing to the floor of the archenteron, thin out significantly where they surround the blastocoel. The hemispherical shaped dorsal lip of blastopore which become visible at the commencement of the gastruation stage carry on to augment, initially becoming semicircular, then transform into a horse shoe shaped and then finally forming into circular band. The band is the accomplished by the blastopore. Various yolk cells of vegetal pole present in the section are crowed into the blastoporal cavity where they form a gathering identified as a yolk plug. Blastopore rapidly reduces in size while the archenteron is still developing and becomes completely formed in the final stage; the yolk plug appears as only a small oval on the gastrula.
Growth and Modification of Frog Species
1. Changes in habits and habitats:
a) In frogs, metamorphosis is related with to the modifications and adaptations as a frog changes environmental habitats from an aquatic to a terrestrial mode. Metamorphosis has modified the adaption of frogs as this alteration it began during the aquatic adaptations in the surface of water to breathe air. Subsequently, it continues the terrestrial surroundings, therefore the frog species become abundant in vegetation area allowing the frog to transform in to amphibious species.
b) The transition during metamorphosis is linked with a change in food chain. The tadpoles being the embryonic origin of frogs are herbivorous, feeding on algae and green matter, which they collect by the adapted feature such as their teeth surrounding their mouths. Adult frogs, alternatively, are classified as carnivorous feeding on insects and worms. Occasionally, they also consume larger prey, for instance smaller frogs species and even little birds and rodents which they dominant and ingest.
2. Changes in Morphology
These include the decrease or complete absence of specific organs or structures which are essential during development of early frog embryology, but not critical in the mature frog species. The significant alterations of this nature include the following developmental structures.
a) The long tail of the tadpole alongside the fin folds is absorbed again during metamorphosis and becomes absent at the final stage of the metamorphosis.
b) The developing gills are resorted, the gill clefts are congested and the branchial cavities start to become absent. The reabsorbtion of gills also takes place by autolysis.
c) The teeth of the perioral disc additionally the homey lining of the jaws are shed.
d) The lateral line sense organs within the skin of tadpoles vanish throughout metamorphosis.
e) The cloacal tube begins to condensed and reduced.
f) Various blood vessels, together with parts of the aortic arches, are reduced during mature development.
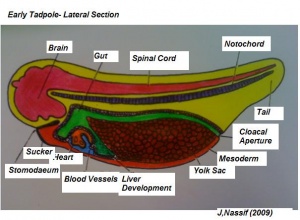
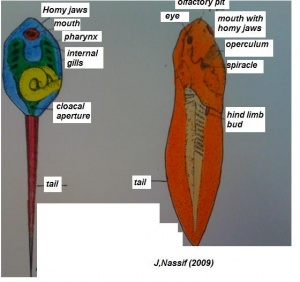
(The figure on the left represents the transformation from a tadpole to a developing structure.)[20]
3. Progressive or constructive changes
This process comprises of the development of specific organs which mature into functional systems only during metamorphosis.
a) During development the limbs continue to augment in size and differentiation. The forelimbs, which in the tadpole mature under the opercular membrane, which then break through to the exterior. Simultaneously there is a increase in the length and strength of the hind limbs, joints develop in them and the toes become visible.
b) The middle ear becomes apparent in relation with the first pharyngeal pouch. The tympanic membrane matures. It is bounded by circular tympanic cartilage which allows the frog to retain air.
c) The visual organs bulge up on the dorsal surface of the head developing the nictitating membrane.
d) There is augmentation of the tongue and the formation of thevomerina teeth.
4. Remodelling of some structures
Systematic organs which operate both in the early embryonic larva and the mature adult change their differentiation during metamorphosis so as to meet the requirements of the adult mode of life and due to the habitat adaptations. The figure on the right illustrates structures developing from specic germ cell layers.
a) The skin of the embryonic tadpole is lined with a double-layered epidermis. The number of layers of cells in the epidermis augments throughout metamorphosis. Superficial surface layers become cornified. Multicellular mucous and serous glands originate in the skin. The pigmentation of the skin continuously changes, new patterns and colour start to form.
b) There is a lengthening of the mouth gap as a result of rotation of the quadrate cartilage and the true jaws become functional.
c) The tongue rapidly progresses and becomes larger and more muscular.
d) The eyes become more specialised.
e) In early tadpoles, the GIT is extremely long and wound up into a spiral folds. The intestine become greatly lengthened in herbivorous species due to the vegetables food chain.[21]
5. Development of the reproductive system
(The figure below represents the reproductive and development cycle of a frog as it transform from egg to frog specie.)
In tadpoles, right after the mouth is being formed, two indentation like thickenings of peritoneum begin to appear near the body cavity (dorsal surface), which is nearest to the mesentery which is near the developing kidneys. These appear in all tadpoles.
