File:Mouse model of ovarian cord formation.jpg
Mouse_model_of_ovarian_cord_formation.jpg (800 × 491 pixels, file size: 85 KB, MIME type: image/jpeg)
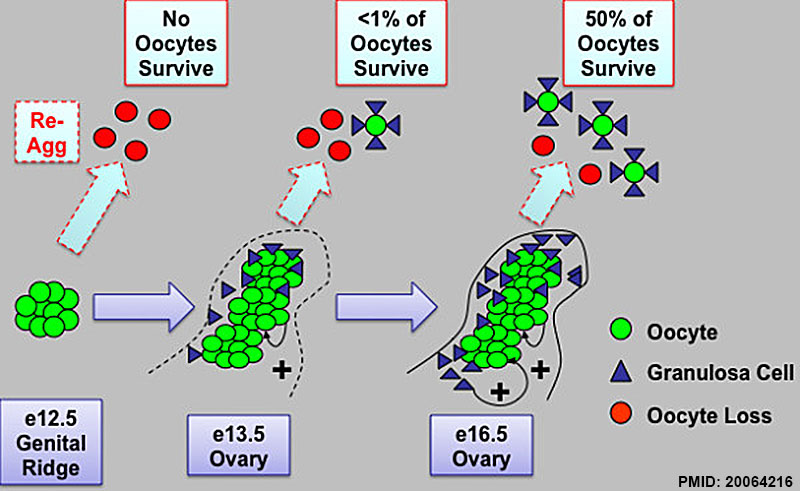
Model of intact ovarian cord formation and maturation promoting oocyte survival and development in follicles. Prior to ovary development on e13.5, germ cells located in cysts in the e12.5 female genital ridge are not yet competent to survive and form follicles when re-aggregated (re-agg). However, ovary maturation on e13.5 and development of intact ovarian cord structures containing oocyte and somatic granulosa cell clusters are sufficient to permit some oocyte survival and follicle formation upon re-aggregation. By e16.5, intact ovarian cord-enclosed meiotic oocyte clusters and granulosa somatic cells are now primed to undergo robust follicle formation and oocyte development in follicles when re-aggregated. The percentage of surviving re-aggregated oocytes was calculated in comparison to intact transplant controls. Oocyte (green) and granulosa cell (blue) contacts and paracrine signaling factors may promote oocyte survival (+) and possibly facilitate programmed ovarian germ cell cyst break down into follicles. The cord and/or oocyte-mediated recruitment of granulosa cell clusters during ovary differentiation may also provide somatic cell signaling factors such as WNT4, R-spondin1, and Follistatin that promote oocyte survival and maturation. In addition, intact ovarian cords may facilitate the protection of oocytes from re-aggregation induced inhibitory signaling from somatic cells in the genital ridge. Thus, we define a critical window from e13.5 to e16.5 of oocyte enclosure in intact fetal ovarian cord structures for the intrinsic programming of oocytes with competence to survive and undergo further development.
http://www.biomedcentral.com/1471-213X/10/2
© 2010 Nicholas et al; licensee BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
1471-213X-10-2-8.jpg
BMC Dev Biol. 2010 Jan 8;10:2. doi: 10.1186/1471-213X-10-2.
Intact fetal ovarian cord formation promotes mouse oocyte survival and development.
Nicholas CR1, Haston KM, Pera RA.
Author information
Abstract
BACKGROUND:
Female reproductive potential, or the ability to propagate life, is limited in mammals with the majority of oocytes lost before birth. In mice, surviving perinatal oocytes are enclosed in ovarian follicles for subsequent oocyte development and function in the adult. Before birth, fetal germ cells of both sexes develop in clusters, or germline cysts, in the undifferentiated gonad. Upon sex determination of the fetal gonad, germ cell cysts become organized into testicular or ovarian cord-like structures and begin to interact with gonadal somatic cells. Although germline cysts and testicular cords are required for spermatogenesis, the role of cyst and ovarian cord formation in mammalian oocyte development and female fertility has not been determined.
RESULTS:
Here, we examine whether intact fetal ovarian germ and somatic cell cord structures are required for oocyte development using mouse gonad re-aggregation and transplantation to disrupt gonadal organization. We observed that germ cells from disrupted female gonad prior to embryonic day e13.5 completed prophase I of meiosis but did not survive following transplantation. Furthermore, re-aggregated ovaries from e13.5 to e15.5 developed with a reduced number of oocytes. Oocyte loss occurred before follicle formation and was associated with an absence of ovarian cord structure and ovary disorganization. However, disrupted ovaries from e16.5 or later were resistant to the re-aggregation impairment and supported robust oocyte survival and development in follicles.
CONCLUSIONS:
Thus, we demonstrate a critical window of oocyte development from e13.5 to e16.5 in the intact fetal mouse ovary, corresponding to the establishment of ovarian cord structure, which promotes oocyte interaction with neighboring ovarian somatic granulosa cells before birth and imparts oocytes with competence to survive and develop in follicles. Because germline cyst and ovarian cord structures are conserved in the human fetal ovary, the identification of genetic components and molecular mechanisms of pre-follicle stage germ and somatic cell structures may be important for understanding human female infertility. In addition, this work provides a foundation for development of a robust fetal ovarian niche and transplantation based system to direct stem cell-derived oocyte differentiation as a potential therapeutic strategy for the treatment of infertility.
PMID 20064216
File history
Yi efo/eka'e gwa ebo wo le nyangagi wuncin ye kamina wunga tinya nan
| Gwalagizhi | Nyangagi | Dimensions | User | Comment | |
|---|---|---|---|---|---|
| current | 08:07, 1 May 2015 |  | 800 × 491 (85 KB) | Z8600021 (talk | contribs) | Model of intact ovarian cord formation and maturation promoting oocyte survival and development in follicles. Prior to ovary development on e13.5, germ cells located in cysts in the e12.5 female genital ridge are not yet competent to survive and form f... |
You cannot overwrite this file.
File usage
The following 2 pages use this file: