2014 Group Project 8: Difference between revisions
No edit summary |
m (Protected "2014 Group Project 8" ([Edit=Allow only administrators] (indefinite) [Move=Allow only administrators] (indefinite))) |
Revision as of 23:20, 24 October 2014
| 2014 Student Projects | ||||
|---|---|---|---|---|
| 2014 Student Projects: Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | Group 6 | Group 7 | Group 8 | ||||
| The Group assessment for 2014 will be an online project on Fetal Development of a specific System.
This page is an undergraduate science embryology student and may contain inaccuracies in either description or acknowledgements. | ||||
Musculoskeletal
This webpage will be focusing on fetal muscular development.
Introduction
The musculoskeletal systems main purpose is to provide the body with structure, stability, support, protection, mineral storage, heat production and movement. It is made up of multiple structures; bone, cartilage, skeletal, tendon, ligaments and joints. This project will be focusing on the fetal development of the Muscular and Tendinous tissue. Both tissue have major embryonic contributions from the somites and during the fetal period undergo myogenesis and fibrillogenesis respectively to form mature tissue. Many molecules, particularly growth factors and proteoglycans regulate the growth of the muscle and tendons. Historical and current research and models in musculoskeletal development will be addressed, with addition of common muscular congenital fetal abnormalities.
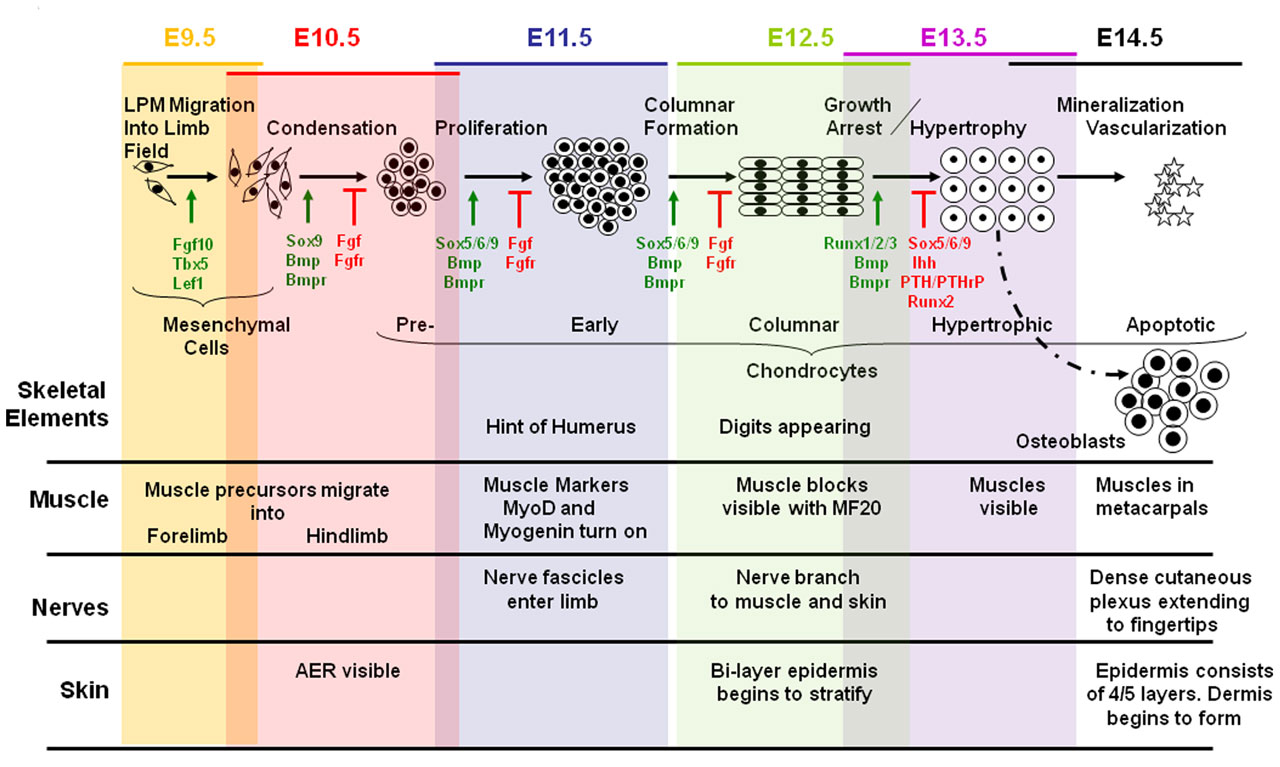
Background Early Embryonic development
Skeletal Muscle develops from a process known as myogenesis. Mesenchymal cells which are embryonic connective tissue cels differentiates into embryonic muscle cells, myoblasts. Myoblasts which have single nuclei fuse and elongate to form myotubes which are multinucleated and cylindrical.