These indentations appear here because of a change in the endothelial cells, everywhere else they are usually flat looking cells but they undergo a change and in this particular area become somewhat cuboidal/columnar.
Later on the indentations become more obvious due to the epithelial cells replicating numerously to form a thick layer. The posterior two thirds of the indentation for the female is the ovary and for the male it is the testis. The third that is left differentiates and becomes fat for the body.

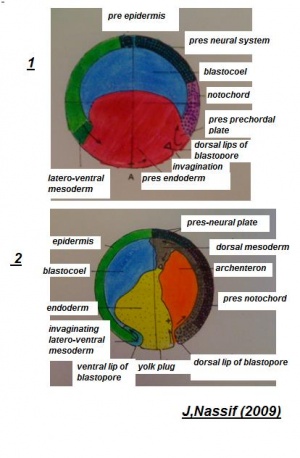
Delamination
Delamination indicates a mass separating a group of cellular matter from other cellular groups. It has been illustrated that the division of notochord, mesoderm and endoderm tissues from each other to form distinct cellular masses is completed by the progression of delamination, subsequent to these materials moving to the inside throughout gastrulation. During the developing gastrula, the germ layers are all recognized. These distinct segments of the embryo develop from these germ tissue layers.
Polarity and Rotation
Throughout fertilisation to the beginning of gastrulation, the frog’s egg continues in the original location in relation to its polarity. Subsequent to gastrulation its polarity begins to differentiate. This progression is linked with migration of materials within the gastrula and can be responsible for the alterations of the centre of gravity.
Closing of Blastopore
Subsequent to the formation of the gastrula, the blastopore is visible as a tiny round circular filled cavity containing the yolk plug. As it continues to reduce in dimension, it represented as a pear-shaped outline through the mutual approach of its lateral lips. Through it final progression these fuse entirely to produce a longitudinal groove, the streak which continue dorsally and ventrally in a small aperture. The inferior aperture closes, resulting in a depression called the anal pit. The superior region remains open for some period and begins to as the neural groove is laid down.
Post Gastrulation or Organogenesis
Throughout the duration of pre-gastrulation, all tissues for different organs vanish from the surface of blastula and migrate inside to take their final arrangement in the embryo structure where organs are developed from their potential regions. Consequently organogenesis transfers an embryo into free larva structure.
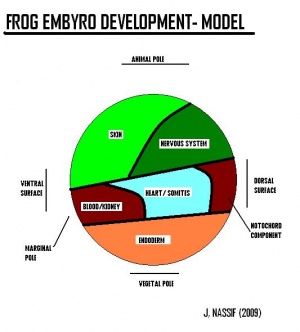
Germ-layer origin of various body tissues
Ectoderm will give raise to structures such as skin, brain, spinal cord, neurons and sense receptors. Mesoderm will give raise to structrues such as notochord, muscles, blood, bone and sex organs. Endoderm will give raise to sturctures such as inner lining of gut, liver, pancreas, lungs and bladder, thyroid and parathyroid glands and thymus.
Structures derived from Germ-layers of frog species
Ectoderm
Establishes the epidermal segments and its derivatives, to the frog’s nervous system. The ectoderm also contributes to :
1. The olfactory and auditory epithelium.
2. The retina and lens of the eye.
3. Sensory organs.
4. The epithelial lining of the oral cavity and the anus.
5. The pineal and pituitary body.
Mesoderm
Mesoderm originates:
1. Connective tissue.
2. Muscles, except the notochord.
3. Blood vessels.
4. Lymphatics.
5. The peritoneum and the urinary and reproductive system.
6. The dermis, parts of the eye excluding lens, cornea, and conjunctiva.
Endoderm
From the endoderm arises:
1. The epithelial lining to the gut and oesophagus, stomach, intestine, bile duct.
2. The hepatic cells of the liver, respiratory tract, larynx, trachea and lungs.
3. The lining of the urinary bladder, pancreas thyroid and thymus.
[The two figures reveal the development of structures deriving from the celluar components of the embryology of the frog].[Structures derived from Germ-layers of frog species ]
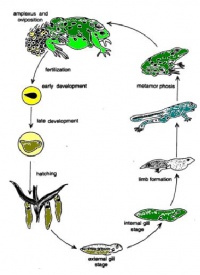
Life cycle of a frog
Fertilization-egg:
The female lays eggs in the spring. A group of fertilized eggs is called spawn;
7-10 days:
Fertilized egg feed on remaining yolk which is in its gut. Their gills, mouth and tail have poorly developed. Begin to swim and feed on algae;
10-30 days(4 weeks):
A layer of skin grows over gills. Teeth begin to appear. A coiled gut start to develop. This is aid in digestion;
30-60 days(6-9 weeks):
Legs being to grow and head is more distinct. Start to eat insects. Arms begin to grow;
60-80 days(12 weeks):
Resemble a frog. Still have remaining tail;
80-140 days(20 weeks):
Fully developed, spend most of time out of water. Majority of frog live between 4-15 years.
