Paper - The development of the hypophysis cerebri of the rabbit
| Embryology - 16 Jun 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Atwell WJ. The development of the hypophysis cerebri of the rabbit (Lepus Cuniculus L.). (1918) Amer. J Anat. 24(2): 271-337
| Online Editor |
|---|
| Wayne J. Attwell (1889 - 1941) student of GC. Huber.
See also Atwell WJ. The development of the hypophysis cerebri in man, with special reference to the pars tuberalis. (1926) Amer. J Anat. 37: 139-193. Links: Pituitary Development | Rabbit Development |
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
The Development of the Hypophysis Cerebri of the Rabbit (Lepus Cuniculus L.)
1. Introduction
2. Review of literature
- A. Special questions concerned with the development of the hypophysis
- 1. Entodermal origin
- 2. The relation of the notochord to the hypophysis
- 3. The lobes of the hypophysis
- a) General
- b) The anterior process
- c) The lateral lobes
- d) The relation of the lateral lobes to the 'pars tuberalis'
- 4. The structure of the intermediate part
- 5. The development of the neural lobe
- B. The development of the hypophysis of the rabbit
3. Material and methods
4. The development of the hypophysis of the rabbit by days
5. Discussion of observations
- A. The relation of the entoderm and the notochord to the hypophysis
- B. Some general features of the development of the hypophysis
- 1. The hypophysial stalk
- 2. The residual lumen
- D. The development of the neural lobe
- E. The neuro-epithelial contacts and their significance
- F. Terminology and phylogeny of the lobes of the hypophysis
6. Summary and conclusions
7. Literature cited
Introduction
Notwithstanding the numerous studies to which the hypophysis has been subjected, many of its deeper problems remain unsolved. Few investigators have confined themselves to the development of the gland in a single species. This is due, no doubt, to the alluring possibilities in broad comparative studies. As a consequence, many sweeping and unwarranted conclusions have been drawn from insufiicient observations, or from observations on a few specimens in widely different vertebrate classes.
The recent recognition of a distinctive third epithelial portion of the gland lying under the membranes of the brain in the region of the tuber cinereum—the ‘pars tuberalis’ of Tilney (’l3)—and the as yet imperfect appreciation of its interesting development, make careful ontogcnctic studies highly desirable as the basis for phylogenetic comparisons.
The Work which forms the basis of the present paper was undertaken in an attempt to trace the development of the hypophysis with reasonable completeness in a single mammal. To this end the rabbit (Lepus cuniculus L.) was chosen, since this animal breeds Well in confined quarters, has a short gestation period, and brings forth its young in large litters. These factors combine to make possible the collection of the carefully timed embryological material so essential to chronological studies in development.
This study will treat particularly of the morphogenesis of the hypophysis from the time of its appearance until birth, giving especial attention to the ontogeny of the ‘pars tuberalis’ and to the development of the neural lobe; it will deal also with the differentiated histological structure of the three parts of the epithelial hypophysis at the time of birth, and finally will attempt to relate certain of the author’s observations to those of other investigators.
I desire to express my sincere thanks to Professor Huber for his continued interest in this Work, for his very material assistance in overcoming technical difficulties, and for the unstinting Way in which he has placed the excellent facilities of the anatomical laboratories at my disposal. My wife has given valuable aid in much of the tedious Work connected with the construction of the wax models.
Review of Literature
A. Special questions concerned with the development of the hypophysis
1. Entoderm origin
An endless chain of discussion has been evoked by the question as to whether the epithelial hypophysis is derived from the entoderm or from the ectoderm, or whether it is compounded of elements derived from both of these germ layers. Since the time of Goette C74), Balfour C74), and Mihalkovics C75), only a few authors have maintained that this portion of the gland is developed from entoderm alone. There have been many, however, to state that the primary ectodermal anlage is later augmented by a larger or smaller contribution from the entoderm.
Hoffman C86) and Ostroumoff C88) believe the hypophysis in certain reptiles to be entodermal in origin. Kupffer C94) describes a growth from the cephalic end of the foregut to meet the dorsal wall of Rathke’s pocket. This he interprets as an attempted recurrence of a primitive pre—oral mouth or ‘paleostoma.’ This suggestion of Kupffer’s has caused many writers to attach great phylogenetic importance to the hypophysis. Furthermore, according to Kupffer, the entoderm makes important contributions to the epithelial lobe of the hypophysis.
In a previous paper (Atwell, ’ 15) reference was made to the observations of Saint—Remy C95), Valenti C95 a, ’95 b, and ’97), Nusbaum C96), and of Bruni C14), according to all of whom the entoderrn of the pouch—like cephalic extremity of the foregut (‘Seessel’s pouch’) fuses with the ectodermal hypophysial anlage and possibly ‘contributes a few cells to it.
Orrù ('00) divides the glandular portion of the hypophysis of Gongylus ocellatus into two lobes, one of which is in relation to the infundibular process, While the other lies ventral to the first. Although he cannot trace a difference histologically in these two lobes, he believes it Very probable that the dorsal lobe, which lies close to the infundibular process is entodermal and that the ventral lobe is ectodermal.