The neural tube and notochord release signaling molecules like Shh, Wnts and [BMP]-4. These signaling molecules act on transcription factors of the MyoD family and Pax [1].The MyoD or MrF family includes MyoD, Myf-5 , myogenin and Myrf4 [2]. The MyoD family of transcription factors, like MyoD are myogenic bHLH (basic helix loop helix) transcription factors. Pax-3 and the MyoD induce myogenesis, formation of myoblasts. Pax-3 also acts on c-met which is a migratory peptide.
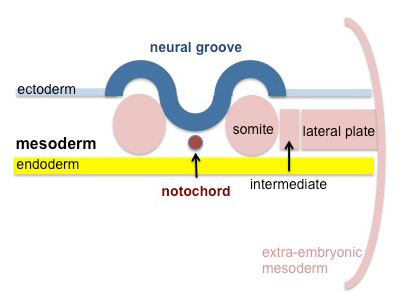
Skeletal muscle is derived from the somites. Paraxial mesoderm segments into somite structures on both sides of the notochord and neural tube.
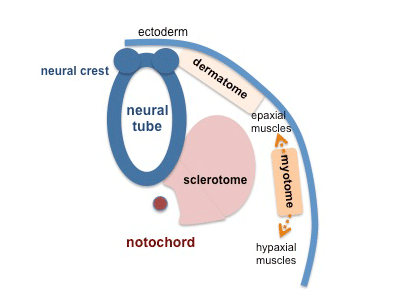
Somites are mesodermal structures where the dorsal most end of the somite, which is known as the dermomyotome, becomes skeletal muscle and dermis [3]. A small anterior portion of the paraxial mesoderm remains un-segmented and eventually forms some muscles of the head [4].
The myotome lies in-between the scleratome which is ventrally located. The scleratome forms the cartilage and bone of the axial skeleton of the embryo. The dermomyotome is located dorsally and forms the first skeletal muscle in the embryo.
The medial part of the dermomyotome forms the dorsal and intecostal muscles whilst the lateral part of the dermomyotome forms the limb and ventral muscles [3].
As nearly all of the muscular system develops from the mesoderm. The Iris muscle comes from neuroectoderm. And Eosophagus skeletal muscle is derived from transdifferentiation of smooth muscle.
Myogenesis occurs in two phases; primary and secondary which occur in embryonic and fetal periods Primary myotubes express MHC slow myosin heavy chains [5]. The primary myotubes form the structure and scaffold upon which secondary myotubes form during secondary myogenesis which occurs in the fetal period [5].
Molecular and Cellular regulation of fetal myogenesis
Skeletal myofiber number is set at birth [6]. This was found using experiments on mice and pigs. Similar trends have been observed in humans as found by Widdowson et al (1972) where a huge increase and then levelling of gastrocnemius myofibers were found in the gestational period [7].