Timeline of frog development
Development of egg and embryo at temperature 18 degree celsius.
0 hours - fertilization of the egg
1 hours - formation of the gray crescent due to pigment migration
3.5 hours - early cleavage
4.5 hours - blastula stage(coeloblastula with eccentric blastocoel
26 hours - gastrulation
26 hours - early - crescent shaped dorsal lip
34 hours - middle- semicircular blastoporal lip
42 hours - late - circular blastoporal lip
50 hours - neurulation
50 hours - early - medullary plate
62 hours - middle- neural folds converging
67 hours - late - neural tube formed and ciliation of embryo
84 hours - tail bud stage(early organogeny)
96 hours - muscular response to tactile stimulation
118 hours - early heart beat, development of gill buds
140 hours - hatching and gill circulation
162 hours - mouth opens and cornea becomes transparent
192 hours - tail fin circulation established
216 hours - degeneration of external gills, formation of operculum, development of embryonic teeth
240 hours - opercular fold over brachial chamber except for spiracle and internal gills
255 hours - prolonged larval stage with refinement of organs
270 hours - development of hindlimbs, internal development of forelimbs in opercular cavity
275 hours - projection of forelimbs through operculum, left side first
280 hours - absorption of the tail and reduction in size of the gut
284 hours - metamorphosis complete, emergence from water as miniature, air breathing frog
Stages of frog embryology
--Mark Hill 08:40, 9 October 2009 (EST) see my comment on images
The rate of development of the egg and embryo will depend upon the temperature at which they are kept. The approximate schedule of development at 23 degree celsius is provided below.
| Stage | Time since fertilisation (hours) | Stage Characteristic | Embryo characteristic | Image of frog stage |
|---|---|---|---|---|
| 1 | 0.00-1.30 | fertilization of the egg, post fertilization | animal hemisphere, gray crescent and vegetal hemisphere are present | |
| 2 | 1.30-2.00 | splits into two cells | appearance of first cleavage furrow | |
| 3 | 2.00-2.15 | becomes four cells | appearance of second cleavage furrow | |
| 4 | 2.15-2.45 | becomes eight cells | appearance of third cleavage furrow | |
| 5 | 2.45-3.00 | becomes sixteen cells | appearance of fourth cleavage furrow | |
| 6 | 3.00-3.30 | becomes thirty-two cells | appearance of fifth cleavage furrow | |
| 6.5 | 3.30-4.00 | blastula stage | Three dorsal folds become visible as a result of endoderm invagination. Pole cells no longer visible on surface | |
| 7 | 4.00-5.00 | Gastrulation stage | two primary germ layers. epiblast and endoderm | |
| 8 | 5.00-7.00 | neurulation | medullary plate, neural folds and neural tube | |
| 9 | 7.00-9.00 | germ layer | complete lip involution encircling yolk | |
| 10 | 9.00-11.00 | early gastrula | two primary germ layers | |
| 10.5 | 11.00-11.45 | gastrula | two germ layers | |
| 11 | 11.45-12.30 | medulla plate | Yolk sac protrudes dorsally, labium moves to midline on ventral side | |
| 11.5 | 12.30-13.15 | continuation of medulla plate | start closing the plate | |
| 12 | 13.15-14.15 | early neurula | thickened ectoderm give rise to CNS | |
| 12.5 | 14.15-14.45 | neural folds | expansion of cavity | |
| 13 | 14.45-16.15 | neural folds | continue expanding cavity | |
| 14 | 16.15-17.30 | neural folds | continuation of cavity | |
| 15 | 17.30-18.15 | tail bud stage | early organogeny | |
| 16 | 18.15-18.45 | tail bud stage | posterior ventral view | |
| 17 | 18.45-19.45 | tail bud stage | anterior view | |
| 18 | 19.45-20.45 | close neural fold | anterior view | |
| 19 | 20.45-21.45 | neural fold close complete | dorsal view | |
| 20 | 21.45-22.30 | early tail bud | anterior view | |
| 21 | 22.30-24.00 | termination of neural crest | progress to next stage | |
| 22 | 24.00-24.45 | prolonged development | elongation | |
| 23 | 24.45-26.15 | elongation of embryo | later view | |
| 24 | 26.15-27.30 | continuation of elongation of embryo | dorsal view | |
| 25 | 27.30-29.30 | organs development | embryo elongates and develops dorsal thickening | |
| 26 | 29.30-31.15 | elongation | dorsally forms neural and brain cavity | |
| 27 | 31.15-32.30 | elongation of embryo | dorsal view | |
| 28 | 32.30-35.00 | further development | lateral view | |
| 29-30 | 35.00-37.30 | tail bud | growth | |
| 31 | 37.30-40.00 | prolonged development | bud development | |
| 32 | 40.00-44.30 | early muscular development | elongation | |