A considerable portion of the controversy concerning the entodermal origin of the glandular part of the hypophysis has centered about the Ganoid, Amia calva. Dean (’96) holds that in this fish the hypophysis is ectodermal. Reighard (’O0) states that the Amian hypophysis is developed entirely from ectoderm, while Prather (’O0) states that it is entirely from entoderm. Gregory (’02) cannot agree with Prather and believes that this part of the hypophysis is both ectoderrnal and entodermal.
Reighard and Mast (’08) have presented the most conclusive observations for this form. They find that the hypophysis of Amia is ectodermal in origin. They show that Prather was in error, 1) from a lack of the early stages which show a connection of the hypophysis anlage with the mother ectoderm, and, 2) because of imperfect fixation which failed to bring out the line of separation between the hyppphysis and the entoderm.
P. E. Smith (’14) has reopened the problem and comes to the conclusion that the anlage is ectodermal, but adds that “it can be said with considerable probability that the entoderm contributes to the composition of the hypophysis.”
Atwell (’15) saw the epithelial connection between Seessel’s and Rathke’s pouches in the chick much as described by Saint Remy, Valenti and Bruni. He adds the observation that the entodermal bud which forms the connecting strand has constantly in relation to it the cephalic extremity of the notochord, both before and after the ecto—entodermal fusion has formed. The entodermal strand loses its connection with Seessel’s pouch and becomes incorporated into the dorsal wall of Rathke’s pouch. A relationship was noted between the anterior end of the notochord and a small entodermal bud in rabbit embyros, but it could not be shown that any entoderm fuses with the hypophysis anlage in this mammal.
It is to be noted that while the above-mentioned investigators believe that entoderm enters into the formation of the hypophysis, the majority of them freely admit that the main portion of the gland is ectodermal. The only one in recent years to claim that a considerable portion of L the definitive mammalian hypophysis is derived from the entoderm is Miller (’16). This author, who studied the hypophysis of the pig (Sus scrofa) has noted that “the notochord pulls away from the pharynx carrying with it a mass of cells (entoderm),” which later becomes fused with the ectodermal anlage. This is much as described by Atwell for the chick. However, Miller further maintains that this entodermal component ‘rotates anteriorly and superiorly,’ becomes encapsulated by growth of the ‘lateral cords,’ and forms the medulla of the anterior lobe. The cells supposed to be derived from the entoderm have a different histological appearance from those derived from-the ectoderm.
P. E. Smith (’16), likewise B. M. Allen C16, ’17), has removed the glandular ectodermal anlage from young larvae of the frog.
In successfully operated ‘animals, the anterior lobe was entirely
lacking. Smith concludes that this apparently demonstrates conclusively that the entoderm has not
the intrinsic power to form a hypophysis. If it enters into the formation
of the gland at all, it must be considered as a tissue inclusion which becomes changed through its adaptability into glandular parenchyma, a conclusion previously drawn by the writer, Smith (’14).
2. The relation of the notochord to the hypophysis
The close proximity of the cephalic extremity of the chorda to the hypophysial anlage has been taken by many to be significant of some influence the chorda may exert on the developing gland.
Koelliker (’7 9) has noted in a rabbit embryo of eleven days a close relation between the anterior end of the notochord and an outgrowth from the inferior part of the dorsal wall of the hypophysis.
In the Normentafel of the rabbit’s development Minot and
Taylor -(’O5) note a “distinct connection between notochord and
hypophysis” in both ten-and-one-half-day and eleven-day
embryos- (Nos. 11 and 12). This connection apparently had
disappeared entirely by eleven and one-half days of development.
Woerdeman (’13), observing embryos of Sus scrofa corresponding in age to Nos. 71 and 78 in Keibel’s Normentafel, has seen a true contact between the chorda and the dorsal wall of Rathke’s pocket. He believes that he is justified in calling it a true contact because at the place of union there is no membrane propria intervening between the two structures; at this place there is a very noticeable thickening of the dorsal wall of Rathke’s pocket, and the arrangement of the nuclei is Very irregular.
The observations of Atwell (’15) and of Miller (’16) have been given in the previous section in treating of the entoderm origin of a portion of the hypophysis.
Baumgartner (’16) studied turtle embryos of various ages and
found that the notochord is usually in direct contact with the
caudal surface of Rathke’s pouch. In a 4.5-mm. embryo he saw
a dorsal projection from near the base of the pouch to which the
end of the notochord is applied.
Parker (’17), in a study of the hypophysis region of the Marsupials, states,
- The relation of the ehorda to the hypophysis is purely secondary, and my own observations lend not the slightest support to the View of Miller that the notochord makes a considerable contribution to the developing hypophysis.
She believes that the anterior end of the notochord is early in relation to the protochordal plate and that the connection often seen between the notochord and the hypophysis is effected by this plate or by a bridge connecting the premandibular somitcs. Of these latter traces were found in young marsupial embryos.