Multipotent mesenchymal cells (MSC) form myoblasts as well as adipocytes and fibroblasts [8]. Therefore specific the nutrients and growth are important in directing the growth of these MSC into either adipocytes or myoblasts [9]. Myoblasts differentiate to form the embryonic, fetal and adult skeletal muscle and in fact myoblasts differentiate to form all three early in development.
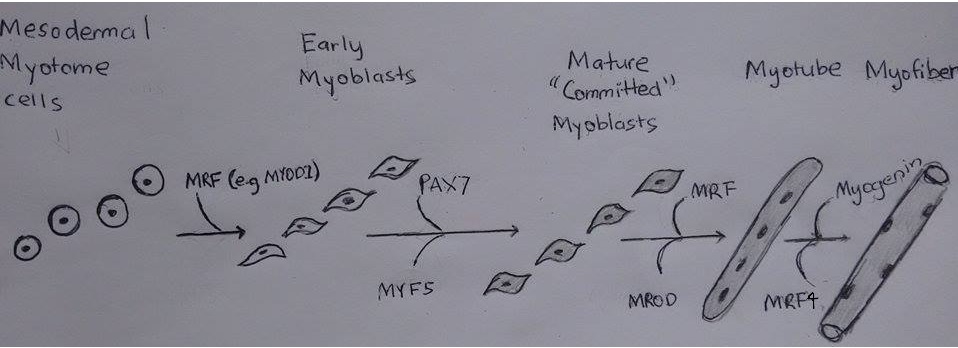
As discussed previously the mesoderm forms somites which divide into the dermomyotome, scleratome and myotome. The myotome becomes the skeletal muscle. Myotome cells migrate to different positions, for example limb buds. Once myotome cells travel to a target destination. Mrf like MYOD1 allow for the transdifferentiation of mesodermal myotome cells to the immature muscle cells, myoblasts.
Then the myoblasts proliferate under the influence of growth factors up until the neonatal period. Following this there is a decrease of growth factors which results in the myoblasts stop proliferating and align. The membranes of the myoblasts fuse together and become myoblasts.
Approximately 100 or more myotubes form a muscle fiber. Note that myoblasts still continually are fusing to this growing mass of muscle cells.Primary myotubes form the scaffolding for which the fetal myoblasts will differentiate into secondary myotubes and add too [10].
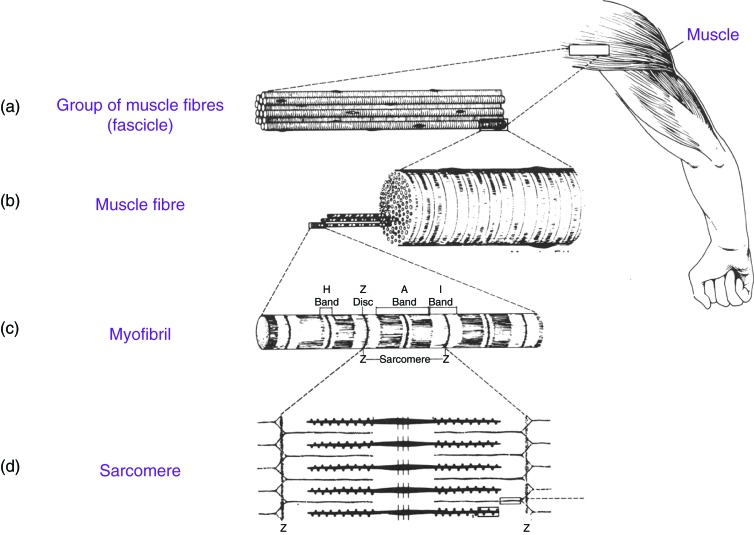
When myoblasts fuse and become myotubes there are number of changes that occur. In the cytoplasm of myotubes features of striated skeletal muscle develops like myofilaments and myofibrils. Myofibrils contain thick myosin and thin actin proteins which repeat along the myofibril to form repeating units known as sarcomeres. The sliding of myosin and actin allows for the contraction of muscle. In sarcomeres the nuclei are pushed to the side.