| 33-34 | 44.30-50.00 | muscular movement | apparatus for locomotion | |
| 35-36 | 50.00-53.30 | muscular movement | apparatus for locomotion | |
| 37-38 | 53.30-56.30 | heart beat | develops apparatus | |
| 39 | 56.30-66.00 | mouth opens | cornea transparent | |
| 40 | 66.00-76.00 | gill circulation | hatching | |
| 41 | 76.00-80.00 | tail, fin circulation | circulation | |
| 42 | 80.00-86.00 | internal gills, operculum | opercular fold, teeth | |
| 43 | 86.00-98.00 | operculum complete | operculum closed on right | |
| 46 | 98.00-106.00 | metamorphosis |
Abnormalities of frog
Abnormalities of frog could be caused by multiple factors. These include change in climate, predators, parasites, bacteria, fungi, viruses or pollution and contaminants such as pesticides, metals and fertilizer.[22]
The Abnormalities are further classified into different categories. These categories are:
1. Infectious Diseases:
Perkinsus Symptoms- caused by perkinsus-like protozoan organism. Symptoms include swollen viscera that leads to a bloated body and and infected swollen heart
Ichthyophonus symptoms- cause by parasitic genus, where symptoms include a swollen tail resorption site that matches the surrounding skin in color and translucency.
2. Surficial Abnormalities:
Surficial abnormalities as the one that are visible on the surface of the skin. This includes abnormal pigmentation, , subcutaneous hemorrhaging, wounds due to trauma or from a predator Edema is a fluid-filled swelling under skin whihc is also a surficial abnormality.
3. Skeletal Abnormalities:
This is further classified into three more categories:
a. Skeletal Malformations-
- Microcephaly (small head or blunt snout)
- Scoliosis (Curved spine in lateral direction)
- Shrunken Limb (Micromelia)
- Amelia (Completely missing limb with no stump)
b. Skeletal Abnormalities of Unclear Etiology-
This condition arises when a frog has ectromelia (missing limb), brachydactyly (missing digits) or other such condition with no visible trauma or external damage, then this will be classified as the Skeletal abnormality of unclear etiology.
c. Skeletal Injuries-
Any limb missing or broken due to trauma or other related reason which shows clear evidence of it is classified as skeletal injury
4. Eye Abnormalities:
Most common includes-
i. Anophthalmia: Skin covers the eye socket as there is no eye
ii. Abnormal Iris Coloration: Reduced pigment in either one eye or both resulting in two different colours of the iris at times
iii. Abnormal size or shape: Both eyes either deviating from each other or size of pupil or iris is different than the other eye
Genetics
There are a wide variety of species that differ from each other when it comes to genetics and their functioning.
TAXONOMY: Phylum Chordata / Sub Phylum Vertebrata / Class Amphibia / Order Anura
Their are more than two dozen different families of frogs, where suborders are:
1. ARCHAEBATRACHIA- most primitive frogs
2. MESOBATRACHIA- linked between the Archaebatrachia and Neobatrachia
3. NEOBATRACHIA- most modern frogs
| Number of frogs' species | 5280 |
| Smallest frog genome size: | 0.95pg, Ornate burrowing frog |
| Largest frog genome size: | 13.40pg, Ornate horned frog |
| Mean of frogs' genome | 4.68pg ± 0.13 |
- Majority of frogs have only 22 to 26 chromosomes and polyploid is very common where they are almost bisexual.
- Polyploid Amphibians reduce their total cell number such that they acheive the same body size as diploids. One of the most unsual forms of polypoidy in amphibians is the water frog Rana esculenta from Europe and Western Asia.
- The species with larger genomes have more genes for e.g. the frog genus Xenopus includes 16 species, with genome sizes ranging from 3.5 x 10^9 bp to 1.6 x 10^10 bp. These differences have arisen by numerous events of polyploidization within the past 40 million years or so. The ancestral chromosome number for the genus seems to have been 18, but there are species with 36, 72 and 105 chromosomes [25]
| SPECIES | X.LAEVIS | X.TROPICALIS |
|---|---|---|
| PLOIDY | Allotetraploid | Diploid |
| NO. OF CHROMOSOMES | 36 chromosomes | 20 chromosomes |
| GENOME SIZE | 3.1 x 10^9 bp | 1.7 x 10^9 bp |
| EGG SIZE | 1-1.3 mm | 0.7-0.8 mm |
| GENERATION TIME | 1-2 years | 4 months |
Genome Sequencing
Since Frog has a huge family with many different species, only few main species that are used widely for the purposes of experiments have their genome sequenced.