3. The lobes of the hypophysis
a) General. It has long been customary to consider the hypophysis as composed of two parts. One, known as the anterior lobe, is that portion derived from the ectodermal mouth invagination and, according to certain authors, augmented by addition from the entoderm. The other, known the posterior lobe, is that part developed from an outgrowth of the floor of the third ventricle of the brain. This usage is still common in modern text-books of anatomy and embryology. That it is entirely inadequate for the description of the gland from either an embryologic or histologic View-point will become evident from the discussion which is to follow.
Peremeschko (’67) studied the hypophysis of a number of domestic animals and of man. He notes the existence of a cleft (‘Kanal’) in the epithelial portion of the gland which divides it into two unequal parts—one, the ‘Korkschicht,’ and the other, the ‘Markschicht.’ The former is the main body of the epithelial lobe, while the latter is a thin" lamina closely applied to the neural lobe. These two portions are also characterized by differences in histological appearance. The cells of the ‘Markschicht’ are poorer in protoplasm, have clearer nuclei, and are not easily changed by reagents.
Lothringer (’86) notes this intraglandular cleft and agrees with Peremesehko that it is not the separationbetween epithelial and brain parts. Instead, it separates the ‘Epithelsauin’ from the ‘Epithelkorper.’ The former corresponds to Peremeschko’s ‘Markschicht’ and the latter to his ‘Korkschieht.’
Herring (’08b) speaks of the epithelial investment of the neural lobe as the ‘pars intermedia’ obviously because of its position between the neural lobe and therelnainder of the epithelial lobe, ‘the anterior lobe proper.’ Pars intermedia and anterior lobe proper are separated by the residual lumen of Rathke’s pocket.
Stendell (’13) calls these two divisions of the anterior lobe the ‘Zwischenlappen’ and the ‘Hauptlappen,’ respectively. The residual lumen he knows as the ‘Hypophysenhohle.’
All of these writers take pains to emphasize the fact that the ‘pars intermedia’ is inseparably bound to the neural lobe. This is particularly evident when an attempt has been made to mechanically separate the so—called anterior and posterior lobes. Almost invariably a thin. epithelial layer, the ‘pars intermedia,’ is found to have reInained adherent to the neural lobe.
b) The anterior process. W. Muller" (’71) describes for 16- and 18-cm. human, sheep, and pig embryos an anterior process of the hypophysis. His description reads: “Erstreckte such ein schmaler, conisch sich verjfingender Fortsatz langs der Vorderen Flache des Processus infundibuli nach oben und vorn gegen das Chiasma hin.” '
Mihalkovics (’75) observed the formation of an anterior process during the development of the hypophysis in the rabbit. In an embryo 2 cm. in length the epithelium of the inferior part of the hypophysial sac, at the place Where the stalk is attached grows forward and upward as a solid process. Continuing, Mihalkovics’ own words are: “Bei Siiugetieren biegt sich zuerst der untere Teil des Sackchens etwas nach Vorn und aufwärts um und Wachst zu einern soliden Fortsatz aus.”
Kraushaar (’85) treats of the development of the hypophysis in the Rodents. His descriptions are mainly of Mus musculus. He notes an anterior process of the developing hypophysis and speaks of it in these words: “Gegen das Chiasma hin entsendet die Hypophysis einen schmalen, soliden Fortsatz.”
Lothringer C86), in describing the hypophysis of the adult dog, names that portion of the gland where ‘Epithelsaum’ and ‘Epithelkorper’ are bound together the ‘Umschlagstheil.’ This ‘Umschlagstheil’ borders the brain substance Very closely. He notices that “eine schmale Fortsetzung desselben breitet sich an »der Unterflache des Tuber cinereum aus, bis whine Vermochten wir, da wir stets an Vom Gehirn getrennten Organen untersuch‘eten, nicht Init Sicherheit festzustellen.” He pictures this process and labels it “Fortsatz des Epithelsaums auf den Trichter.”
Haller (’97), describing the mouse, speaks of a thin part of the
hypophysis which extends forward and is closely applied to the
brain wall. This ‘Vorderer Lappen,’ as he calls it, pours its
secretion into the subdural space. This last statement has not
been verified by subsequent observers. In ’09, Haller saw a
‘vorderer Fortsatz’ in Erinaceus, Mustela, and Vesperugo noctula, and mentions the structure again (’10) in describing embryos of the mouse and of the roe.
Salzer (’98) figures and describes a solid anterior process which
consists of glandular substance and which extends toward the
optic chiasm. He also speaks of a plate-like part which lies
ventral to the main body of the hypophysis. Woerdeman (’14)
has interpreted this ‘Platte’ as the remains of the hypophysial
stalk. .
Gronberg (’01) studied the development of the brain and its appendages‘ in Erinaccus europaeus. He considers the hypophysis only secondarily, but he thinks noteworthy a process which the hypophysis sends forward almost to the middle of the chiasm. Transverse sections show this process to be a. broad horizontal plate. It grows forward from the place of attachment of the hypophysial stalk.
Joris (’07) saw in the meninges of the brain a mass of glandular
cells which is attached at the anterior end of the hypophysis.
This cell mass extends from the optic chiasm to the base of the
infundibulum and divides into two diverging branches. These
make an angle, open posteriorly, embracing the neck of the infundibulum. To this cell mass J oris gave the name of ‘lobule de la
tige.’ He believed that it becomes united with the hypophysis
secondarily.