What is known as primary myofibre forms when after growth factors attract the nerve and it attaches to the bed of muscle. The nerve gives of branches to secondary myofibres that form later.
| Muscle Myogenesis Movie |
|---|
| <html5media height="400" width="400">File:MYOGENESIS_video.mp4</html5media> |
External laminae develops for the myotubes and separates the myotubes from surrounding mesenchymal tissue. Sheaths containing the myofibers develop; endomysium, epimysium and perimysium. External laminae and reticular fibers form the endomysium. Perimysium and epimysium layers are the formed by fibroblasts.
There are a greater deal of secondary myofibers than primary and are receptive to nutrients and growth factors. Muscle regulatory factors (MRFs) control the proliferation of secondary myoblasts. MRF’s are helix-loop-helix transcription factors.
| Cell type/Stage | Associated Molecules | Action |
|---|---|---|
| Mesodermal Myotome cells | MYOD1 | Allow for transdifferentiation into myoblast |
| Early Myoblasts | PAX7 , MYF5 | Act on CDK4 and Cyclin D1 to dephosphorylate Rb and induce cell proliferation [11] [12] [13] |
| Mature ‘Committed’ Myoblasts | MRF, MROD | MYOD acts on myostatin to take myoblast out of cell cycle and ready for differentiation [11] |
| Myotubes | Myogenin, MRF4 | Inhibit cell cycle and proliferation by acting on regulatory proteins like P21 to stop the cell cycle allowing for the conversion of myotube to myofiber. [11] [14] |
Following the formation of myofibers, growth factors, amino acids and stretch/load activity act on myofibers to affect subsequent hypertrophy maturation of myofibers [11]
Myofiber hypertrophy is occurs when protein synthesis is greater than protein degradation. The resulting accumulation of protein results in hypertrophy. Therefore maintaining protein levels is important in the hypertrophy of myofibers. This is regulated by nutrients and growth factors (GFs). Growth factors There are a large number of growth factors that affect fetal myogenesis. Some of the main GFs as Brown (2014) describes in her article “IGF1, insulin, basic fibroblast growth factor (bFGF), transforming growth factor-β (TGF- β)” [11] [15] [16] Experiments have shown that if IGF1 is removed reduced muscle mass and hypoplasia has resulted. And increased IFG1 expression has resulted in hyperplasia and increased skeletal muscle. IFG1 has also been found to enhance protein synthesis. [11][17][18] Insulin similarly has been found to promote fetal muscle growth and protein synthesis. Basic fibroblast growth factor (bFGF) and transforming growth factor-β (TGF- β) induce proliferation and myogenesis by upregulating cyclin D.
Nutrients Being the building block of proteins, amino acids are important in muscle protein synthesis. But they are more important in adult muscle protein synthesis than fetal muscle protein synthesis. Experiments have shown for example an amino acid infusion did not always result in a fetal muscle growth, only when there was a rise in the insulin levels. And little is known of the interaction of amino acids and growth factors in the context of fetal myogenesis. Research has shown that fetal or secondary myofibers are more prone to suffer from nutrient deficiency than primary myofibers in pigs and sheep. [19][20] [21]
Fetal myofiber number have been observed to decrease with detal nutrient deficiency. This is concerning as myofiber numbers are set at birth. Nutrients are also important for fetal myofiber hypertrophy. Stretch and loading Stretch and loading also affect hypertrophy. [22]
Tendon Development
Tendons are connective tissue which join muscle and bone allowing the transmission of force. They organised fibrils which form fibers, which along with fibroblasts are surrounded by connective tissue to form fascicles[23]. Tendons primary embryonic structure originate from mesenchymal progenitor somite cells with further contributions of Neural Crest and Lateral plate mesoderm.