Xenopus tropicalis has one of the smallest genomes among amphibians and has the shortest generation time- four to six months and the only diploid genome among the 14 Xenopus species. This means it has no more than two copies of most genes, whereas the other species of Xenopus have four copies of most genes (pseudotetraploid). US Department of Energy's Joint Genome Institute (JGI) in Walnut Creek, California, initiated the X. tropicalis Genome Project, finding 1,700 million base pairs. [27]
Xenopus oocytes
- The oocytes provide an important expression system for molecular biology.
- By injecting DNA or mRNA into the oocyte or developing embryo, scientists can study the protein products in a controlled system. This allows rapid functional expression of manipulated DNAs (or mRNA). This is particularly useful in electrophysiology, where the ease of recording from the oocyte makes expression of membrane channels attractive.
- One challenge of oocyte work is eliminating native proteins that might confound results, such as membrane channels native to the oocyte.
- Translation of proteins can be blocked or splicing of pre-mRNA can be modified by injection of Morpholino antisense oligos into the oocyte (for distribution throughout the embryo) or early embryo (for distribution only into daughter cells of the injected cell).
Xenopus Laevis
- Xenopus Laevis is an important model organism in developmental biology. X. laevis is tetraploid and reaches sexual maturity in 1 to 2 years. What makes it important in developmental biology is its large and easily manipulable embryo.
- Extracts from the eggs of X. laevis frogs are also commonly used for biochemical studies of DNA replication and repair, as these extracts fully support DNA replication and other related processes in a cell-free environment which allows easier manipulation.
- The Human chorionic gonadotropin hormone (hCG) is present in the urine of the pregnant women in large quantities only. This hormone in the urine induces X.laevis oocyte production which formed the basis of first well-documented method of pregnancy testing X. laevis is also notable for its use as the first well-documented method of pregnancy testing when it was discovered. Today, commercially available HCG is injected into Xenopus males and females to induce mating behavior and breed these frogs in captivity at any time of the year.
Gene cluster
- The alpha and beta globin genes are closely linked in small cluster of frogs compared to human and chicken
- The similarity of architecture of two clusters found in X.laevis supports the tetraploid origin
- The two loci encoding the muscle specific creatine kinase isoenzymes and have large differences in developmental profile, therefore suggesting that duplicate loci that have not undergone silencing are not simply redundant copies but have acquired specialization.
- Contains twice number of genes for proteins i.e. haemoglobin and sarcomeric actin serum albumin compared with other species
Current Embrology Research
Currently, a number of fields have benefited from the development of frog embrology. These include cloning, verification of messenger RNA, and Cell cycle.
Cloning
In 1952, Robert Briggs and Thomas J King cloned northern leopard frogs using a method of nuclear transfer. Briggs and King's experiment was similar to that envisioned - and piloted using salamanders - by Hans Spemann in his 1938 proposal for a 'fantastical experiment'. Later, John Gurdon extended this work and showed that nuclei from differentiated cells could support development, although less well than those from early embryos. [29]
Lemaitre et a. (2005) from MRC Cancer Cell Unit, Cambridge, U.K. worked on experiments that demonstrated that importance of serial nuclear transplantation for the sucessful cloning of frogs. He demonstrated that exposure of somatic-cell nuclei (erythrocyte nuclei) and sperm nuclei to an extract of mitotic cell extract reorganizes the chromatin into shorter loops and allows replication at much shorter intervals along the DNA. This increases the efficiency of DNA replication in mammalian cell-free systems.
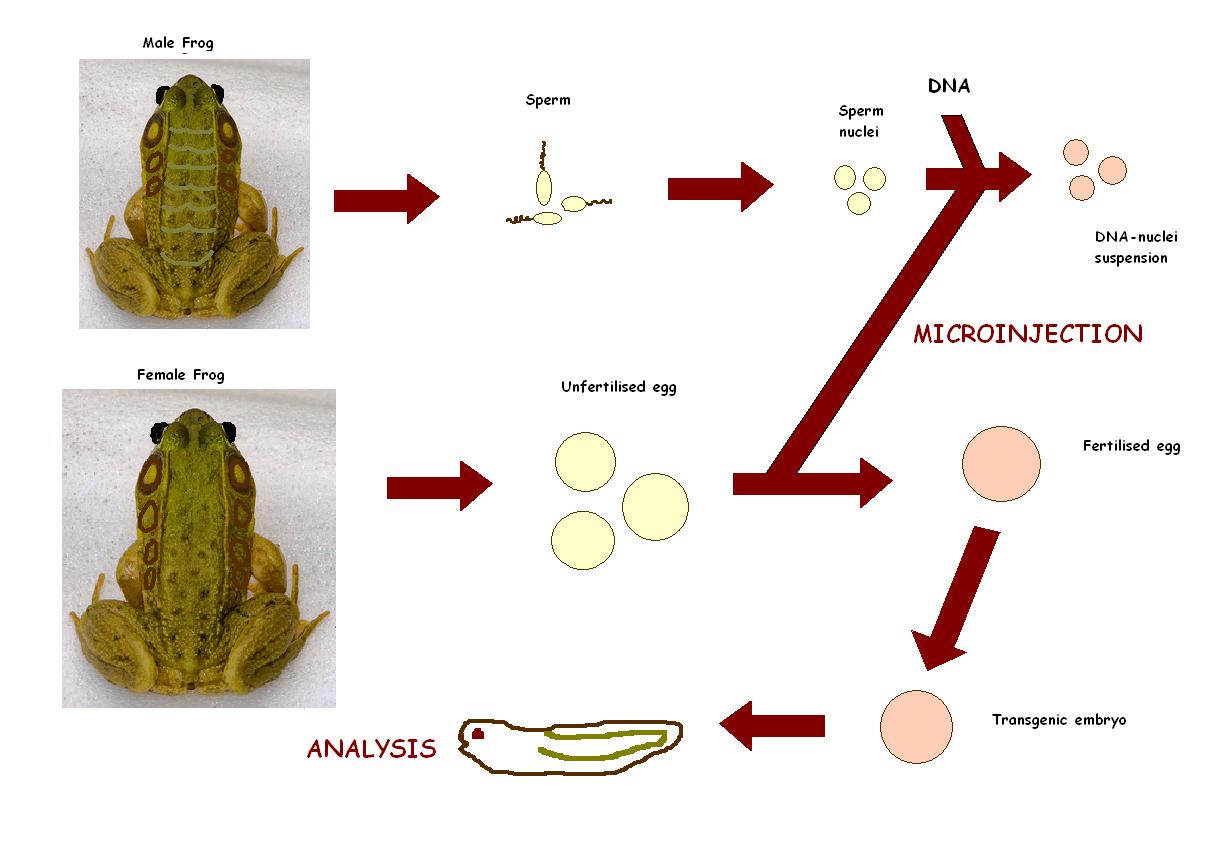
Transgenesis techniques for functional genomics in Xenopus
Transgenesis in Xenopus is made possible due to large embryos, a reliable fate map, ease of microinjection, ease of dissection/micromanipulation and existence of the neuroendocrine reflex of background adaptation as a model for activation/inact.
Recently, Hajime Ogino and Haruki Ochi from Nura Institute of Science and Technology in Japan focused on the genomic resources and principles of the transgenesis techniques in Xenopus, and discusses their applications to genome wide network analysis, with emphasis on the use of bioinformatics tools. This is will to understand the gene regulatory networks that control vertebrate development. [30]
Xenopus used to be a problem in earlier days when it came to transgenesis. Current methods involves isolated sperm nuclei from Xenopus testis that are microinjected into unfertilized eggs. The generated transgenic tadpoles contain 5-35 copies of the integrated plasmid that are expressed in all cells. If desired, expression of the transgene can be directed to the appropriate tissue and at the appropriate time by using specific promoters, mostly the Xenopus POMC gene promoter (isolated from a Xenopus genomic DNA library) to specifically direct expression of green fluorescent protein (GFP) at high levels to the intermediate pituitary cells, which can either be over expressed or inhibited.[31]
Verification of messenger RNA
While the existence and role of messenger RNA (mRNA) was known in bacteria, in the 1960s it was still debated whether it also existed in vertebrates. Taking haemoglobin mRNA from immature red blood cells and injecting it into a Xenopus oocyte, John Gurdon showed that the haemoglobin protein was indeed produced. Producing proteins in Xenopus oocytes has proved to be extremely useful in cell biology, in particular for the study of receptor proteins.[32]
Cell Cycle
As they develop outside the mother, frog eggs are well stocked with the proteins needed to drive the development of the embryo. Studies of these processes has shed considerable light on the processes involved in cell division - termed the cell cycle. [33]
Limb development in Xenopus Laevis

Donald D. Brown (2005),Department of Embryology, Carnegie Institution of Washington demonstrated factors related to limb development of Xenopus Laevis. Thyroid hormone (TH) is found to be required for limb development in this frog. Specific cell types in the growing limb were targeted for expression of a dominant negative form of the TH receptor by sperm-mediated transgenesis. Limb muscle development, the innervation of muscle from the spinal cord, and cartilage growth can be inhibited without affecting patterning of the limb or differentiation of other cell types. Remodeling of the skin occurs late in metamorphosis after the limb has formed. The coordination of these independent programs is affected in part by the control that TH exerts over DNA replication in all cell types of the limb. [34]
Glossary
- Amphibian:
Relating to or characteristic of animals of the class amphibia.Amphibians are found in the taxonomic class of amphibia, amphibians are capable of both occupying and successfully living in both land and aquatic communities.amphibian
- Augmentation:
Enlargement/Increase in cellular size.
- Autolysis:
breakdown of a part or whole cell or tissue by self-produced enzymes
- Blastomeres:
The undifferentiated cells formed by cleavage of the fertilised ovum. This includes cells in the cleavage, morula, and blastula stages of the embryo
- Carnivorous:
Flesh-eating; subsisting on animals as food.
- Cleavage:
he repeated division of a fertilised ovum
- Cleft:
an opening, fissure, or V-shaped indentation made by or as if by splitting
- Chordate:
Members of a diverse phylum of animals that, as embryos, possess a (1) notochord; (2) a dorsal, hollow nerve cord, (3) pharyngeal gill slits; and (4) a post-anal tail
The act or state of splitting or dividing of a cell, particularly during the telophase of (animal) cell division.
- Cytoplasm:
The cytoplasm (of both eukaryotes and prokaryotes) is where the functions for cell expansion, growth, metabolism, and replication are carried out
- Epiboly:
The expansion of one cell sheet over other cells, as takes place during gastrulation
- Fertilization:
A process in sexual reproduction that involves the union of male (sperm) and female (ovum) gametes (each with a single, haploid set of chromosomes) to produce a diploid zygote.
- Gamete:
A reproductive cell (male (sperm) or female (egg)) that has only half the usual number of chromosomes
- Gametogenesis:
process leading to the production of gametes. The development and maturation of sex cells through meiosis.Another name for meiosis where a diploid cell is divided into two haploid cells with half the chromosome content of the diploid parent cell.
- Herbivorous:
eating plants; of or pertaining to the herbivora.
- Holoblastic:
The complete division of an isolecithal or microlecithal egg into blastomeres
- Invagination:
One of the methods by which the various germinal layers of the ovum are differentiated.
- Isoenzymes:
Isozymes (also known as isoenzymes) are enzymes that differ in amino acid sequence but catalyze the same chemical reaction.
- Mesentery:
membranous fold attaching various organs to the body wall.
- Metamorphosis:
A change in the form and often habits of an animal after the embryonic stage during normal development.
- Neuroendocrine:
Neuroendocrine [IPA nʊəroʊˈɛndəkrɪn] cells are cells that release a hormone into the circulating blood in response to a neural stimulus.
- Organogenesis:
The part of embryonic development where the body's main organs develop.
- Peritoneum:
The smooth serous membrane which lines the cavity of the abdomen, or the whole body cavity when there is no diaphragm, and, turning back, surrounds the viscera, forming a closed, or nearly closed, sac.
- Perivitelline space:
The perivitelline space is the space between the zona pellucida and the plasma membrane (sometimes called the vitelline membrane) in a fertilized ovum.
- Polyploidy:
Cells with three or more sets of chromosomes.
- Pronucleus:
the nucleus of the ovum or sperm after fertilization but before they fuse to form the nucleus of the zygote
- Protoplasm:
The fluid living content of the cell that consists of two major divisions, the cytoplasm and the nucleoplasm (cell nucleus). It is composed mainly of nucleic acids, proteins, lipids, carbohydrates, and inorganic salts
- Segmentation:
division of some metazoan bodies (such as annelida and Arthropoda) into repeated parts, segments. Segmentation can be homomeric (more or less the same) or heteromeric(different from each other).
- Terrestrial:
Of or on the ground, of the habitat of a plant, on land as opposed to in water, or on the ground as opposed to on another plant.
- Transgenesis:
Transgenesis is the process of introducing an exogenous gene - called a transgene - into a living organism so that the organism will exhibit a new property and transmit that property to its offspring.
- Yolk:
nutritive material of an ovum stored for the nutrition of an embryo
- Zygote:
The cell from which an organism develops, that results from the fertilization of the egg by the sperm.
Primary Resource "Biology- Online Dictionary[35]
References - Frog Embryology:

1. Savage, J. M. (2002). The Amphibians and Reptiles of Costa Rica. University of Chicago Press, Chicago [36]
2. Ford, L.S.; D.C. Cannatella (1993). "The major clades of frogs". Herpetological Monographs 7: 94–117 [37]
3. Tyler, M. J. (1994). Australian Frogs A Natural History. Reed Books [38]
4. Cogger, H.G.; R.G. Zweifel, and D. Kirschner (2004). Encyclopedia of Reptiles & Amphibians Second Edition. Fog City Press [39]
5. Beltz, Ellin (2005). Frogs: Inside their Remarkable World. Firefly Books [40]
6. Tyler, M. J. (1994). Australian Frogs A Natural History. Reed Books [41]
7. Moury JD, Hanken J (1995) Early cranial neural crest migration in the direct-developing frog, Eleutherodactylus coqui. Acta
Anatomica (Basel) 153, 243-253.