Staderini (’08) describes somewhat similar relations and speaks
of a ‘lobus chiasmaticus’ which extends forward and of a ‘lobus
praemammillaris’ the cells of which are within the brain coverings
and surround the infundibular neck.
Herring (’08a) describes and figures a lobe which he names the
“tongue—like process of the pars intermedia.” It extends forward and is closely applied to the brain wall. He notes that this
part is more vascular than the ‘pars intermedia.’
Bolk C10) speaks of a ‘lobulus bifurcatus’ in primate embryos.
The two arms of the lobe embrace the infundibulum near its
attachment to the brain. Bolk believes that this ‘lobulus bifur—
catus’ becomes detached to form the cell masses found embedded
in the meninges.
Tilney C13) differentiates histologically three portions of the
glandular hypophysis in birds and mammal. His ‘pars distalis’
and ‘pars infundibularis’ correspond to the ‘anterior lobe proper’
and the ‘pars intermedia’ of Herring, respectively. The ‘pars
tuberalis’ is closely applied to the tuber cinereum and extends
foward toward the optic chiasm. Tilney believed that he was
presenting the histological structure of a “hitherto undescribed
portion of the hypophysis.” Woerdeman (’14) points out that
the ‘lobule de la tige’ of J oris, Standerini’s ‘lobus praemammilaris’
and ‘lobus chiasmaticus,’ and Bolk’s ‘lobulus bifurcatus’ are
without doubt the same structure as Tilney’s ‘pars tuberalis.’
Baumgartner (’16) expresses himself as in accord with this view
of Woerdeman’s. Tilney’s account of the development of the
‘pars tuberalis’ will be referred to later.
c) The laterallobes. Gaupp (’93) states that the hypophysis
of the lizardhas a three-fold anlage—a large round ‘Mittelknospe’
and two long ‘Lateralknospen’ which bud out from the mouth
epithelium. These parts are separated by a venous ring. Later
thereuappears a fourth part anterior to the ‘Mittelknospe,’ the
‘Vordere Knospe.’ The two lateral buds fi.rst unite with the
gland and later separate as solid bodies. They attach themselves
closelyto the brain floor. It seems that they are present in the
adult animal, but further observation is required to establish
this point.
Chiarugi (’94) notes two epithelial strands in Cavia cobaya located ‘at the place where the hypophysis is constricted off from the epithelium of the mouth. He believes that these are homologous to the ‘Lateralknospen’ of Gaupp.
Weber (’98) saw, in Chiroptera, a tripartite hypophysis fundament. He does not hold, as does Gaupp, that these three parts are separate at the beginning. Rather, a single anlage early differentiates into two ‘bourrelets lateraux’ and a ‘crete mediane.’
Rossi (’96) describes a median part and two lateral parts for the hypophysis of the chick.
Nusbaum C98) (referred to by Woerdeman, ’14) states that the hypophysis develops from two sources, namely, from an unpaired out-pouehing of mouth epithelium and from a pair of epithelial thickenings derived from the primitive gut.
Economo C99) speaks of two ‘Seitensprossen’ to be seen during the development of the hypophysis i.n doves and chicks. In dove embryos the buds, or sprouts, appear between the fourth and seventh days. They are said to be arranged on each side of the infundibular process. During a part of their development they possess lumina which communicate with the hypophysis cavity. He notes a similarity to Gaupp’s observations on the Reptiles.
Standerini CO3) traces the developing hypophysis in reptiles. The anlage is simple, but later the gland consists of a median part and two lateral parts.
Bolk (’10)' describes an hypophysial anlage of three divisions in young embryos of Macaeus cynomolgus.
Tilney (’11) observed in Aspidonectes two accessory pouches which arise from the main oral evagination. He believed that the importance of these and similar accessory pouches has been exaggerated, “since it is a common tendency in many forms for the anlage of the gland to present multiple diverticula.”
Bruni (’13) noted that in the Sauropsida Rathke’s pouch is early differentiated into a single ‘lobo medio’ and two ‘lobi laterali.’ In mammals the lateral lobes appear much later.
d) The relation of the lateral lobes to the ‘pars tuberalis.’
Gisi (’07), in a dissertation treating of the brain of Hatteria
punctata, speaks of a thin anterior process of the hypophysis
which is termed the ‘pars terminalis.’ Of this part it is stated:
“Wahrscheinlich ist diese Pars terminalis der Hypophyse das
Endproduct der seiten Knospeni an den friiheren Embryonalstadien.”
Herring (’08 a) speaks only briefly concerning the development of his “tongue-like process of the pars interniedia.” He says:
- The anterior lobe also grows forward and laterally. The neck of the sac retains a tubular character for some time, and becomes somewhat convoluted. One of these convolutions (fig. 5, la) applies itself to the under surface of the brain and gives rise to the tongue-shaped process which extends forwards from the anterior lobe towards the optic chiasma.
To Tilney (’13) must be given the credit for first clearly showing that the ‘pars tuberalis’ has its origin from two lateral buds.