Appearance of tendons begins in the 20th Carnegie stage and marks the beginning of fibrillogenesis. This process is initiated by fibroblasts in series of extracellular compartments; they enlarge the cells domain into extracellular space. Channels deep in the cytoplasm drive the process of elongation, these channels location is associated with Golgi bodies. [24]
First type of compartments are formed by collagen containing secretory vacuoles which fuse with surrounding cell membranes. Initial fibrillogenesis is mediated my macromolecular interactions based on vacuole content, with a lesser input from receptor membrane interactions. Fibril groups as fibres close to the cell surface and Secondary extracellular compartments form; at this stage fibroblast are arranged adjacently[24] . Third level of compartmentalization forms later when fibroblast are adjacent with 2 or more other fibroblasts. As the tendon matures fibres coalesce invading each other with interdigitating processes. Secretory vacuole persist, laterally aggregating with further growth preserving sites for fibril deposit. [25] Collagen fiber assembly branch creating fibre networks along fascicles. Fibres branch within tendon fascicle
The main regulatory factors of tendon fibrillogenesis are Leucine-rich repeat proteoglycans.
| Non-fibrillar components | Molecular Characteristics | Function |
|---|---|---|
| Fibromodulin | Keratin sulphate, proteoglycan | Fibromodulin modulates the site-specific cross-linking ultrastructure of collagen, ensuring mechanical strength. Control the pattern of lysyl oxidase-mediated collagen cross-linking by reducing access of the enzyme to telopeptides, by binding to the collagen. [26] |
| Decorin | Chondoirin sulphate, proteoglycan | Regulates expression of multiple leucine-rich proteoglycansins(SLRP) during tendon fibrillogenesis, via Class I and II Small(SLRP). Competes with Biglycan for binding sights on collagen types I-VI . Concentration increases as fetal development continues. [27] |
| Biglycan | Chrondroitin sulphate and Dermatan sulphate, proteoglycan | Process of regulation closely mimic Decorin though concentration is maximal expression at day 16-18 during embryonic development, reducing after this point. Competes with Decorin for bonding sights on collagen I-VI. [28] |
Historical findings
Predating 1970 the adult musculoskeletal system was heavily researched which provided some overlap with prenatal development, though few studies existed which focused on the fetal stages. During the 70's a majority of research in the field focused on the histologist differences and development of differing fiber types. Research on morphological development was very limited with eh exception of two Czechoslovakian studies in the late 1980's which displayed muscle formation for multiple primordia and sexual differentiation.
The differing developments of alpha and beta fibers was revealed in a study by University of California published in 1972 using lamb fetuses as an experimental model. Beta muscle fibers are formed during the first stages of fusion, the individual Beta fibers create a network for the alpha fibers to develop on. Red muscle fasciculi are formed by merging of small fiber bundles. White muscles are formed by ongoing addition of alpha fibers. Additionally it was concluded that fetal muscle contraction didn't significantly effect number of fibers present. Most fibers had already been formed by the 20th week, during this period the limit muscle contractions result in little mechanical tension.