8.Raynaud A (1985) Development of limbs and embryonic limb reduction. In Biology of the Reptilia (ed. Gans C, Billett F), pp.
59-148. New York: John Wiley.[42]
9. Hanken J (1986) Developmental evidence for amphibian origins. In Evolutionary Biology (ed. Hecht MK, Wallace B, Prance GT), 20, pp. 389-417. New York: Plenum Press. [Hanken J (1986) Developmental evidence for amphibian origins. In Evolutionary Biology (ed. Hecht MK, Wallace B, Prance GT), 20, pp. 389-417. New York: Plenum Press.]
10. Elinson RP (1990) Direct development in frogs : wiping the recapitulationist slate clean. Seminars in Developmental Biology
1, 263-270.
11. Beebee, T. J. C. 1996. Ecology and Conservation of Amphibians. Chapman and Hall. London. [43]
12. Lehtinen, R. M. 2002. The use of screw pines (Pandanus spp.) by amphibians and reptiles in Madagascar. Herpetological Bulletin 2002:20–25.
13. Parris, K. M. and M. A. McCarthy. 1999. What influences the structure of frog assemblages at forest streams. Australian Journal of Ecology 24:495–502. CrossRef
14. Zug, G. R. 1993. Herpetology: An Introductory Biology of Amphibians and Reptiles. Academic Press. San Diego, CA.
15. Slack,J.M.W., Darlington,B.G., Heath,J.K. and Godsave,S.F. (1987)Mesoderm induction in early Xenopus embryos by heparin-binding growth factors. Nature, 326, 197-200.
16. Melton,D.A. (1990) Activins are expressed early in Xenopus embryogenesis and can induce axial mesoderm and anterior structures. Cell, 63, 485-493.
17. Harland,R.M. and Misher,L. (1988) Stability of RNA in developing Xenopus embryos and identification of a destabilizing sequence in TFIIIA RNA. Development, 102, 837-852.
18. Cunliffe,V. and Smith,J.C. (1992) Ectopic mesoderm formation in Xenopus embryos caused by widespread expression of a Brachyury homologue. Nature, 358, 427-430.
19. Kinoshita,K., Bessho,T. and Asashima,M. (1993) Competence prepattern in the animal hemisphere of the 8-cell-stage Xenopus embryo. Devel. Biol., 160, 276-284.
20. LaBonne,C., Burke,B. and Whitman,M. (1995) Role of MAP kinase in mesoderm induction and axial patterning in Xenopus development. Development, 121, 1475-1486.
21. Green,J.B.A., New,H.V. and Smith,J.C. (1992) Responses of embryonic Xenopus cells to activin and FGF are separated by multiple dose thresholds and correspond to distinct axes of the mesoderm. Cell, 71, 731-739.
22. Grainger R., Blumberg B., Harland R., Amemiya C., Importance of Xenopus Tropicalis to biomedical and biological research, Benaroya Research Institute, Virginia Mason Research Center [44]
23. Gregory, T.R. (2005). Animal Genome Size Database. [45]
24. U.S Fish and Wildlife Services, ABNORMALITY CLASSIFICATION SOP, [46]
25. Donald D. Brown*, Liquan Cai*, Biswajit Das*, Nicholas Marsh-Armstrong‡, Alexander M. Schreiber*, and Rejeanne Juste*(2005), Thyroid hormone controls multiple independent programs required for limb development in Xenopus laevis metamorphosis [47]
26. Ogino, Hajime; Ochi, Haruki, Resources and transgenesis techniques for functional genomics in Xenopus, Development Growth & Differentiation, Volume 51, Number 4, May 2009 , pp. 387-401(15) [48]
27. Giles Newton (2004) Why the frog? The Human Genome, [49]
28. Xenbase, [50]
29. Donders Center for Neuroscience & Nijmegen Center for Molecular Life Sciences (NCMLS), Beyond the genome (Xenopus transgenesis for functional genomics),Faculty of Science, Radboud University [51]
External Links

1. Frog embryology Frog Embryology
2. Frog cellular materials xenbase
3. The zoology of frog species zoology frogs
4. Frog cycle bioethics frog Just a little cycle of FROG'S life cycle
5. Frog Embryology UNSW Embryology
6. Frog Research carleton library exhibit
ANAT2341 group projects
Project 1 - Rabbit | Project 2 - Fly | Project 3 - Zebrafish | Group Project 4 - Mouse | Project 5 - Frog | Students Page | Animal Development