Tilney has traced the development of the ‘pars tuberalis’ in the cat and in the chick. It arises as a relatively late structure. It has its origin in two secondary diverticula or sprouts from the body of the pituitary sac. These sprouts, the tuberal processes, ultimately fuse with each other across the median line, displace the body of the pituitary sac ventrad and thus secondarily assume their juxta-neural position.
Tilney emphasizes both the histological and developmental separateness of ‘pars tuberalis’ from ‘pars infundibularis’ (Herring’s ‘pars intermedia’).
‘In his interesting study of the comparative development of the hypophysis, Woerdeman (’14) has seen the ‘lobuli laterali’ in several mammals and traces their development into the ‘lobulus bifurcatus’ of Bolk. This lobule divides into cell masses which lie in the meninges of the brain and which, in some cases, lose connection with the main body of the hypophysis. Woerdeman also has traced the early history of the lateral lobes. They arise from two enlargements of the thickened epithelial plate which lies anterior to Rathke’s pocket and which later becomes incorporated into the hypophysial anlage.
Miller (’16), in his study of the hypophysis of Sue scrofa, speaks
only briefly of the ‘lateral cords.’ According to his description,
these cords ‘grow round and encapsulate’ that mass of cells supposedly derived from the entoderm. They eventually form the
cortical layer of the anterior lobe.
Baumgartner (’16) has described the development of the lateral
lobes of the reptilian hypophysis. He sees the lateral buds early
separated from Rathke’s pouch by furrows which begin on the
cranial side. He states:
In the later development of the lateral buds in turtles, the tips grow
forward and form a thin layer closely applied to the floor of the brain
(the part termed by Tilney ‘pars tuberalis’) and to a thin cortical zone
around the middle of the anterior lobe.
In alligators, the lateral buds give rise to the pars tuberalis and two bands encircling the anterior lobe; in lizards, they appear to persist as isolated masses or to disappear, while in snakes, they completely disappear.
Baumgartner believes that the cortical zone or bands described by him for turtles and alligators have been overlooked in other vertebrates, since Miller (’16) is the only observer who has described a similar structure (Sus scrofa).
Parker (’17) finds that the development of the ‘pars tubercles’ in the Marsupials begins at an early stage. The portion of Rathke’s pouch lying posterior to the duct becomes subdivided into two lobes, which are respectively distal and proximal in relation to the hypophysial duct, and are separated from each other by a horizontal constriction. While the distal lobe thickens and forms the glandular tissue of the pars buccalis (probably meaning Tilney"s ‘pars distalis’) as well as the pars infundibularis, the proximal lobe remains thin walled. It is drawn out laterally and curves up to reach the brain wall on each side. Later the two sides fuse to surround the infundibulum.
4. The structure of the intermediate part
Besides the glandular cells and the small amount of connective tissue accompanying the few blood-vessels, a number of observers have noted in the pars intermedia certain distinctive cells which have been variously interpreted as nerve cells, sensory cells, or supporting cells. Lothringer (’86) describes scattered marginal cells in addition to the cylindrical secreting cells. They may ei-ther reach the surface or be bent back upon themselves.
Pirone (’05) sees in the intermediate portioniof the hypophysis, cylindrical cells whichpresent the structure characteristic to the supporting cells of sensory epithelium. He used Cajal’s method and states that he is able to confirm the findings of Gentes and Gemelli.
Gemelli (’05, ’06) makes mention of nerve fibers entering the
pars intermedia from the neural lobe and also of ‘glio-epitheliari’
cells in this part. He considers that the posterior lobe of the
hypophysis is sensory in nature.
Retzius (’94) describes and figures structures in the pars intermedia which he has called neuroglia cells. They are shown by the Golgi method. One type consists of long, fine spindle—shaped cells Which extend through the entire thickness of the pars intermedia. Others are peculiar, branched forms which touch only one surface of the epithelium or neither. The nuclei for the most part lie near the surface bordering the cleft. The end of the spindle-shaped cells towards the neural lobe often widens out into a three-cornered foot.
Herring (’08) finds long, thin nucleated cells in the pars intermedia of the kitten’s hypophysis. These cells, which are brought out by use of Cajal’s silver method, are numerous and take a vertical course through the epithelium. They appear to be of ectodermal origin and to act as supporting cells. Similar cells may be found in the adult, but are better seen in the young animal (p. 139).
Trautmann (’09), in studying the hypophysis of the cat, has seen ‘fadenartige’ cells which extend through the entire width of the intermediate part. These are well brought out by the Golgi method. Another type of cell also was seen which Trainman describes in these words:
- Im Epithelsaum der Katze konnt.e ich ferner durch die Golgische Methode zwischen den obengenannten fadenartigen, Zeltgebilden verastelte Gebilde darstellen, die verschiedenartig zu den ersten verlaufen, mannigfaltige Gestalten aufweisen und Weder Basis noch Peripherie erreichen. (In the epithelial seam of the cat, I could also, by means of the Golgi method, represent between the above-mentioned filamentous tent-like structures a variety which is different from the first, have manifold forms, and neither reach the base nor the periphery.)
Cajal (’11) figures bi-polar cells in the intermediate lobe of the mouse. These are made visible by the use of Golgi’s- method. He has also seen nerve fibers which extend from neural lobe to intermediate part. For these reasons, he considers that the superior (posterior) lobe of the hypophysis has a sensory nature.
On this point Cajal says:
- Deux fait semblentindiquer que lo lobe superieur de Phypophyse doit etre un organe sensorial; c’est, d’unev part, la richesse Tu plexus axile inclus dans le lobe nerveux et l’epithelium adjacent; c’est, d’autre part, l’existence de nombreuses cellules bipolaires epitheliales particulières, signalees par Retzius et nous dans l’epithelium de la gland. (Two facts suggest that the pituitary upper lobe must be a sensorial organ; that is, of unev hand, wealth you plexus axile included in the nervous lobe and the adjacent epithelium; that is, on the other hand, the existence of many bipolar cells specific epithelial, reported by Retzius and we in the epithelium of the glans.)
Miller (’16) remarks that the ‘spindle—shaped supporting cells’ constitute one of the interesting structures in the intermediate lobe of the hypophysis of the pig.
Vanderburgh (’17) sees in the pars intermedia of the guineapig’s hypophysis, supporting cells which extend from the cleft
towards the pars nervosa. The branched variety appear as “little black triangles which are molded to fit the interspace between the cells.” The unbranched were “much elongated and usually more transparent.” The stains used by Vandenbergh did not produce satisfactory evidence as to the nature of these cells.
Stendell (’14) gives a summary of the observations concerned
with these special cells of the pars intermedia. He says that true
ectodermal supporting cells having the nature of the ependyma
of the central nervous system have been definitely observed only
in the hypophysis of mammals, more -specifically in the Carnivora
and in a few Rodents. They are best seen in the cat and dog and
are most readily demonstrated by silver impregnation methods.
He considers that without doubt these elements are true supporting cells. More remarkable and quite foreign to the nature of
the pars intermedia are the glia cells which have been described. They have not often been called neuroglia cells, but are described as ‘branched structures’ which are brought out by the Golgi methods (Trautmann and others). Of the probable origin of these cells Stendell says:
- Da jedoeh die ependymaren Stutzzellen als von Hirnlappen her eingewanderte Elemente anzusehen sind, ist auch fur die gliosen, die ja mit jcnen genetisch ein System ausmachen, die Erklarung gegeben. Sie sind entweder mit jene zusammen eingedrungen oder direct inner halb dcs Zwischenlappens Von der Oberflache in profunde Lagen verdrangte Ependymzellen. {Since, however, the ependymal supporting cells are to be viewed as elements which have migrated from the lobes of the brain, the explanations are also given to the gliosis, which, with them, are genetically a system. They either have penetrated with them, or directly within the intermediate lobes. Ependym cells, which have been displaced from the surface into profound layers, are penetrated)
5. The development of the neural lobe
Recent studies on the morphogenesis of the hypophysis have been confined almost entirely to the epithelial portion of the gland. As a consequence, the literature treating of the development of the neural lobe is very scanty.
Muller (’71) believed that in mammals the specific neural tissue of the lobe is much reduced in amount during the latter
half of fetal life and that connective tissue is substituted. Muller
calls the fully developed infundibular process a “connectivetissue appendage of the brain.”
Mihalkovics (’75), treating of the development of the neural
lobe of the rabbit’s hypophysis, states that its earliest appearance
is due to the pressure of the hypophysial pouch against the Wall
of the forebrain, and not to a relation with the anterior extremity
of the notochord. A protrusion of the brain Wall results above
the apex of the epithelial pouch and may be termed the ‘primitive
Trichter.’ It represents not only the later ‘Trichter fortsatz,’
but also that portion of the Ventricular floor which is to become
the tuber cinereum.
Gronberg (’01) states that from the beginning the processus infundibuli is a hollow sac which can be compared in shape to the finger of a glove‘. In his stage ‘D’ the lumen begins to disappear at the caudal extremity of the lobe. Further description is omitted, but he makes reference to his figures 33 to 36 for details as to the disappearance of the cavity of the lobe.
Herring (’08 a) saw great importance in the early and close union between the buccal and cerebral portions of the hypophysis.
Like Salzer, he could find no connective tissue between the
infundibular process and the hypophysial sac in early stages.
He believes that this close connection has some morphological
significance, perhaps bespeaking the bucconeural duct observed
by Andriezen in Ammocoetes, Amphioxus, and Balanoglossus.
He denies that the neural lobe degenerates into a connectivetissue appendage. He finds that the connective tissue is small in
amount. Treatment with Cajal and Golgi methods shows the
structures formerly described as connective—tissue cells to be
ependymal and neurogliar. elements.
Stendell (’14) traces the form and position of the neural lobe
from the lowest fishes to the Mammals. In the fishes, the neural
portion can be considered as little more than a modified region of
the floor of the third ventricle. In the Elasmobranchs and the
Ganoids, the neural part sends numerous hollow processes into
the substances of the intermediate lobe. In the Teleosts, the
processes are solid and branched. Stendell considers that first
in the Amphibia does one find a true neural lobe, that is, an unbranched, solid thickening of the ventricular floor to which the
epithelial part attaches itself and develops (p. 27). This he
considers the usual arrangement of parts in all the higher
vertebrates.