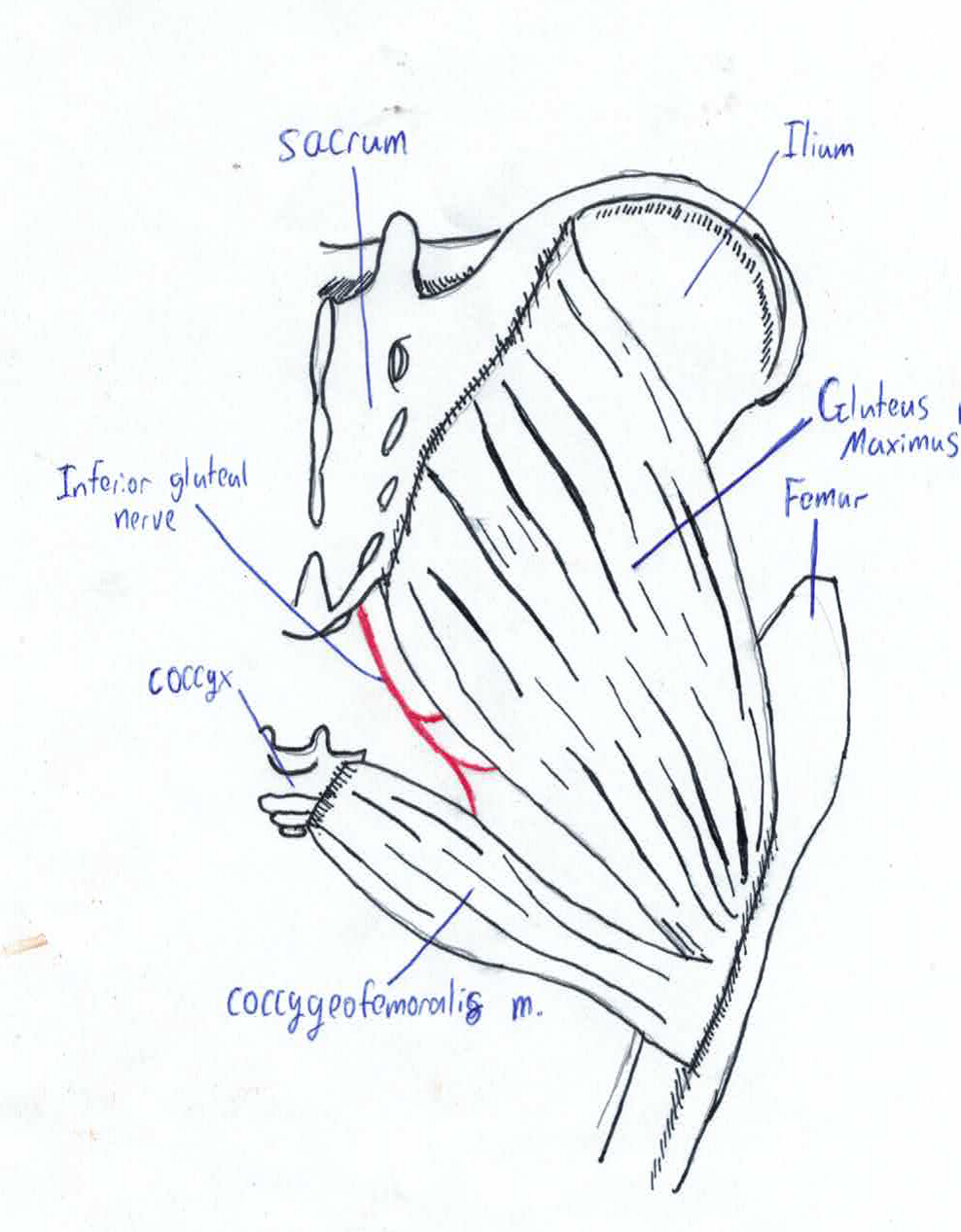
Gluteus Maximus muscle Morphogenesis
A 1985 Czechoslovakian study investigated the development of Gluteus Maximus during the embryonic and fetal periods. Pelvic micro-dissection of human embryos and foetuses with crown length varying from 22-215mm were compared with newborns and adults. The presence of a muscle not present in post-natal adults[29] was discovered called the coccygeofemoralis or pars coccygea; this muscle originates from sides of coccyx and inserts onto the gluteal tuberosity. In the adult human this muscle fuses with the larger pars sacroiliac or fetal gluteus maximus to create the adult gluteus maximus. Pars sacroiliac originates from ilium and sacrum and inserts onto the gluteal tuberosity[30].