B. Development of the hypophysis in the rabbit
So far as I have been able to discover, no author has confined himself to describing the development of the hypophysis in the rabbit alone. Several investigators have included, however, a consideration of this animal in their comparative studies.
Mention has already been made of a connection between notochord and dorsal wall of the hypophysis which was observed in
an eleven-day rabbit embryo by Koelliker. Likewise it has been
noted that peculiar ‘neuroglia-like’ cells were seen by Retzius in
the pars intermedia of a rabbit eight days after birth.
Muller (’7 1) used embryos of the pig, the sheep, and the rabbit
in his study of the development of the hypophysis. He gives a
common description for all embryos of the same length. For example, his description of a 16—mm. stage may be applied equally
Well to any one of these three forms.
The rabbit was the mammalian type chosen by Mihalkovics
(’75) in his study of the hypophysis. His descriptions begin with
an embryo 5 mm. in length. The oral membrane is still intact.
The first appearance of the hypophysis is the ‘Hypophysenwin—
kel’ a shallow infolding of the oral ectoderm just anterior to the
oral plate. In a 6—mm. embryo, the oral membrane has just
ruptured. Both upper and lower stumps are still to be seen.
In this stage the earliest appearance of the primitive infundib—
ulum is to be noted. A 12—mm. embryo shows a definitely
formed ‘Hypophysentasehe’ which communicates with the oral
cavity by a much—constricted opening. The ‘Trichterfortsatz’
is small and conical. A 16-mm. embryo presents a longer ‘Trichterfortsatz’ and the hypophysial pocket has been further constricted off from the mouth epithelium, so that the connecting
‘Hypophysengang’ contains only a minute lumen. The further
cutting—off of the hypophysial pouch, the formation of the anterior
process and of the ‘Driisenschlauche’ are traced by a description
of 2-, 3-, and 4-cm. embryos.
Although not studying the development of the hypophysis, Lothringer C86), Rogowitsch (’89), and Stieda (’90) made important early observations on the anatomy and histology of the gland in the rabbit.
Minot and Taylor (’O5) give definite but necessarily brief
statements concerning the development of the hypophysis in
their “Normal Plates of the Development of the Rabbit.” The
hypophysis of the rabbit is first visible in their embryo No. 9,
nine and one-half days of development, as a “very small evagination of ectoderm on the dorsal side of the mouth just in front of
the oral plate,” which has begun to rupture. In an embryo of
ten days no essential difference is to be noted. At ten and onehalf days the diverticulum of the hypophysis is “well marked,
rather four—sided in cross-section, and closely approximated to the
Wall of the forebrain.” During the next day the pouch becomes
longer and more closely applied to the brain wall. In embryo
No. 14 ( twelve days) the anlage of the infundibulum is “present as a very small evagination of the floor of the forebrain. The
upper end of the hypophysis is slightly expanded laterally,
slightly concave toward the forebrain, and is ‘joined to the infuridibulum.” During the next day and a half the infundibular
evagination becomes more distinct and the mouth of the hypophysial pouch more constricted. At fourteen days the hypophysis is no longer open to the mouth, but is-connected with the
oral ectoderin by a solid epithelial cord. In embryo No. 19
(fifteen days) the infundibulum is a little longer than in preceding
stages and overlies the hypophysis (epithelial portion) more.
The hypophysis is bent concave toward the forebrain. By sixteen days there can be seen a beginning of the cords of the hypophysis which first appear as solid outgrowths. These cords are
larger and more Vascular in a seventeen—day embryo. At eighteen
days the connecting cord between hypophysis and oral ectoderm
is broken through just above the ectoderm. The Normentafel
studies do not extend beyond twenty days of development. At
this stage some embryos still show a small connecting strand between hypophysis and oral ectoderm. The infundibulum is open
to the third ventricle. The hypophysis is much bent and contains a cavity. Two lateral upward prolongations of the hypophysis are to be seen on either side of the infundibulum. The cords
of the hypophysis appear as a vascularized outgrowth of the
anterior wall, irregular in shape, solid except for the contained
vessels. The pituitary fossa is well marked at this time.
3. Material and Methods
The rabbit embryos used in this study were obtained from the rabbit colony of the Department of Anatomy of the University of Michigan. The majority of them were obtained during the course of the study and accurate records have been kept of their ages.
Females which have already born young were selected and the date of birth of the previous litter obtained if recent. It is known that a female will generally submit to coition shortly after parturition, but such a procedure is undesirable, since the suckling of young probably lengthens the gestation period (compare King
Fig 1 page
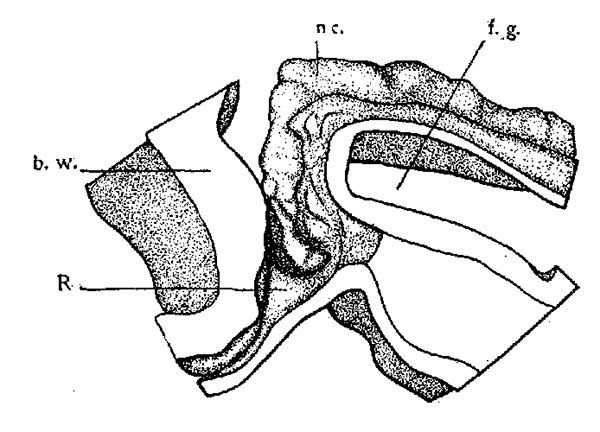
mouth invagination, and the adjacent brain Wall, is shown in figure 1. The hypophysis anlage is present as a shallow pouch much wider from side to side than from front to back. The oral plate is intact. The cephalic extremity of the notochord bends around the foregut and terminates at the dorsal wall of the hypophysis fundament. A contact between notochord and hypophysis cannot be observed in this embryo.
Fig. 1. Model of hypophysis region of a sixteen-somite rabbit (embryo A). x 100. The anterior end of the notochord and portions of the epithelium of the foregut, oral pit, and brain wall are shown. Viewed from the left side. nc., notochord; f.g., foregut; R,Rathka’s pouch, and b.w., brain wall.
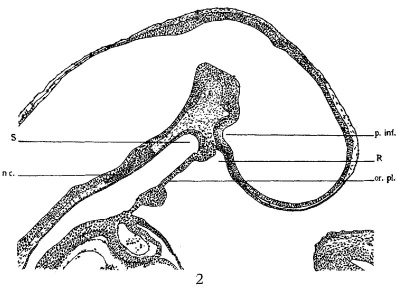
Figure 2 presents a sagittal section of a timed ten—day embryo (27D). It is slightly older than the preceding, but the oral membrane is still unbroken. A comparatively large evagination of the brain wall is seen dorsal to Rathke’s pocket. This corresponds to the ‘primitive Trichter’ of Mihalkovics. In this embryo the extremity of the notochord is in close contact with the wall of hypophysis anlage.
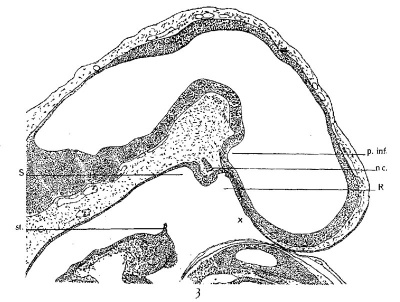
Another ten-day embryo (27A) shows the oral membrane in the process of rupture. A sagittal section of this embryo is given in figure 3. A noteworthy feature is the presence of a thickened epithelium, continuous with the hypophysial wall, which extends nasalward from Rathke’s pocket for some distance. Its rather abrupt termination is marked by x (fig. 3).
A wax—plate reconstruction prepared from this embryo shows that the hypophysial pouch has deepened, and on each side, at its nasal border, has developed a ridge-like protuberance. As seen
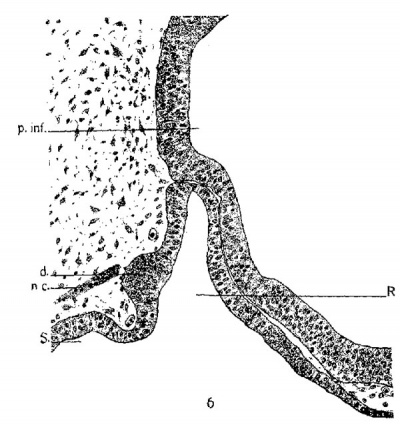
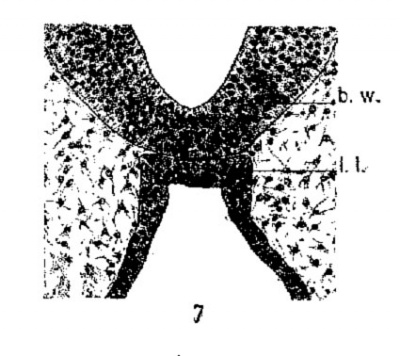
fig6-7
constriction from the oral epithelium. These prominent elevations I interpret as the homologues of the lateral lobes of lower forms. Later stages of development of the rabbit will show how these early lateral lobes give rise to the pars tuberalis of complete development. A transverse section through the region of the lateral lobes is shown in figure 7. The lobes are constricted from the thickened epithelium just nasal to the early hypophyseal pouch.
In embryo 25A (figs. 5 and 6) the notochord ends close to a large thickened evagination of the dorsal wall of the hypophysis from which it is separated by a very narrow space. The condition presented by this embryo corresponds very closely to that of the eleven-day rabbit embryo described by Koelliker (’79).
In embryo C, which is only slightly more advanced, the notochord divides into two parts near its cephalic termination. One branch ends at the apex of Seessel’s pouch, while the other is directed dorsally, forming almost a right angle with the first part.
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
Cite this page: Hill, M.A. (2024, June 16) Embryology Paper - The development of the hypophysis cerebri of the rabbit. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Paper_-_The_development_of_the_hypophysis_cerebri_of_the_rabbit
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G