In fetus with crown length of less than 45 mm the two muscle primordia are separated by small amount of loose connective tissue. This point onwards the muscles become fused by a small furrow which persists until 215mm crown length. By the time of birth the furrow is absence and the muscles are entirely fused [31]. The coccygeofemoralis in long tailed mammals remains separate from the gluteus maximus, known as the caudofemeralis muscle [32]. In these animals its function is lateral flexion of the tail [33]. This is the sole morphogenesis study on any of the large muscles; it is likely other muscles with multiple origins and insertion have separate fetal muscle primordia.
Morphogenesis of human sphincter urethrae muscle
This study was completed in 1989 at the same Czechoslovakian institute of that of Gluteus maximus morphogenesis. . Study displayed three developmental phasess differentiating them by morphogenesis, histology and sexual dimorphism. External urethral sphincter in embryos and fetuses with crown length varying 18-320mm, neonates, children and adults was fixed in formaldehyde and embedded in paraplast. Then cut in series and stained with hematoxylin and eosin for histological analysis. Morphogenesis and Sexual dimorphism specimens were micro-dissected using a stereomicroscope. [34]
| Phase | Time Period | Characteristics |
|---|---|---|
| Indifferent Phase | Before 10th week | Muscle primordia discernible from neighbouring muscle by week 8. Grow to forms a shallow arch; connecting the urethra and urogenital diaphragm. Consists of condensation of myoblasts up to 9.5 weeks, past this point myotubes and muscle fibres appear. |
| Sexual dimorphic Phase | 10th week to Birth | Associated with the development of prostate and vagina. Primordia spread along urethra wall posteriorly.
In Males: Spreads to create the infraprostatic part of external urethral sphincter. Arches anteriorly to join prostate and urethra In Female: Spreads to create upper part of external urethral sphincter. Lower sixth of sphincter connects anterior and lateral urethral walls, additionally projects to lateral vaginal walls. |
| Definite Structuring Phase | After birth | Position of urethral sphincter does not alter in relation with prostate and inferior part of vagina. Infraprostatic region in males and upper part in females grow to form a complete ring. |
Current Research Models and Findings
Abnormalities
References
- ↑ <pubmed>10809386</pubmed>
- ↑ <pubmed>7748174</pubmed>
- ↑ 3.0 3.1 <pubmed> 9094722</pubmed>
- ↑ <pubmed> 12587921</pubmed>
- ↑ 5.0 5.1 <pubmed> 21204650</pubmed>
- ↑ <pubmed>5804561</pubmed>
- ↑ <pubmed>5046781</pubmed>
- ↑ <pubmed>10102814</pubmed>
- ↑ <pubmed>23100595</pubmed>
- ↑ <pubmed>640968 </pubmed>
- ↑ 11.0 11.1 11.2 11.3 11.4 11.5 <pubmed>24532817</pubmed>
- ↑ <pubmed>22445545</pubmed>
- ↑ 13.0 13.1 <pubmed>12242286</pubmed>
- ↑ <pubmed>10733231</pubmed>
- ↑ <pubmed>2190237</pubmed>
- ↑ <pubmed>22682632</pubmed>
- ↑ <pubmed>3546571</pubmed>
- ↑ <pubmed> 7744859</pubmed>
- ↑ <pubmed> 2041547</pubmed>
- ↑ <pubmed>8014156</pubmed>
- ↑ <pubmed>15317692</pubmed>
- ↑ <pubmed>23629510</pubmed>
- ↑ <pubmed>2337297</pubmed>
- ↑ 24.0 24.1 <pubmed>8115369</pubmed>
- ↑ <pubmed>7780173</pubmed>
- ↑ <pubmed>24849606</pubmed>
- ↑ <pubmed>16518859</pubmed>
- ↑ <pubmed>16810681</pubmed>
- ↑ <pubmed>5043313</pubmed>
- ↑ <pubmed>15788867</pubmed>
- ↑ <pubmed>4083527</pubmed>
- ↑ <pubmed>1255730</pubmed>
- ↑ <pubmed>8843689</pubmed>
- ↑ <pubmed>2610390</pubmed>